3 D Structure calculation 1 81 Structure Calculation




![Local fluctuating magnetic fields • Bloc(t) = Bloc[iso] + Bloc(t)[aniso] - Isotropic part is Local fluctuating magnetic fields • Bloc(t) = Bloc[iso] + Bloc(t)[aniso] - Isotropic part is](https://slidetodoc.com/presentation_image_h/fd3cc8fce034d9c5c2c1a430085f182e/image-22.jpg)
- Slides: 38

3 D Structure calculation 1 /81

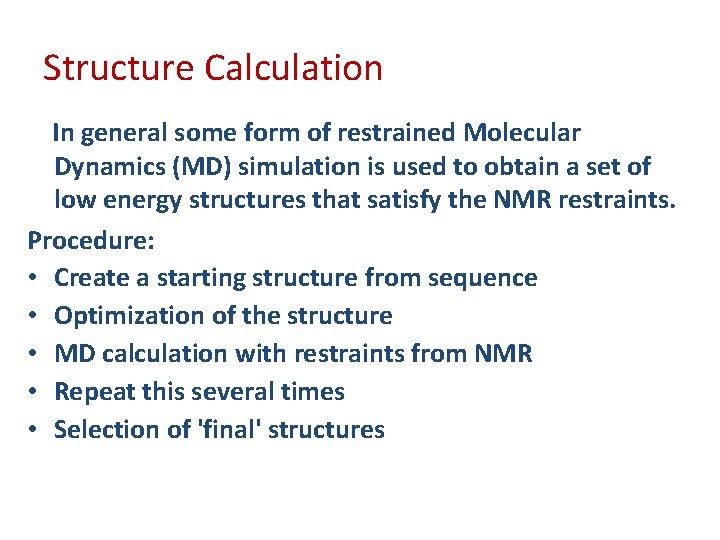
Structure Calculation In general some form of restrained Molecular Dynamics (MD) simulation is used to obtain a set of low energy structures that satisfy the NMR restraints. Procedure: • Create a starting structure from sequence • Optimization of the structure • MD calculation with restraints from NMR • Repeat this several times • Selection of 'final' structures

Starting structure and optimization • The amino acid sequence of the protein is used by the user- interface (Builder) of the modelling program to create an 'extended' starting structure. • Optimization is then done by Energy Minimization (Molecular Mechanics).

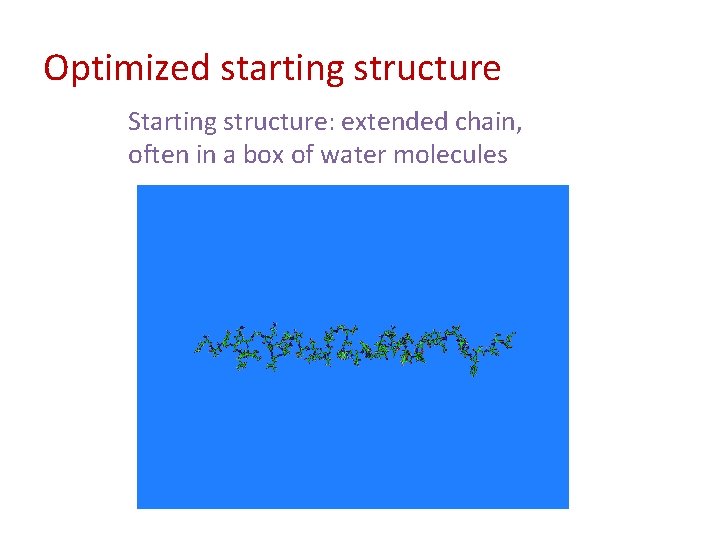
Optimized starting structure Starting structure: extended chain, often in a box of water molecules

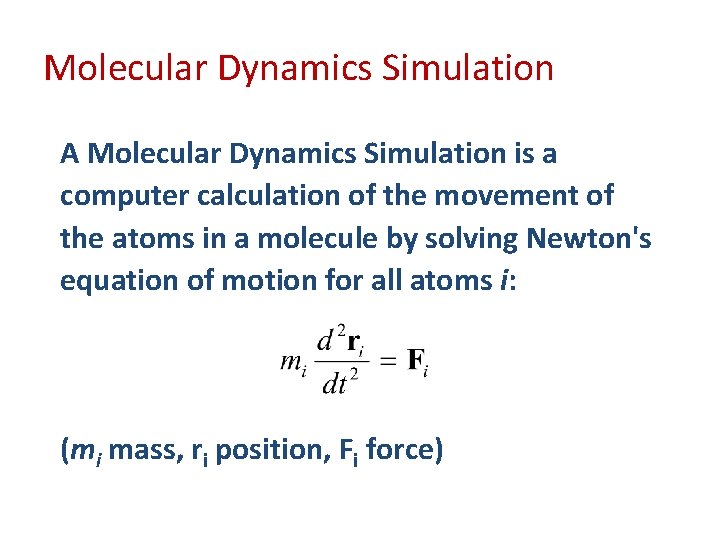
Molecular Dynamics Simulation A Molecular Dynamics Simulation is a computer calculation of the movement of the atoms in a molecule by solving Newton's equation of motion for all atoms i: (mi mass, ri position, Fi force)

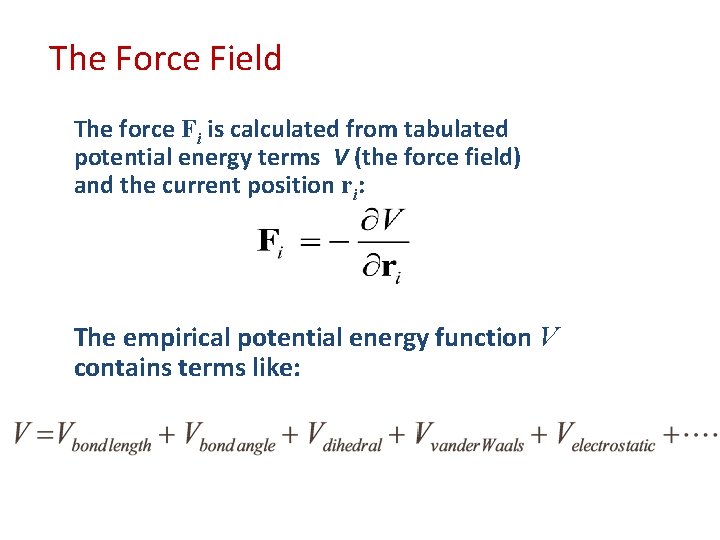
The Force Field The force Fi is calculated from tabulated potential energy terms V (the force field) and the current position ri: The empirical potential energy function V contains terms like:

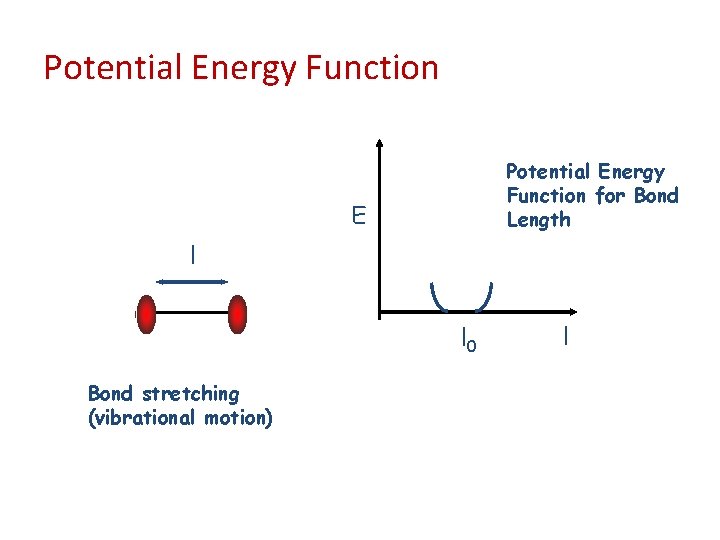
Potential Energy Function for Bond Length E l l 0 Bond stretching (vibrational motion) l

The NMR Restraints In addition to potentials of the force field: • Non-physical restraints for distances and dihedral angles (and others) from NMR • These are extra terms in the potential energy function V Restrained MD

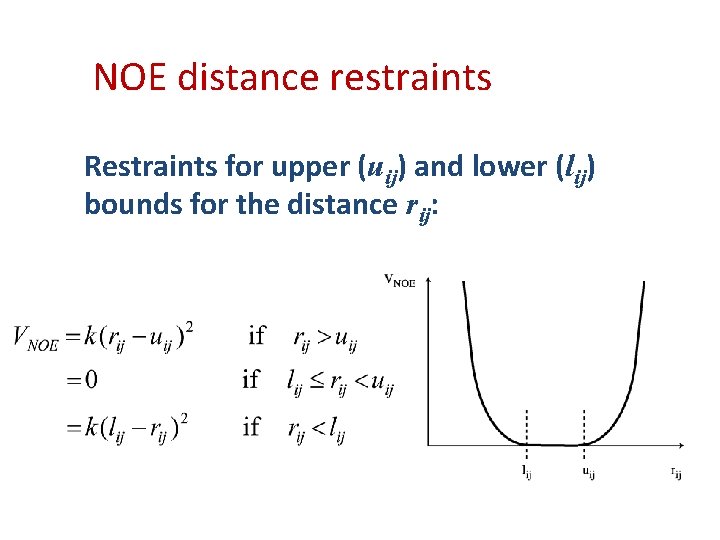
NOE distance restraints Restraints for upper (uij) and lower (lij) bounds for the distance rij:

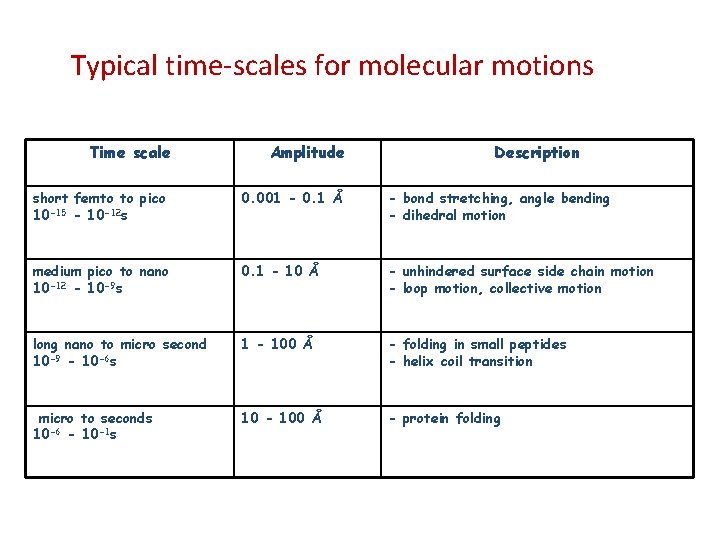
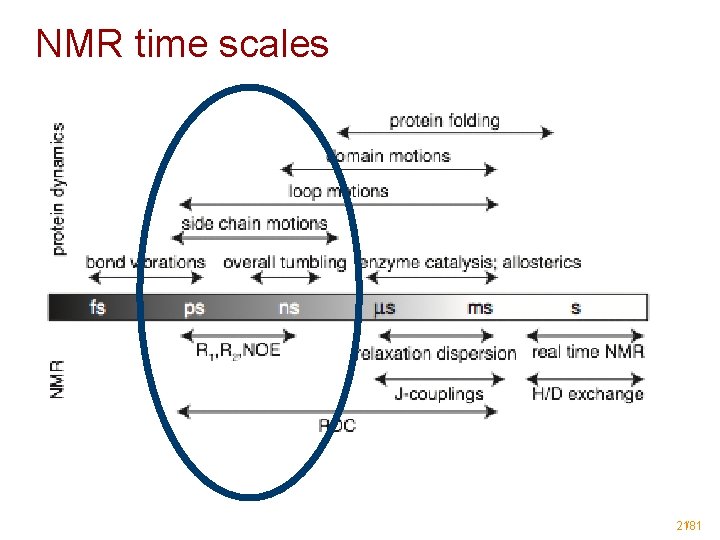
Typical time-scales for molecular motions Time scale short femto to pico 10 -15 - 10 -12 s Amplitude Description 0. 001 - 0. 1 Å - bond stretching, angle bending - dihedral motion medium pico to nano 10 -12 - 10 -9 s 0. 1 - 10 Å - unhindered surface side chain motion - loop motion, collective motion long nano to micro second 10 -9 - 10 -6 s 1 - 100 Å - folding in small peptides - helix coil transition micro to seconds 10 -6 - 10 -1 s 10 - 100 Å - protein folding Molecular Dynamics

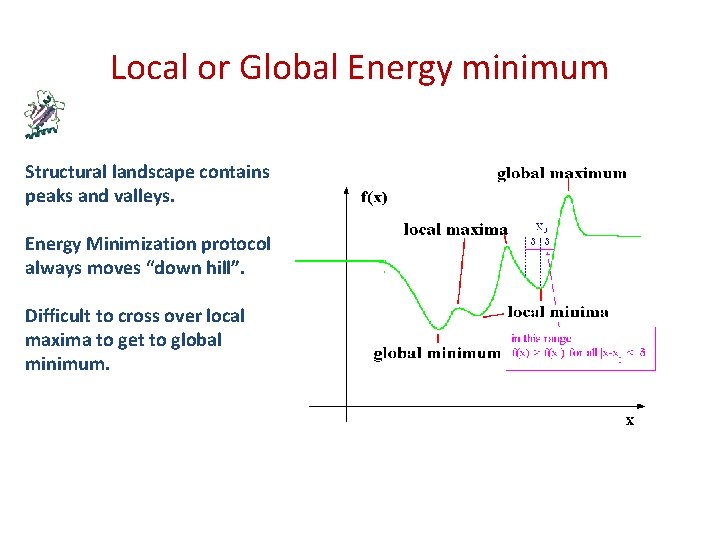
Local or Global Energy minimum Structural landscape contains peaks and valleys. Energy Minimization protocol always moves “down hill”. Difficult to cross over local maxima to get to global minimum.

Therefore: Simulated Annealing • Often used with Restrained MD • Potentials are 'down-scaled' in the beginning • Higher degree of freedom ('sampling a bigger conformational space') • In later steps the potentials are slowly brought to their final values. This is like first heating up the molecule and then cooling it down in small steps.

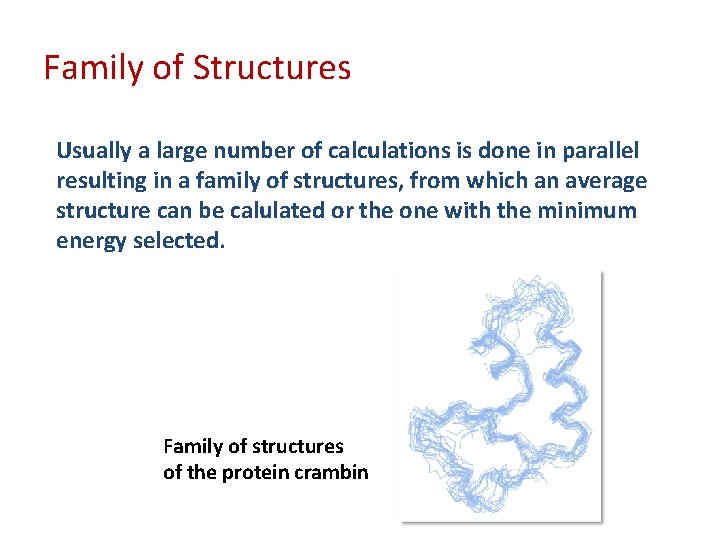
Family of Structures Usually a large number of calculations is done in parallel resulting in a family of structures, from which an average structure can be calulated or the one with the minimum energy selected. Family of structures of the protein crambin

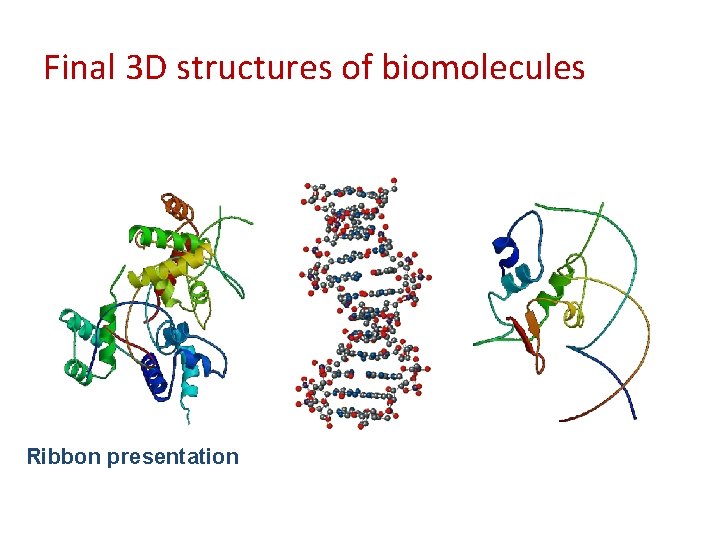
Final 3 D structures of biomolecules Ribbon presentation

Summary: Restrained Molecular Dynamics Choose a force-field Add constraints from NMR data Starting coordinates of all atoms (starting structure) Starting velocities of all atoms ('random seed numbers', Maxwell Boltzmann distribution) • Solve Newton's classical equation of motion for very small steps (few femto-seconds) • Calculate new coordinates, forces and velocities • Repeat the last two steps to find structure with lowest energy • •

Increasing the NMR size limit 16/81

Advances in hardware & techniques • Higher magnetic field strength - Increased resolution & sensitivity Maximum now 1000 MHz (1 GHz) • Cryoprobes - Cooling of probe coil with He gas (~20 K) Reduces thermal noise generated by electric circuits Increase of sensitivity by factor of 3 -4 • Dynamic nuclear polarization (DNP) - Transferring spin polarization from electrons to nuclei Requires saturation of electron spins by Gyrotron irradiation So far only for solid-state NMR 17/81

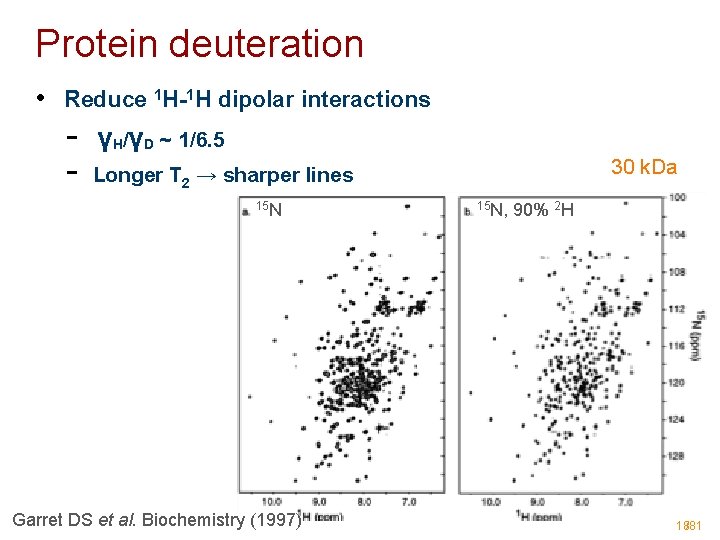
Protein deuteration • Reduce 1 H-1 H dipolar interactions - γH/γD ~ 1/6. 5 - 30 k. Da Longer T 2 → sharper lines 15 N Garret DS et al. Biochemistry (1997) 15 N, 90% 2 H 18/81

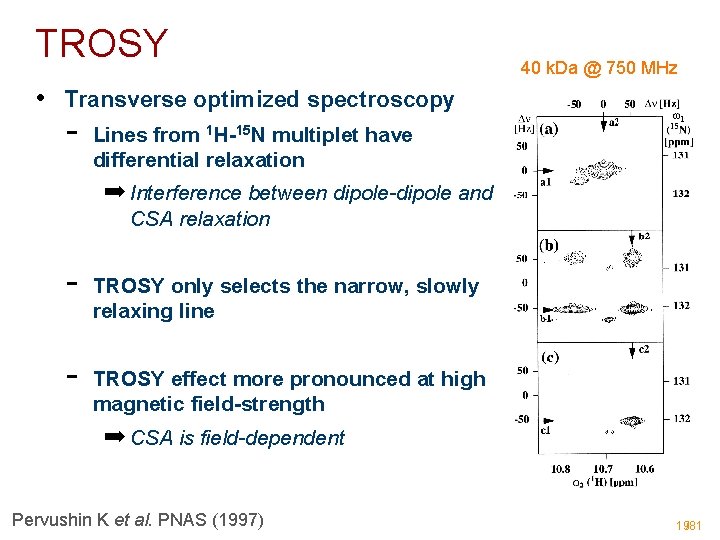
TROSY 40 k. Da @ 750 MHz • Transverse optimized spectroscopy - Lines from 1 H-15 N multiplet have differential relaxation ➡ Interference between dipole-dipole and CSA relaxation - TROSY only selects the narrow, slowly relaxing line TROSY effect more pronounced at high magnetic field-strength ➡ CSA is field-dependent Pervushin K et al. PNAS (1997) 19/81

Relaxation & Dynamics 20/81

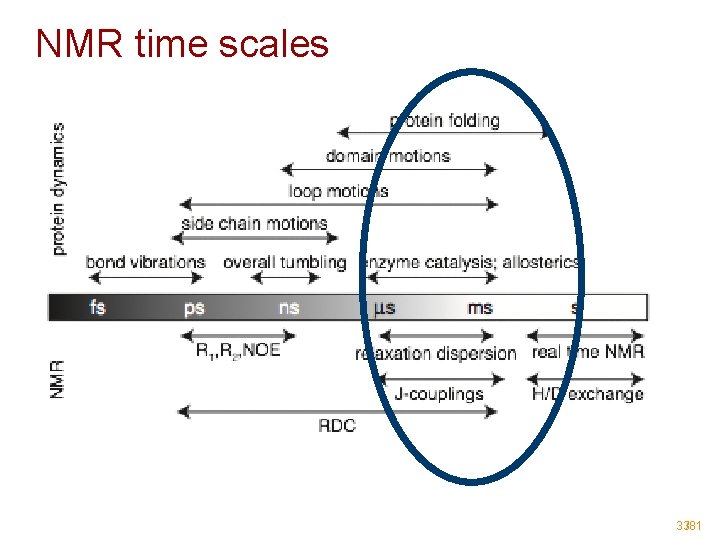
NMR time scales 21/81
![Local fluctuating magnetic fields Bloct Blociso Bloctaniso Isotropic part is Local fluctuating magnetic fields • Bloc(t) = Bloc[iso] + Bloc(t)[aniso] - Isotropic part is](https://slidetodoc.com/presentation_image_h/fd3cc8fce034d9c5c2c1a430085f182e/image-22.jpg)
Local fluctuating magnetic fields • Bloc(t) = Bloc[iso] + Bloc(t)[aniso] - Isotropic part is not time dependent ➡ chemical shift ➡ J-coupling Only the anisotropic part is time dependent ➡ chemical shift anisotropy (CSA) ➡ dipolar interaction (DD) B 0 13 C CSA anisotropic interactions r dipole-dipole 22/81

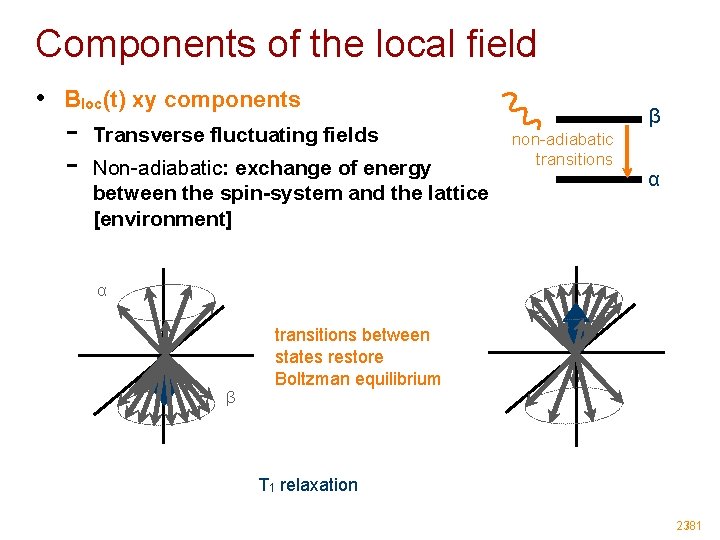
Components of the local field • Bloc(t) xy components - Transverse fluctuating fields Non-adiabatic: exchange of energy between the spin-system and the lattice [environment] β non-adiabatic transitions α α β transitions between states restore Boltzman equilibrium T 1 relaxation 23/81

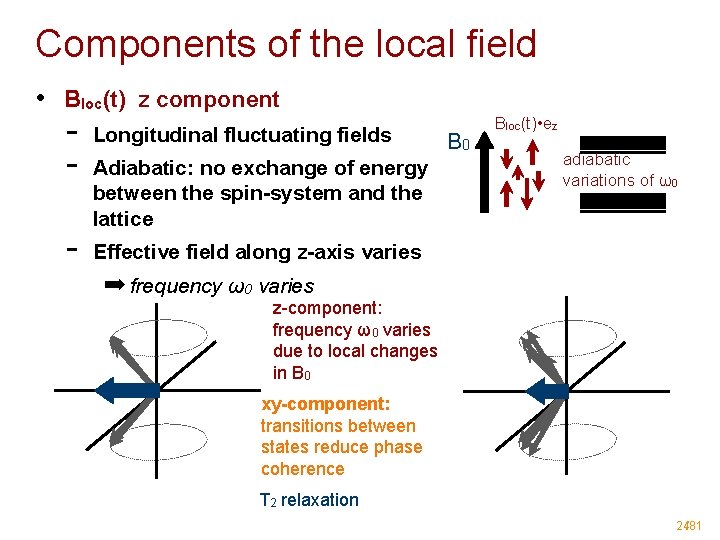
Components of the local field • Bloc(t) z component - Longitudinal fluctuating fields Adiabatic: no exchange of energy between the spin-system and the lattice B 0 Bloc(t) • ez adiabatic variations of ω0 Effective field along z-axis varies ➡ frequency ω0 varies z-component: frequency ω0 varies due to local changes in B 0 xy-component: transitions between states reduce phase coherence T 2 relaxation 24/81

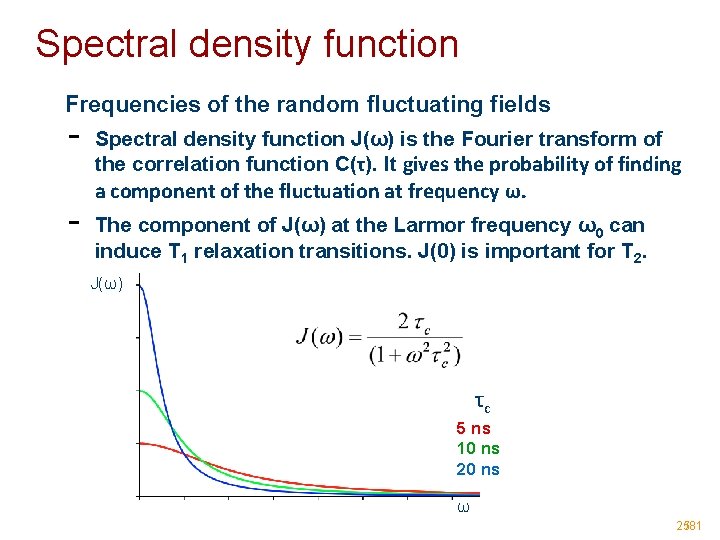
Spectral density function Frequencies of the random fluctuating fields - Spectral density function J(ω) is the Fourier transform of the correlation function C(τ). It gives the probability of finding a component of the fluctuation at frequency ω. The component of J(ω) at the Larmor frequency ω0 can induce T 1 relaxation transitions. J(0) is important for T 2. J(ω) τc 5 ns 10 ns 20 ns ω 25/81

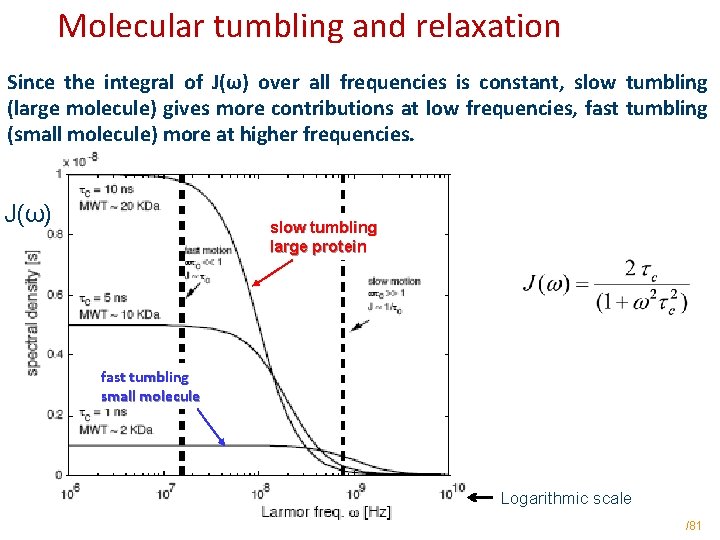
Molecular tumbling and relaxation Since the integral of J(ω) over all frequencies is constant, slow tumbling (large molecule) gives more contributions at low frequencies, fast tumbling (small molecule) more at higher frequencies. J(ω) slow tumbling large protein fast tumbling small molecule Logarithmic scale /81

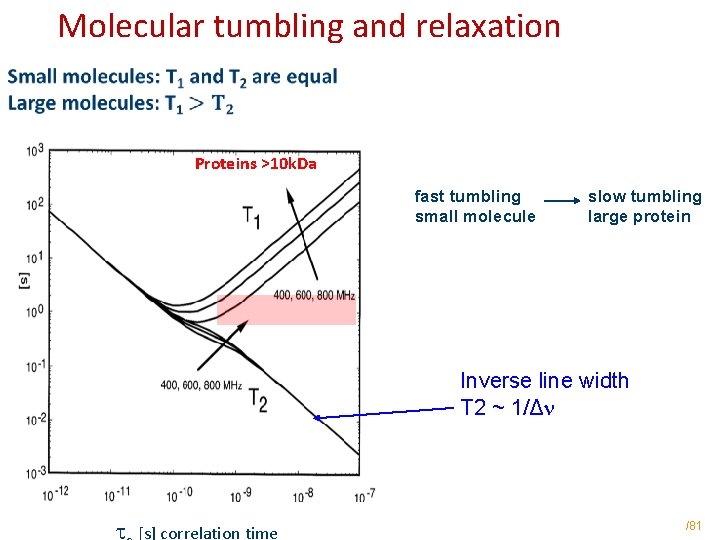
Molecular tumbling and relaxation Proteins >10 k. Da fast tumbling small molecule slow tumbling large protein Inverse line width T 2 ~ 1/Δn t [s] correlation time /81

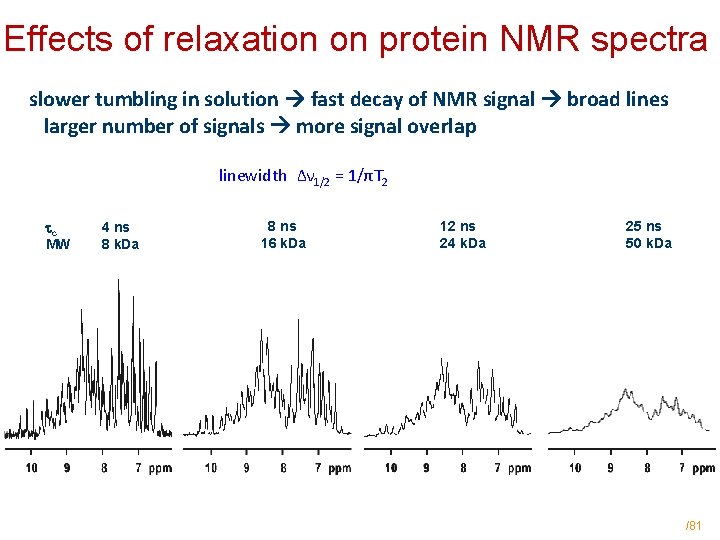
Effects of relaxation on protein NMR spectra slower tumbling in solution fast decay of NMR signal broad lines larger number of signals more signal overlap linewidth Δν 1/2 = 1/πT 2 c MW 4 ns 8 k. Da 8 ns 16 k. Da 12 ns 24 k. Da 25 ns 50 k. Da /81

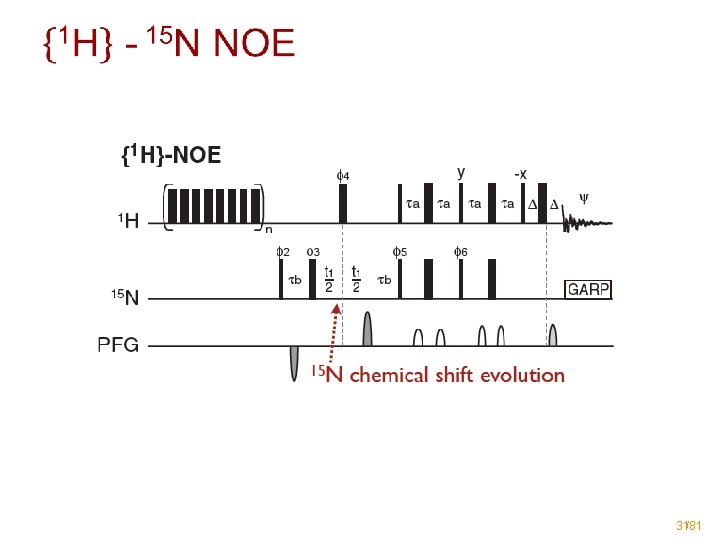
Protein backbone dynamics • 15 N relaxation to describe ps-ns dynamics - R 1: longitudinal relaxation rate R 2: transversal relaxation rate hetero-nuclear NOE: {1 H}-15 N • Measured as a 2 D 1 H-15 N spectrum - R 1, R 2: Repeat experiment several times with increasing relaxation-delay Fit the signal intensity as a function of the relaxation delay ➡ I 0. exp(-Rt) {1 H}-15 N NOE: Intensity ratio between saturated and nonsaturated experiment 29/81

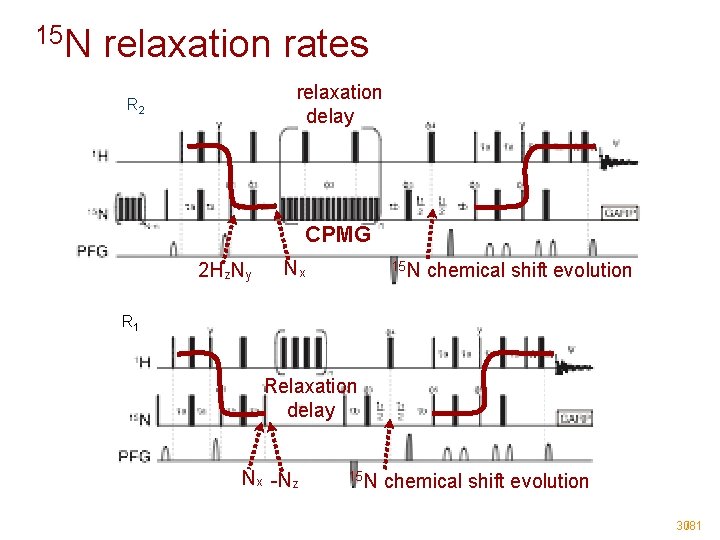
15 N relaxation rates relaxation delay R 2 CPMG 2 Hz. Ny Nx 15 N chemical shift evolution R 1 Relaxation delay Nx -Nz 15 N chemical shift evolution 30/81

31/81

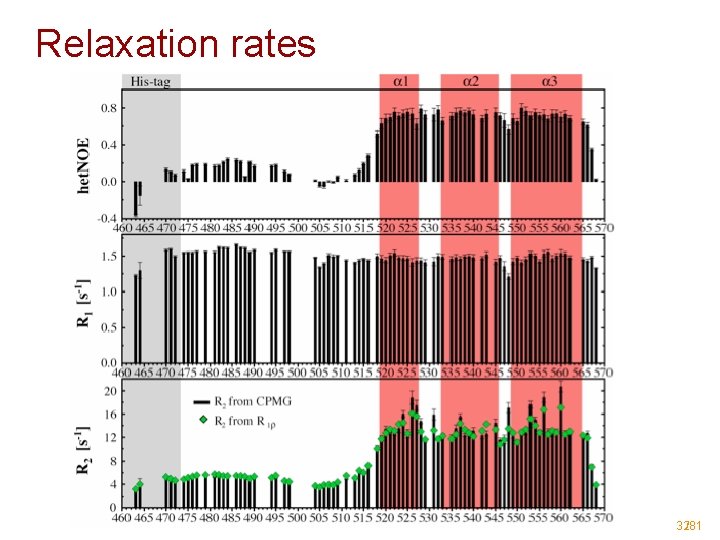
Relaxation rates 32/81

NMR time scales 33/81

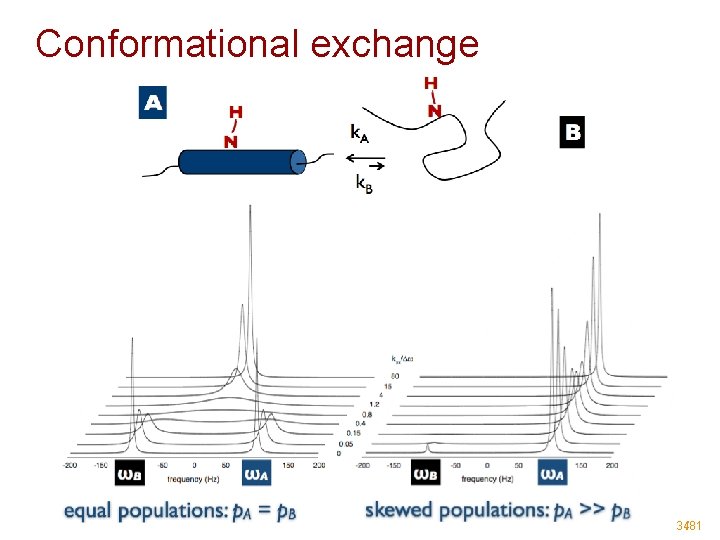
Conformational exchange 34/81

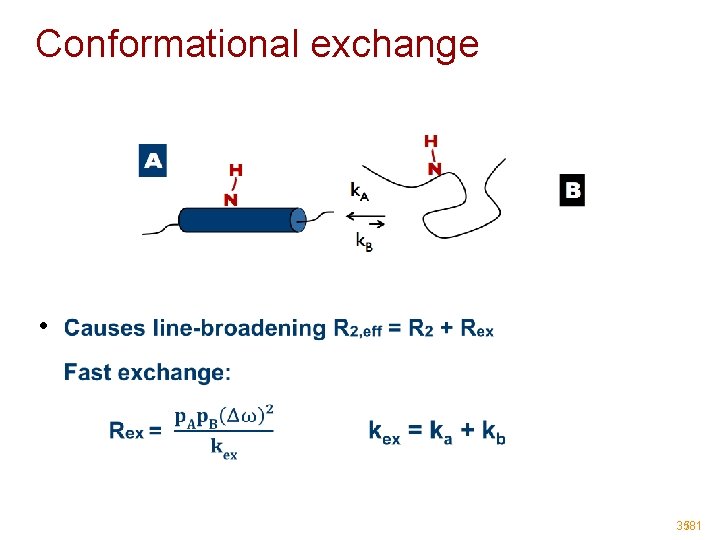
Conformational exchange • 35/81

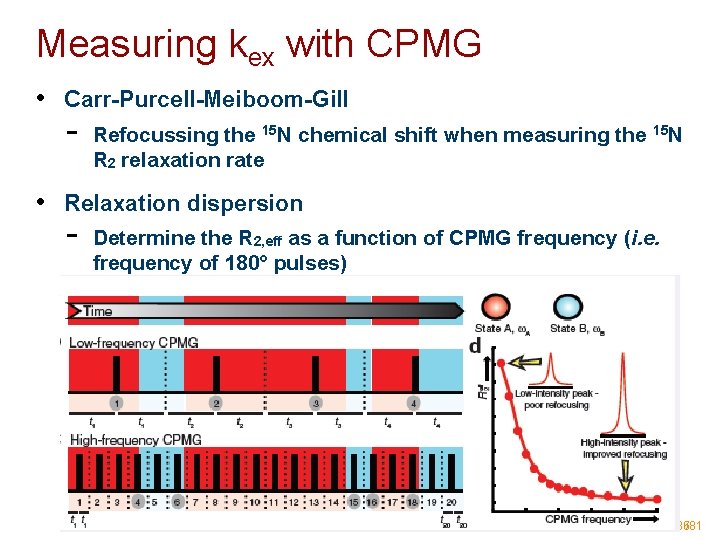
Measuring kex with CPMG • Carr-Purcell-Meiboom-Gill - Refocussing the 15 N chemical shift when measuring the 15 N R 2 relaxation rate • Relaxation dispersion - Determine the R 2, eff as a function of CPMG frequency (i. e. frequency of 180° pulses) 36/81

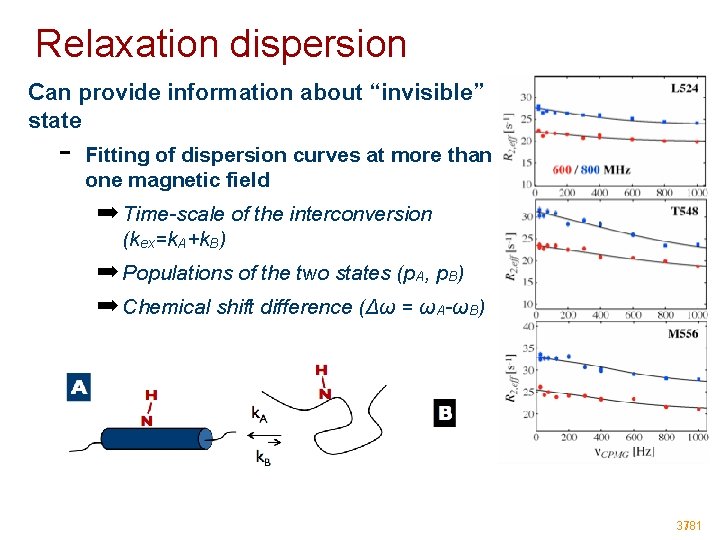
Relaxation dispersion Can provide information about “invisible” state - Fitting of dispersion curves at more than one magnetic field ➡ Time-scale of the interconversion (kex=k. A+k. B) ➡ Populations of the two states (p. A, p. B) ➡ Chemical shift difference (Δω = ωA-ωB) 37/81

Key concepts relaxation • Wide range of time scales • Fluctuating magnetic fields • Correlation function, spectral density function • rotational correlation time (ns) • fast time scale (ps-ns): flexibility (fast backbone motions) from 15 N relaxation and 1 H-15 N NOE • slow time scale (μs-ms): conformational exchange from relaxation dispersion CPMG 38/81