Alkanes Alkanes are molecules that contain only CC








- Slides: 53

Alkanes

Alkanes are molecules that contain only C-C and C-H bonds. Alkanes are either acyclic or cyclic.

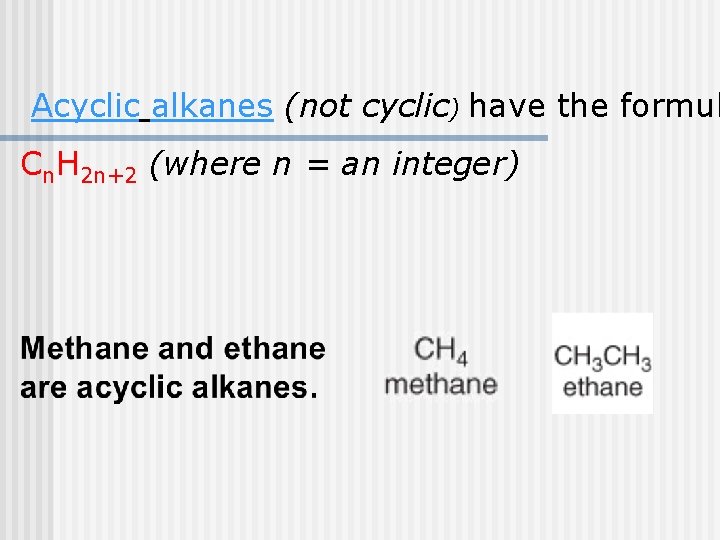
Acyclic alkanes (not cyclic) have the formul Cn. H 2 n+2 (where n = an integer)

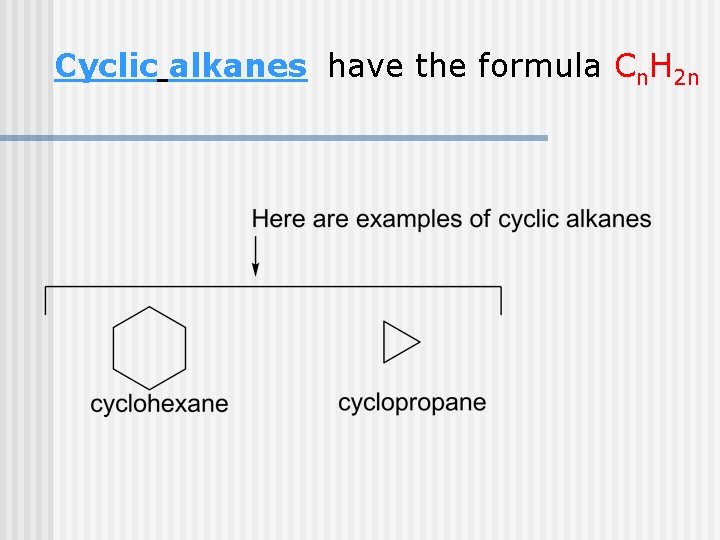
Cyclic alkanes have the formula Cn. H 2 n

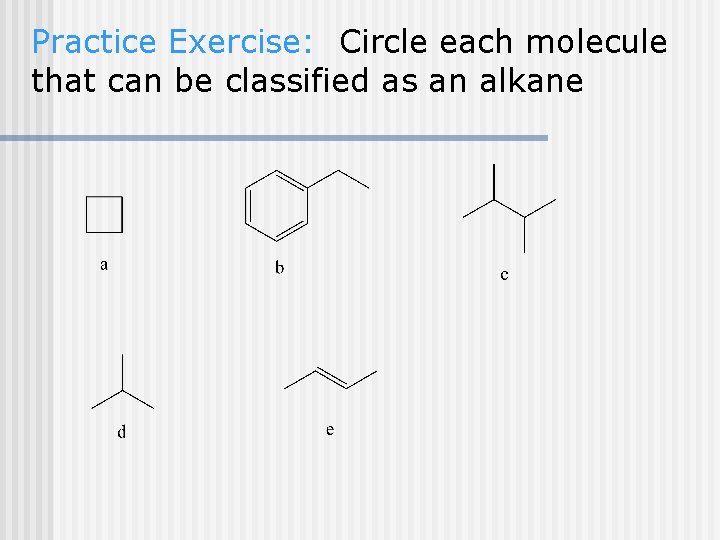
Practice Exercise: Circle each molecule that can be classified as an alkane

Classification of Alkanes

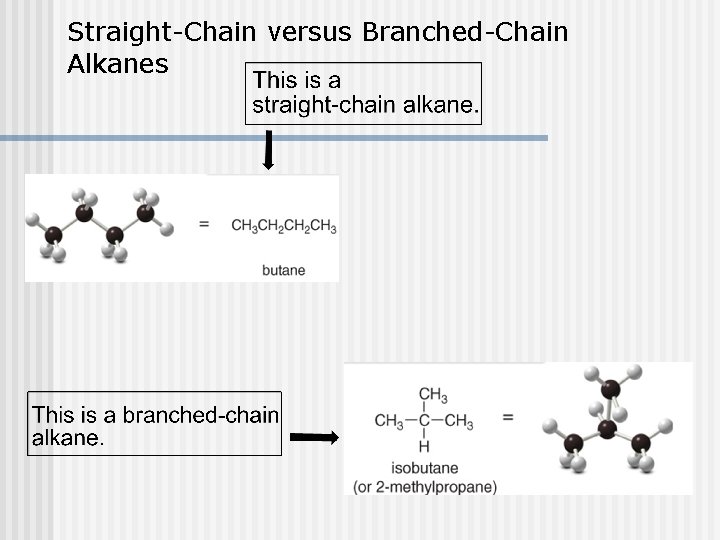
Straight-Chain versus Branched-Chain Alkanes

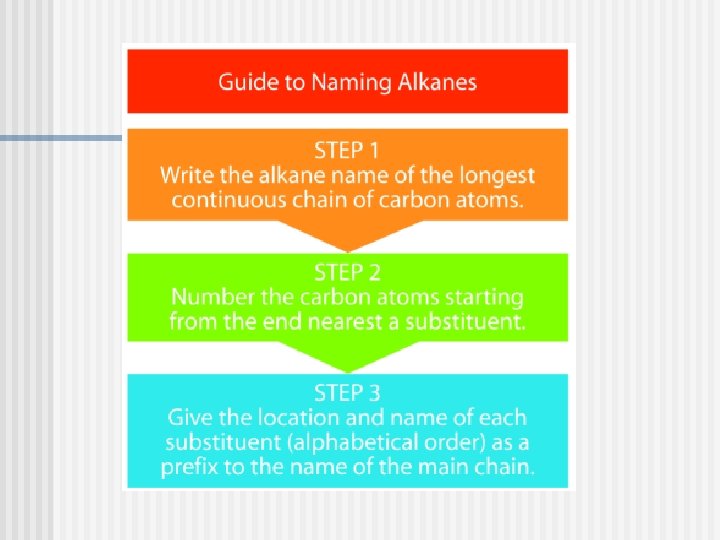
Naming Alkanes (Cn. H 2 n+2)

Before you learn to name alkanes you must…….

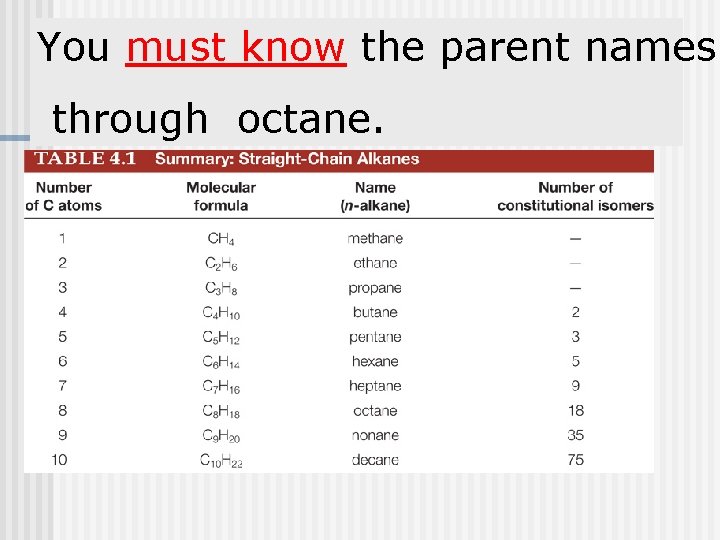
You must know the parent names through octane.

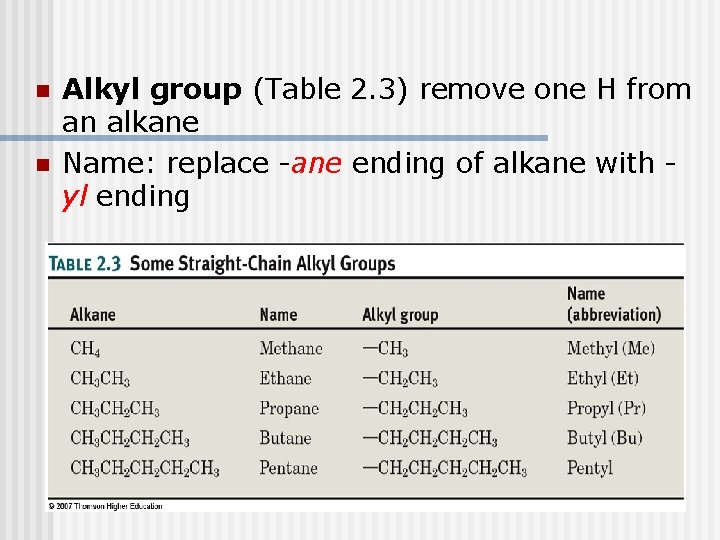
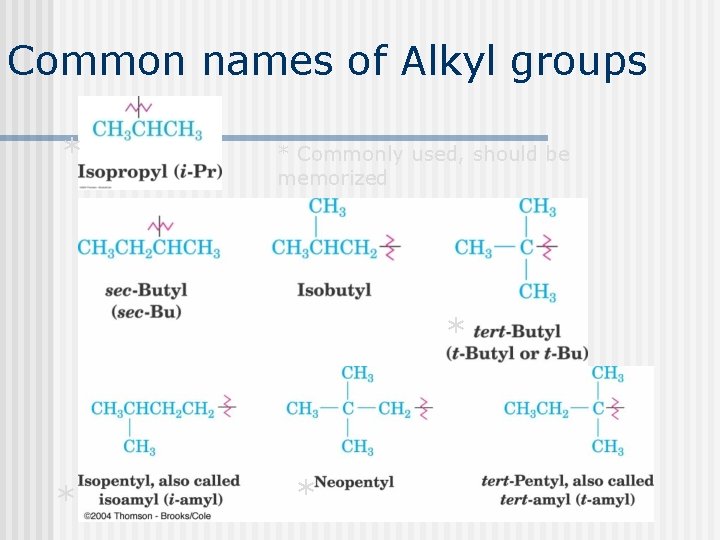
Naming Substituents (Alkyl Groups) Groups along C backbone are called substituents Alkane substituents are called alkyl groups Alkyl groups are the parent RH (alkane) minus a H atom

n n Alkyl group (Table 2. 3) remove one H from an alkane Name: replace -ane ending of alkane with yl ending

Common names of Alkyl groups * * Commonly used, should be memorized * * *

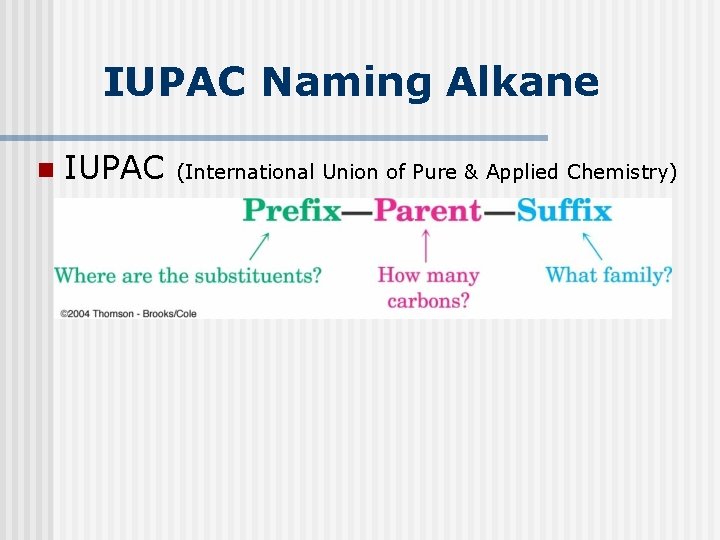
IUPAC Naming Alkane n IUPAC (International Union of Pure & Applied Chemistry)


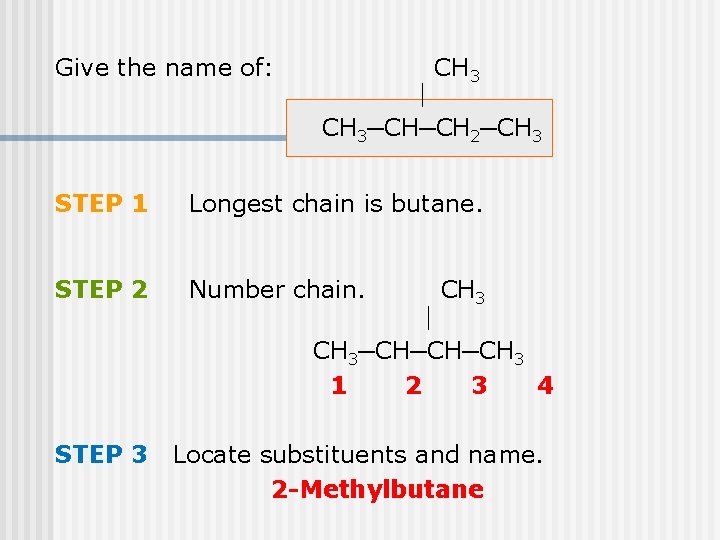
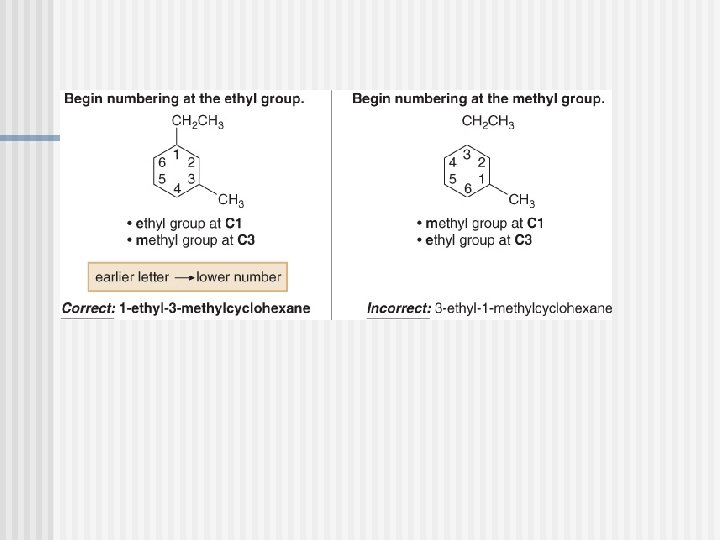
Give the name of: CH 3─CH─CH 2─CH 3 STEP 1 Longest chain is butane. STEP 2 Number chain. CH 3─CH─CH─CH 3 1 2 3 4 STEP 3 Locate substituents and name. 2 -Methylbutane

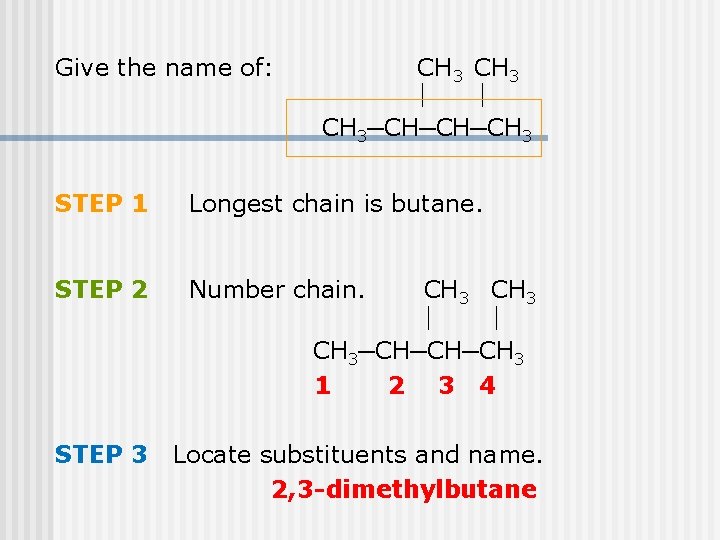
Give the name of: CH 3─CH─CH─CH 3 STEP 1 Longest chain is butane. STEP 2 Number chain. STEP 3 CH 3─CH─CH─CH 3 1 2 3 4 Locate substituents and name. 2, 3 -dimethylbutane

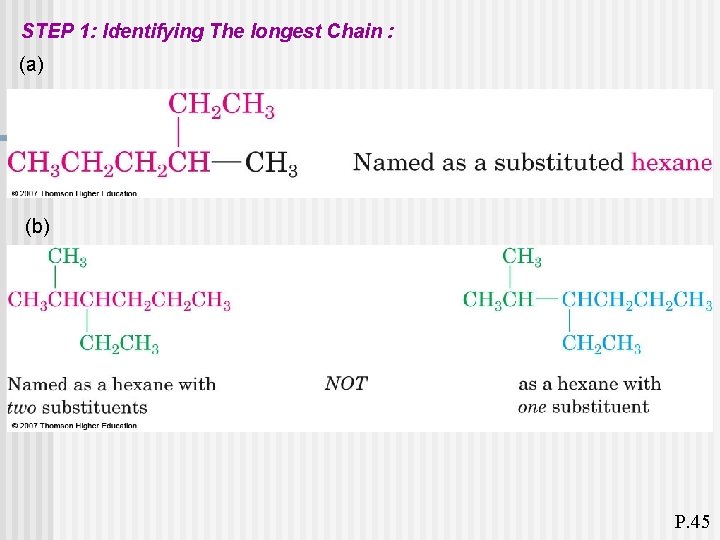
. STEP 1: Identifying The longest Chain : (a) (b) P. 45

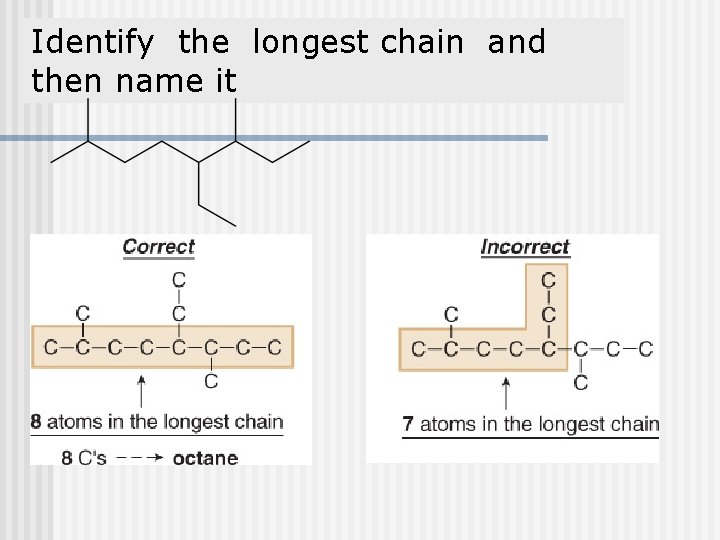
Identify the longest chain and then name it

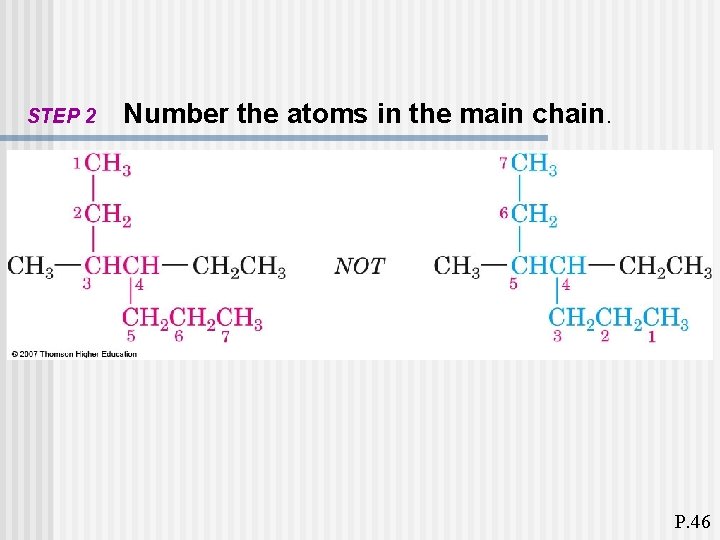
STEP 2 Number the atoms in the main chain. P. 46

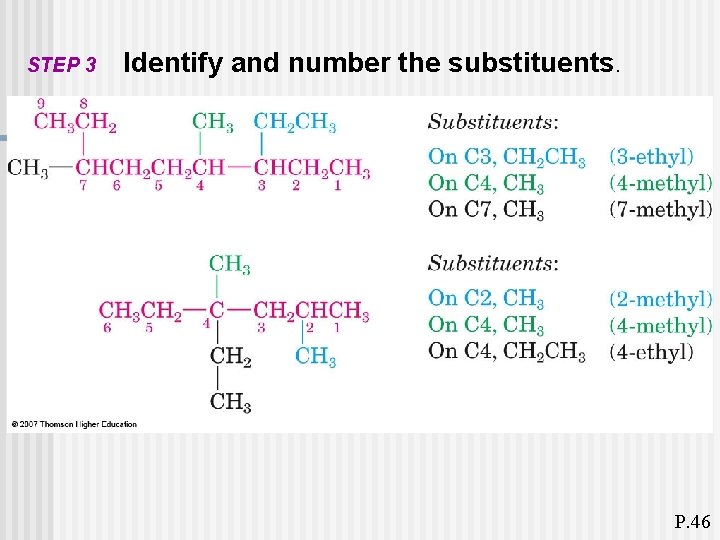
STEP 3 Identify and number the substituents. P. 46

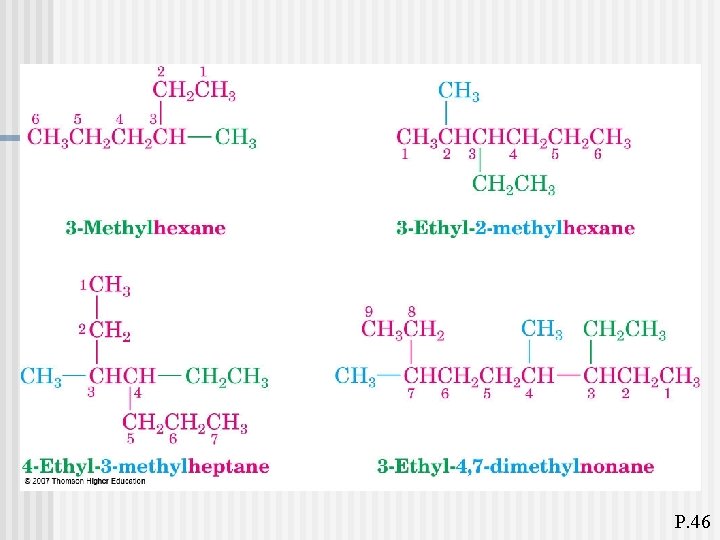
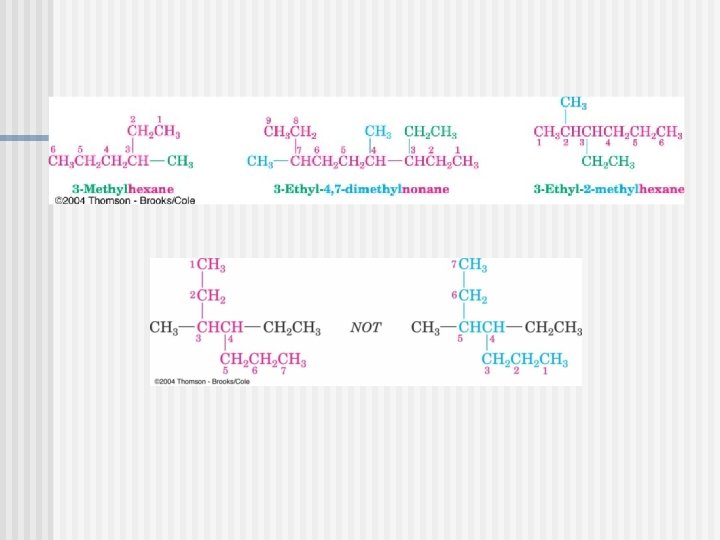
P. 46

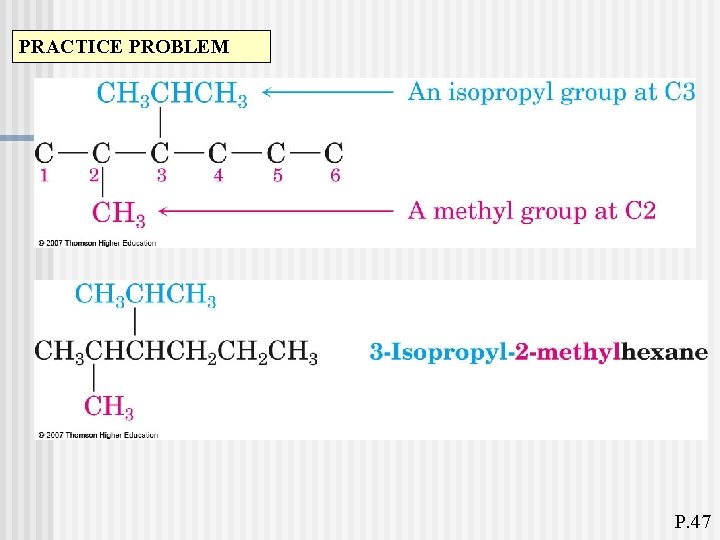
PRACTICE PROBLEM P. 47


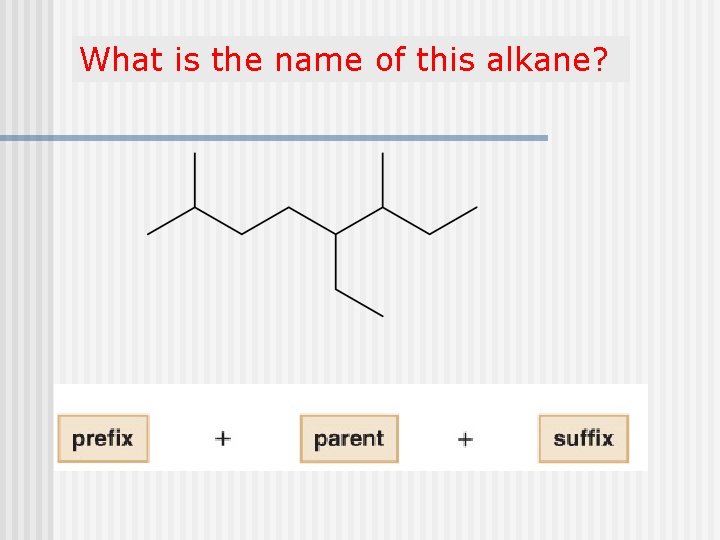
What is the name of this alkane?

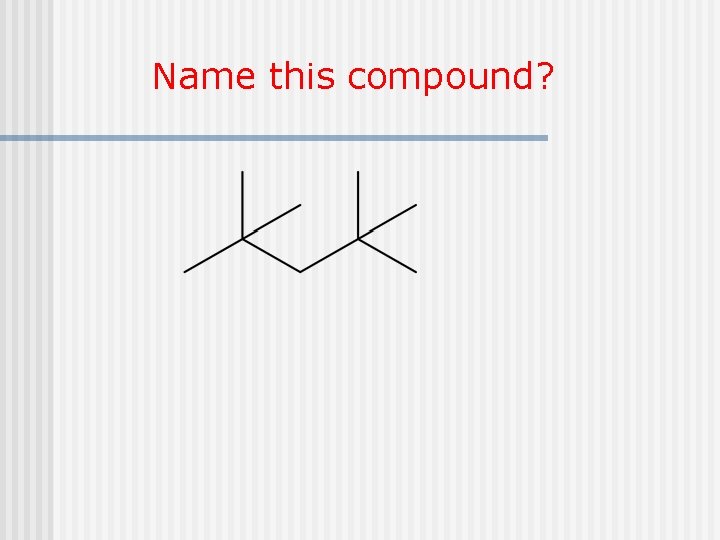
Name this compound?

Ex: Draw the skeletal structure of this compound? 3 -ethyl-2 -methylhexane

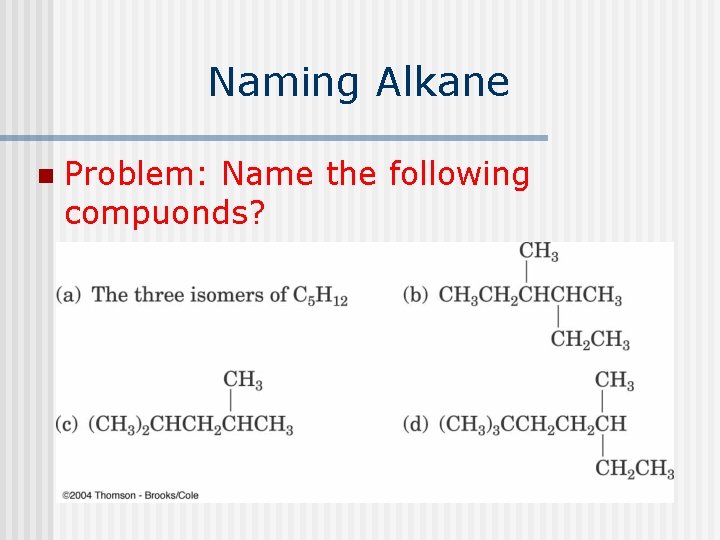
Problem: Name the following compounds?

Naming Alkane n Problem: Name the following compuonds?

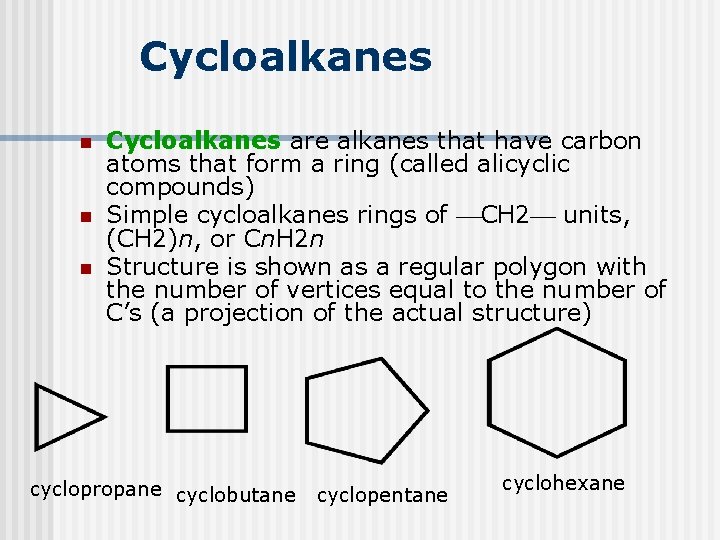
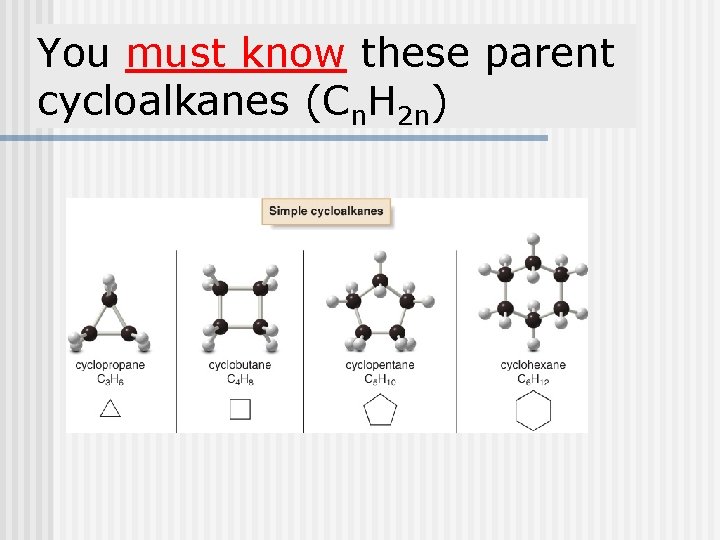
Cycloalkanes n n n Cycloalkanes are alkanes that have carbon atoms that form a ring (called alicyclic compounds) Simple cycloalkanes rings of CH 2 units, (CH 2)n, or Cn. H 2 n Structure is shown as a regular polygon with the number of vertices equal to the number of C’s (a projection of the actual structure) cyclopropane cyclobutane cyclopentane cyclohexane

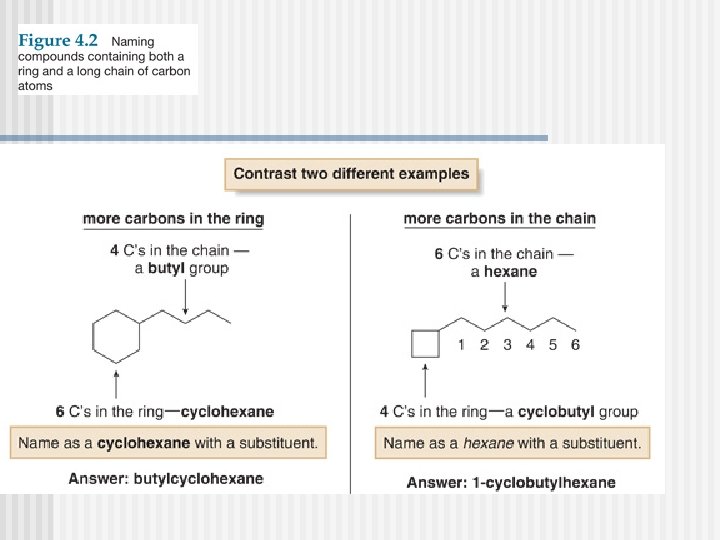
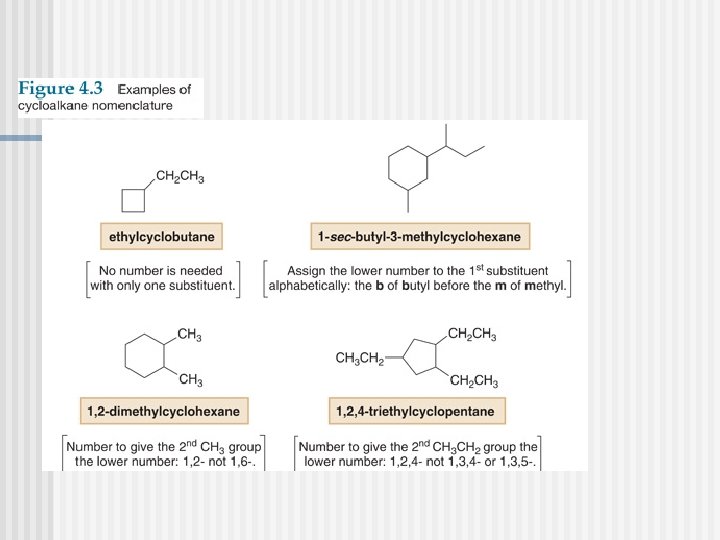
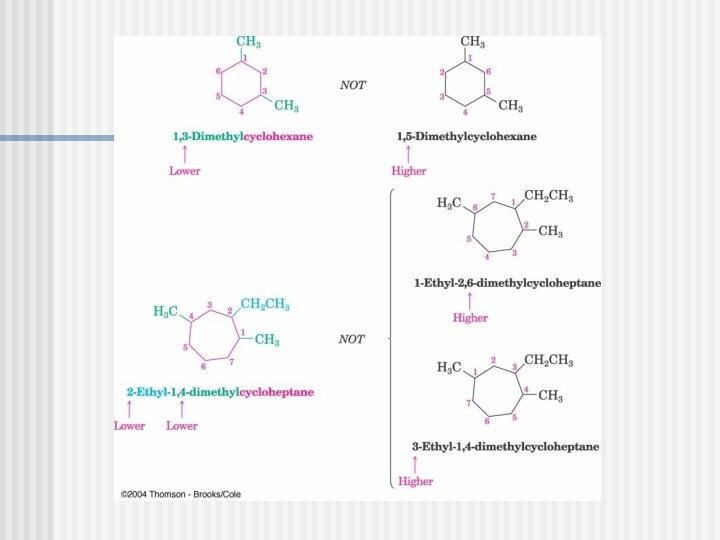
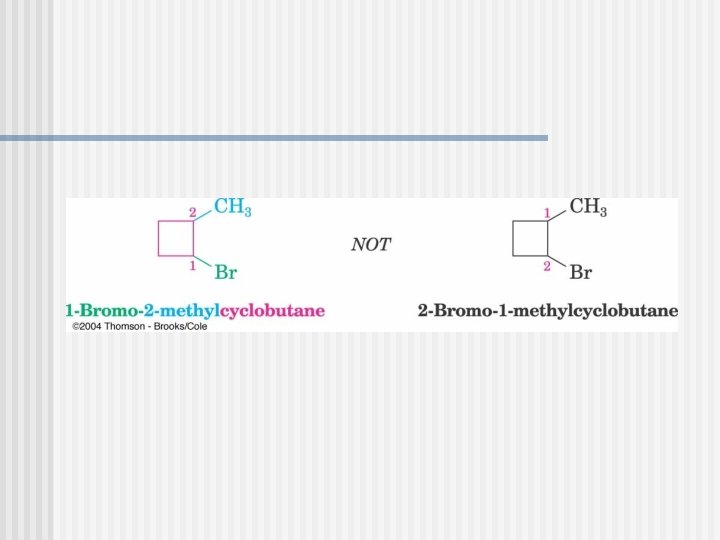
Naming Cycloalkanes

You must know these parent cycloalkanes (Cn. H 2 n)






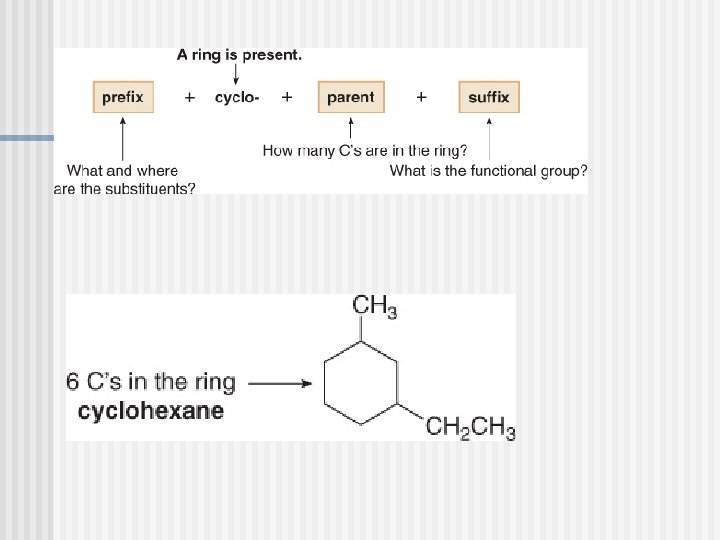
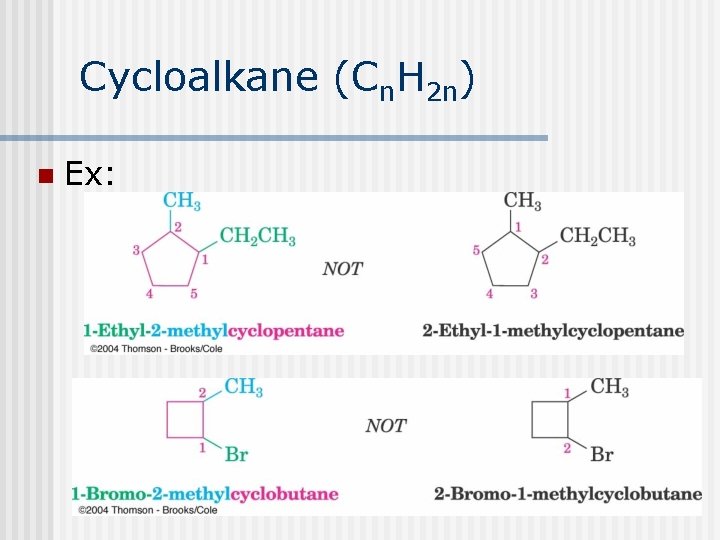
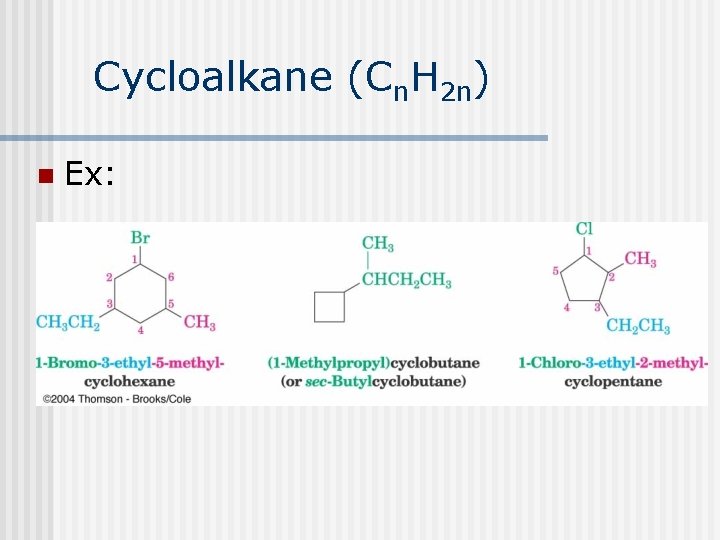
Cycloalkane (Cn. H 2 n) n Ex:

Cycloalkane (Cn. H 2 n) n Ex:



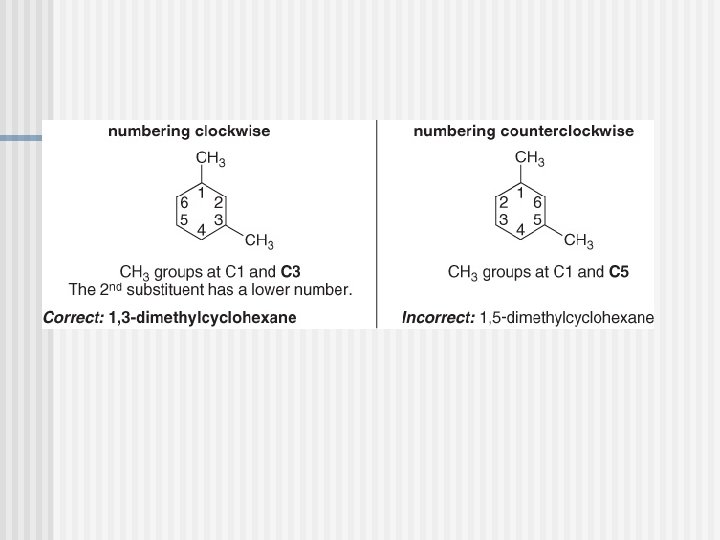
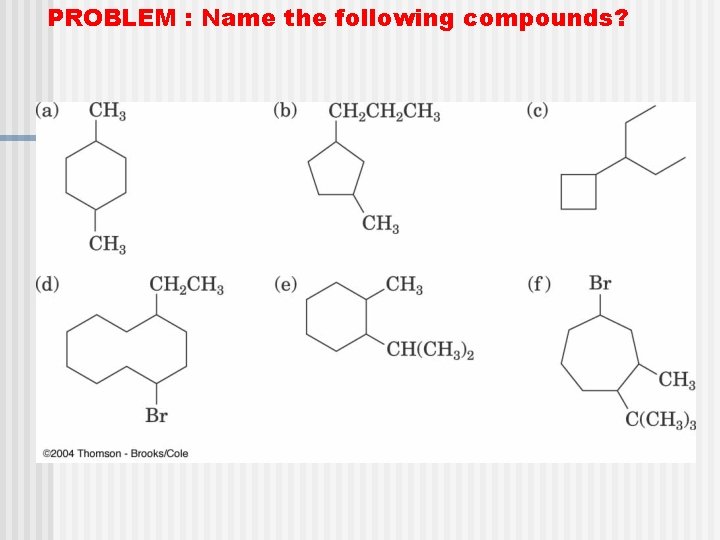
PROBLEM : Name the following compounds?

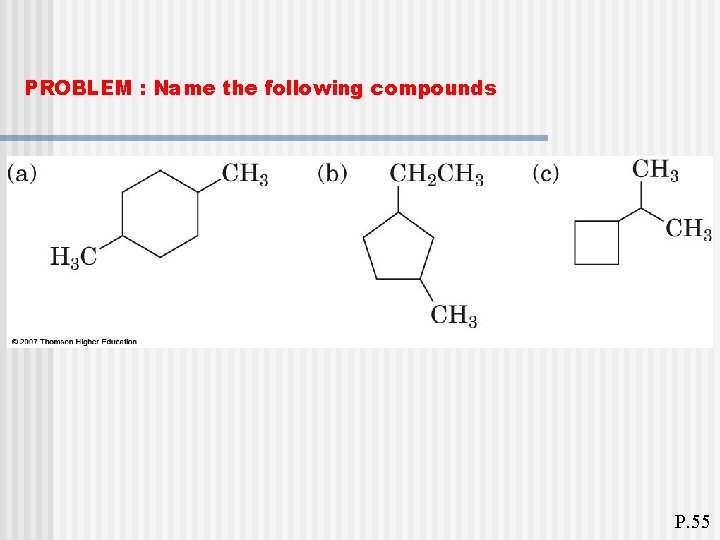
PROBLEM : Name the following compounds P. 55

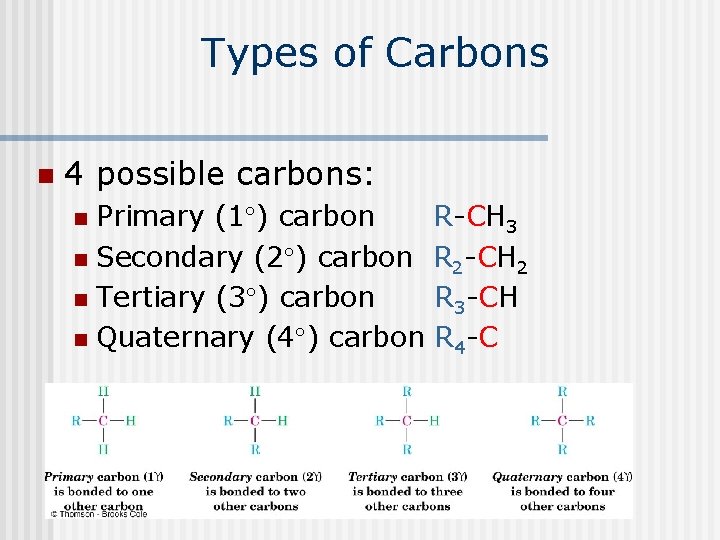
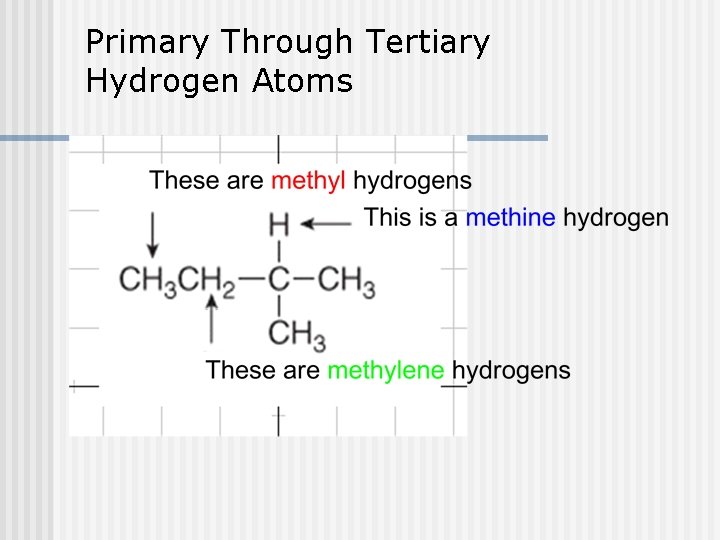
Types of Carbons n 4 possible carbons: Primary (1 ) carbon n Secondary (2 ) carbon n Tertiary (3 ) carbon n Quaternary (4 ) carbon n R-CH 3 R 2 -CH 2 R 3 -CH R 4 -C


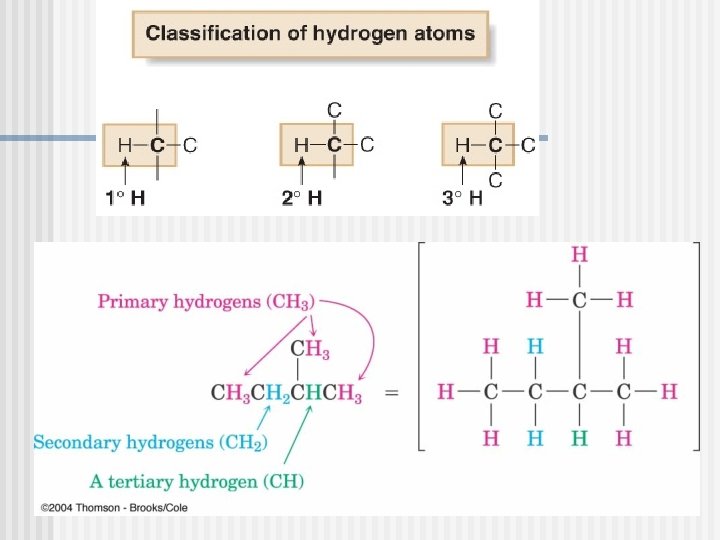
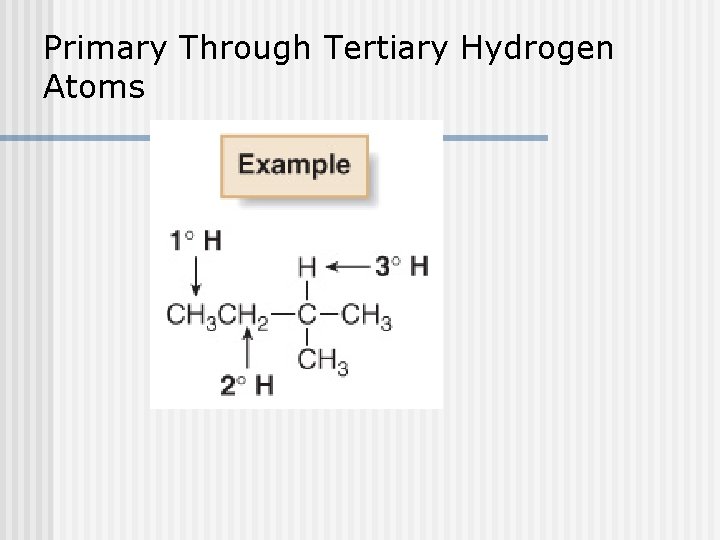
Primary Through Tertiary Hydrogen Atoms

Primary Through Tertiary Hydrogen Atoms

Physical Properties of Alkanes • • • Nonpolar Insoluble in water. Lower density than water. Low boiling and melting points. Gases with 1 -4 carbon atoms. (methane, propane, butane) • Liquids with 5 -17 carbon atoms. (kerosene, diesel, and jet fuels) • Solids with 18 or more carbon atoms. (wax, paraffin, Vaseline)

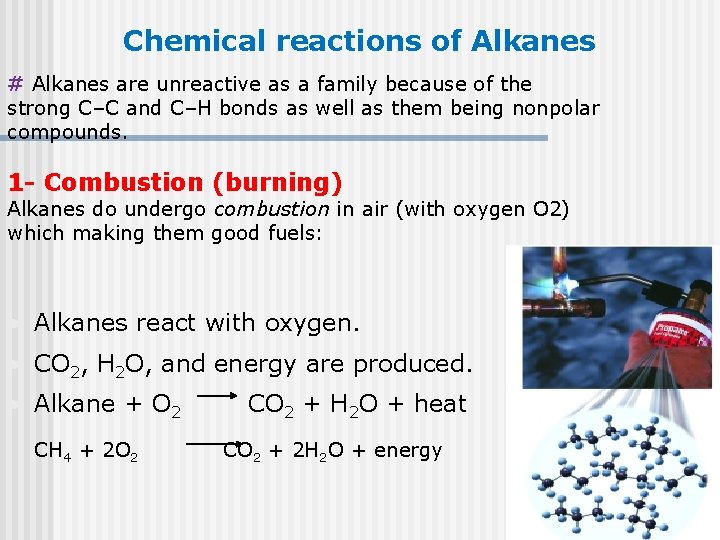
Chemical reactions of Alkanes # Alkanes are unreactive as a family because of the strong C–C and C–H bonds as well as them being nonpolar compounds. 1 - Combustion (burning) Alkanes do undergo combustion in air (with oxygen O 2) which making them good fuels: • Alkanes react with oxygen. • CO 2, H 2 O, and energy are produced. • Alkane + O 2 CH 4 + 2 O 2 CO 2 + H 2 O + heat CO 2 + 2 H 2 O + energy

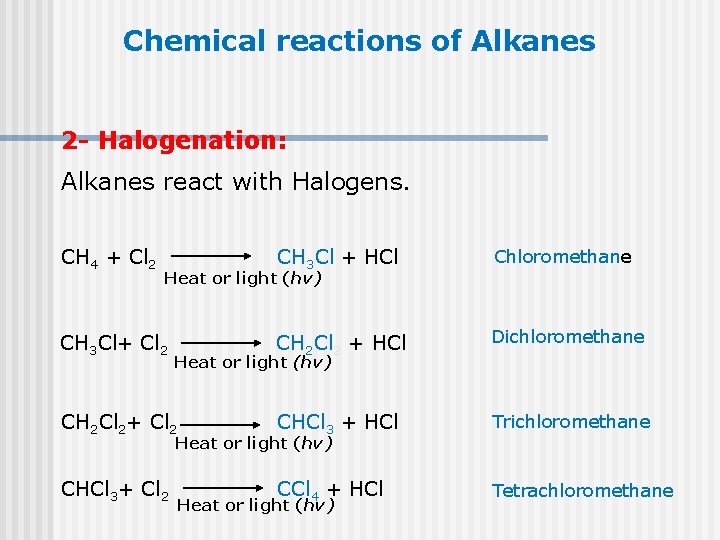
Chemical reactions of Alkanes 2 - Halogenation: Alkanes react with Halogens. CH 4 + Cl 2 CH 3 Cl + HCl Chloromethane CH 2 Cl 2 + HCl Dichloromethane CH 2 Cl 2+ Cl 2 CHCl 3 + HCl Trichloromethane CHCl 3+ Cl 2 CCl 4 + HCl Tetrachloromethane Heat or light (hv) CH 3 Cl+ Cl 2 Heat or light (hv)

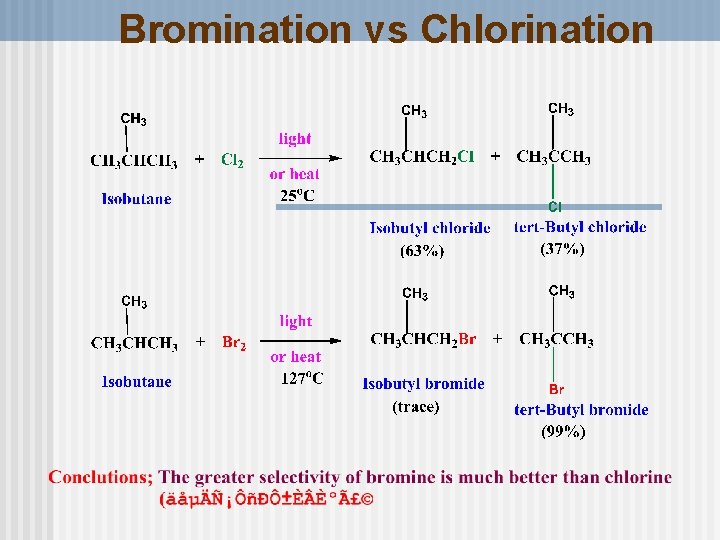
Bromination vs Chlorination

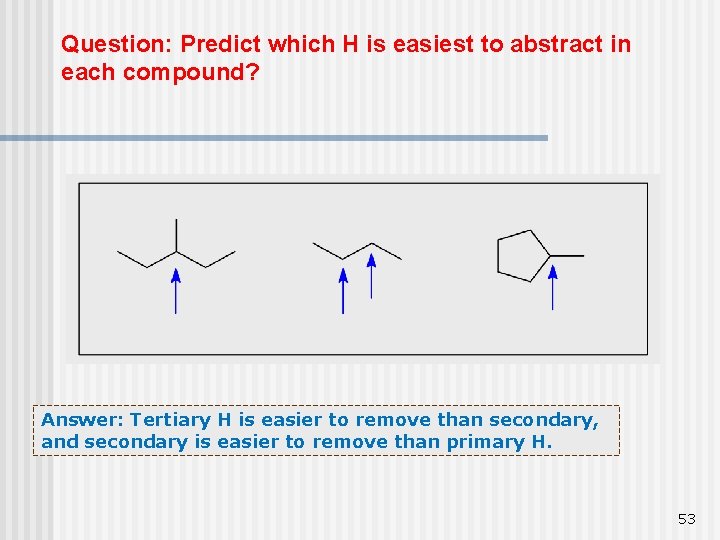
Selectivity of bromine Bromination is slower and more selective than chlorination. n Selectivity is in the order 3 Co > 2 Co > 1 o C because Tertiary H is easier to n remove than secondary, and secondary is easier to remove than primary H 52

Question: Predict which H is easiest to abstract in each compound? Answer: Tertiary H is easier to remove than secondary, and secondary is easier to remove than primary H. 53