Organic Compounds Organic Molecules Generally molecules that contain





- Slides: 21

Organic Compounds

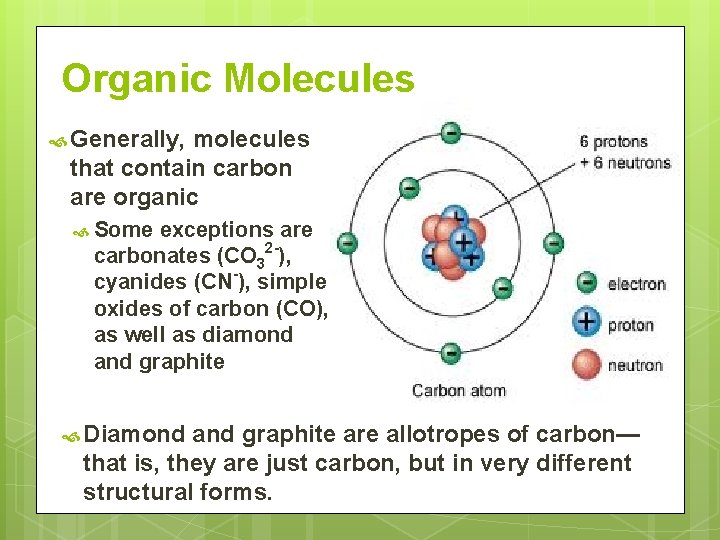
Organic Molecules Generally, molecules that contain carbon are organic Some exceptions are carbonates (CO 32 -), cyanides (CN-), simple oxides of carbon (CO), as well as diamond and graphite Diamond and graphite are allotropes of carbon— that is, they are just carbon, but in very different structural forms.

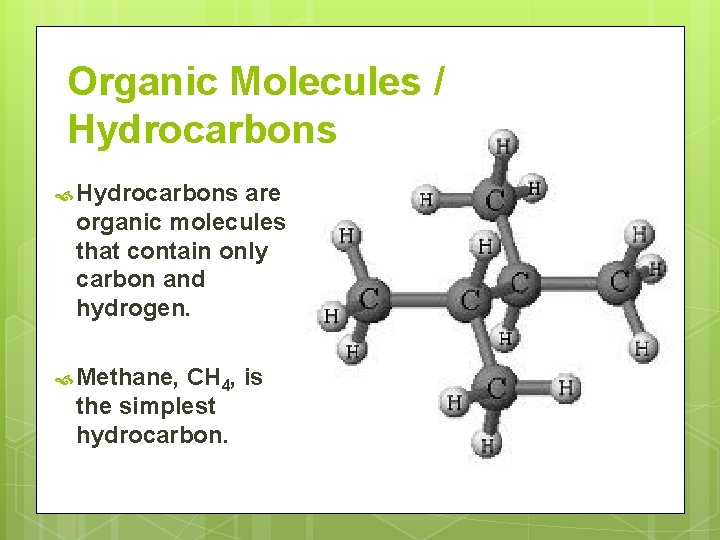
Organic Molecules / Hydrocarbons are organic molecules that contain only carbon and hydrogen. Methane, CH 4, is the simplest hydrocarbon.

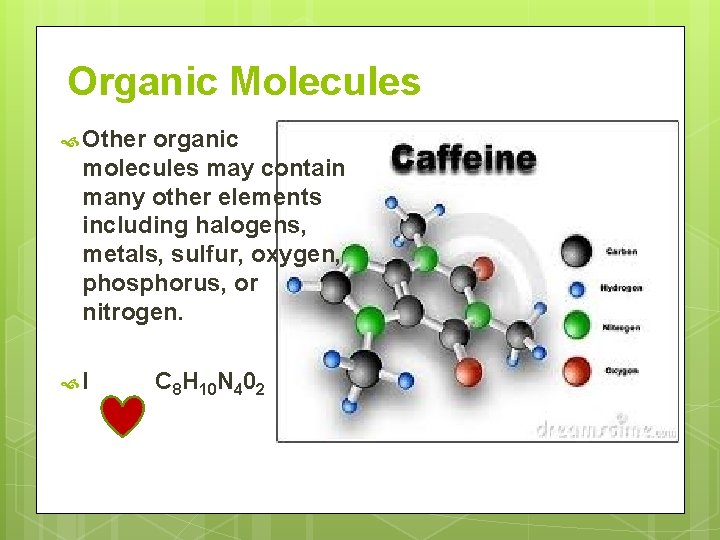
Organic Molecules Other organic molecules may contain many other elements including halogens, metals, sulfur, oxygen, phosphorus, or nitrogen. I C 8 H 10 N 402

Organic Compounds Organic compounds make up the whole or part of innumerable products—some natural and some synthetic. Synthetic examples include: Plastics Explosives Paints Petrochemicals – hydrocarbons derived from petroleum that are used to make synthetic compounds such as plastic

Natural Examples: ? ? Remember in Biology!! 4 types of Organic Molecule: 1) Carbohydrates: sugars, starches (C, H, O) 2) Proteins: amino acids (C, H, O, P) 3) Nucleic Acids: DNA & RNA (C, H, O, N) 4) Lipids: Fats/Oils/Wax, Cell Membranes, Hormones. . (C, H, O, P, S) Pharmaceuticals aspirin, vitamins, insulin

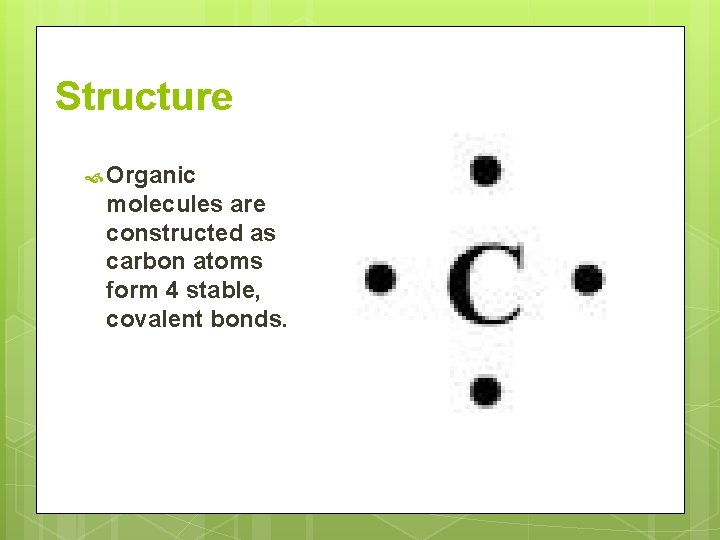
Structure Organic molecules are constructed as carbon atoms form 4 stable, covalent bonds.

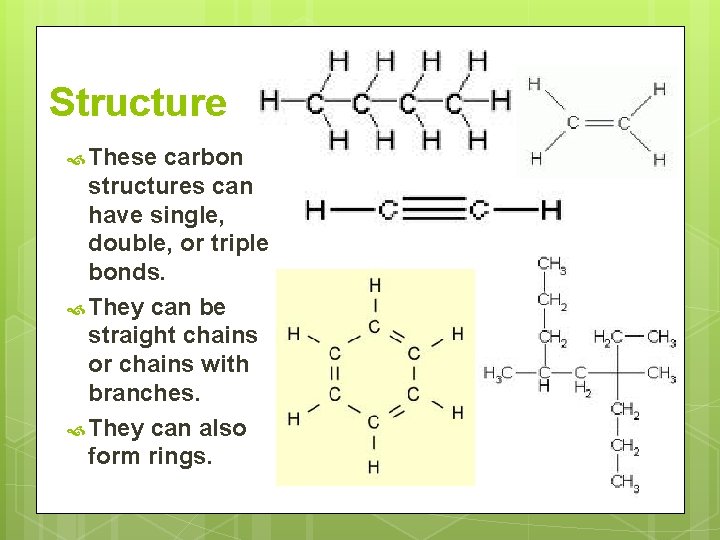
Structure These carbon structures can have single, double, or triple bonds. They can be straight chains or chains with branches. They can also form rings.

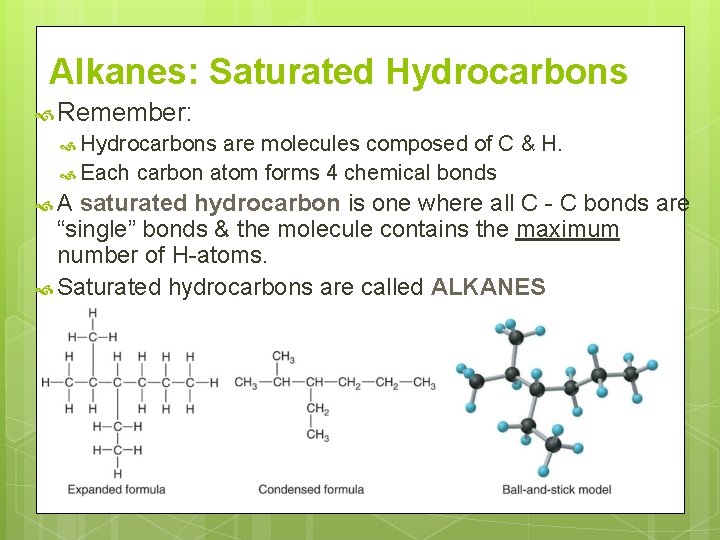
Alkanes: Saturated Hydrocarbons Remember: Hydrocarbons are molecules composed of C & H. Each carbon atom forms 4 chemical bonds A saturated hydrocarbon is one where all C - C bonds are “single” bonds & the molecule contains the maximum number of H-atoms. Saturated hydrocarbons are called ALKANES

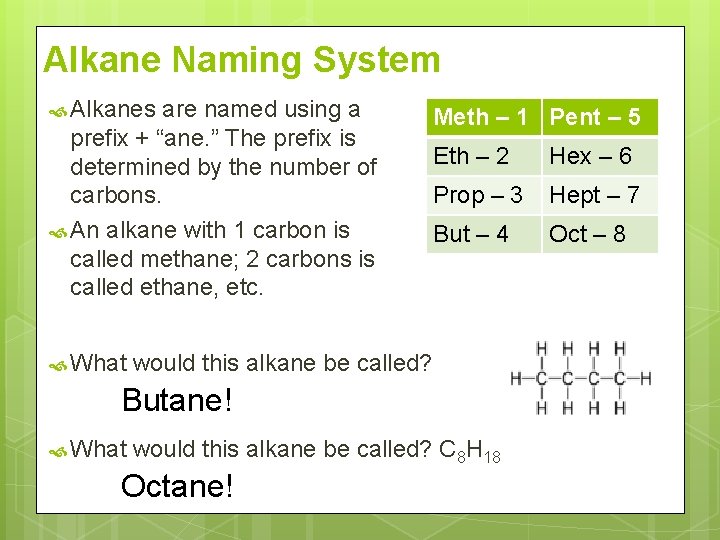
Alkane Naming System Alkanes are named using a prefix + “ane. ” The prefix is determined by the number of carbons. An alkane with 1 carbon is called methane; 2 carbons is called ethane, etc. What Meth – 1 Pent – 5 Eth – 2 Hex – 6 Prop – 3 Hept – 7 But – 4 Oct – 8 would this alkane be called? Butane! What would this alkane be called? C 8 H 18 Octane!

Drawing Alkane Structures Using the prefix, determine how many carbons are in the chain. Connect each carbon with a single bond. Add hydrogens so that each carbon is saturated. Remember that carbon forms 4 bonds. For example, draw propane.

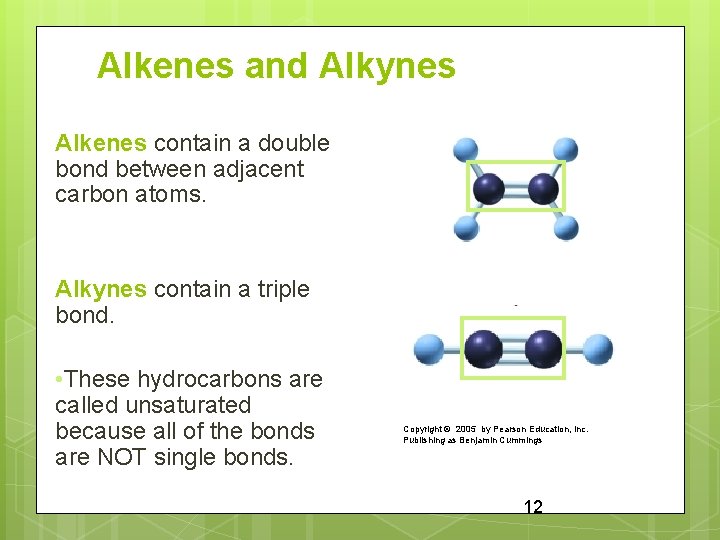
Alkenes and Alkynes Alkenes contain a double bond between adjacent carbon atoms. Alkynes contain a triple bond. • These hydrocarbons are called unsaturated because all of the bonds are NOT single bonds. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 12

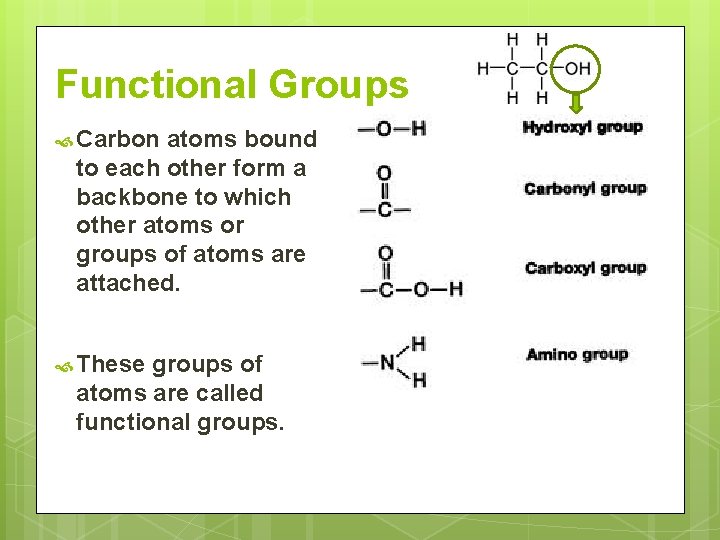
Functional Groups Carbon atoms bound to each other form a backbone to which other atoms or groups of atoms are attached. These groups of atoms are called functional groups.

Dashes and Wedges Used to show 3 -D Straight lines are in the same plane Wedges come out of the plane Dashes go back into the plane

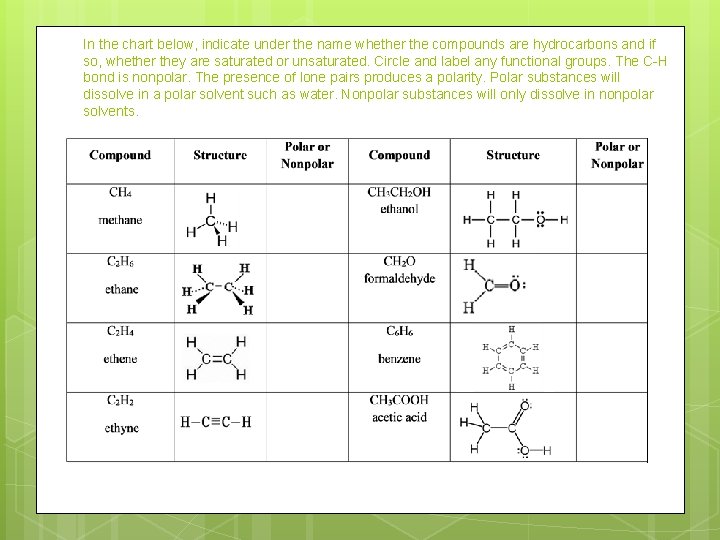
In the chart below, indicate under the name whether the compounds are hydrocarbons and if so, whether they are saturated or unsaturated. Circle and label any functional groups. The C-H bond is nonpolar. The presence of lone pairs produces a polarity. Polar substances will dissolve in a polar solvent such as water. Nonpolar substances will only dissolve in nonpolar solvents.

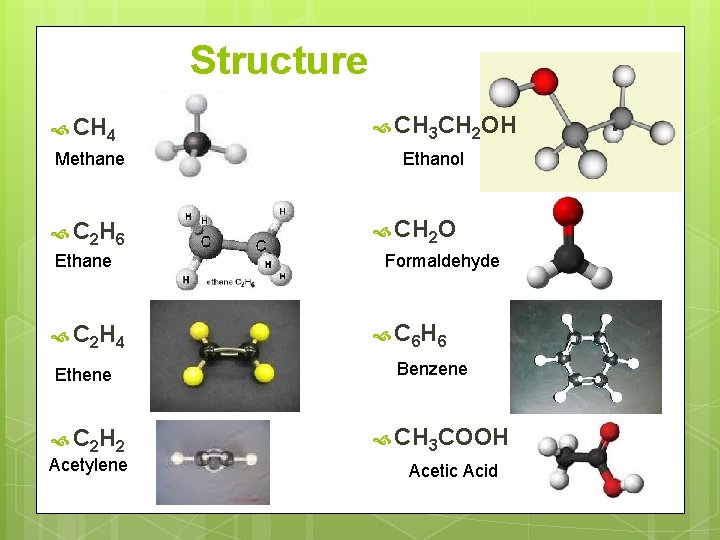
Structure CH 4 Methane C 2 H 6 Ethane C 2 H 4 Ethene C 2 H 2 Acetylene CH 3 CH 2 OH Ethanol CH 2 O Formaldehyde C 6 H 6 Benzene CH 3 COOH Acetic Acid

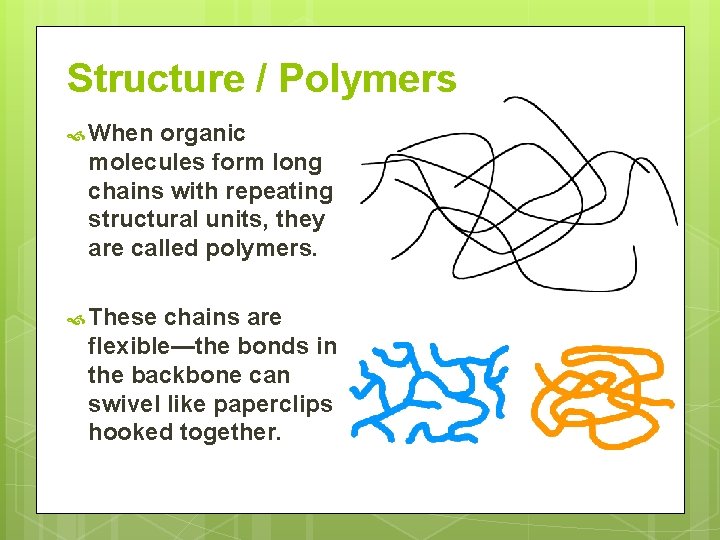
Structure / Polymers When organic molecules form long chains with repeating structural units, they are called polymers. These chains are flexible—the bonds in the backbone can swivel like paperclips hooked together.

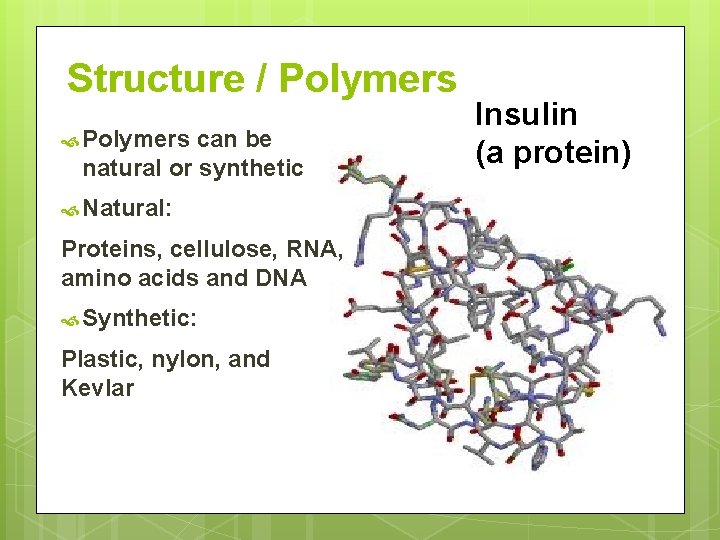
Structure / Polymers can be natural or synthetic Natural: Proteins, cellulose, RNA, amino acids and DNA Synthetic: Plastic, nylon, and Kevlar Insulin (a protein)

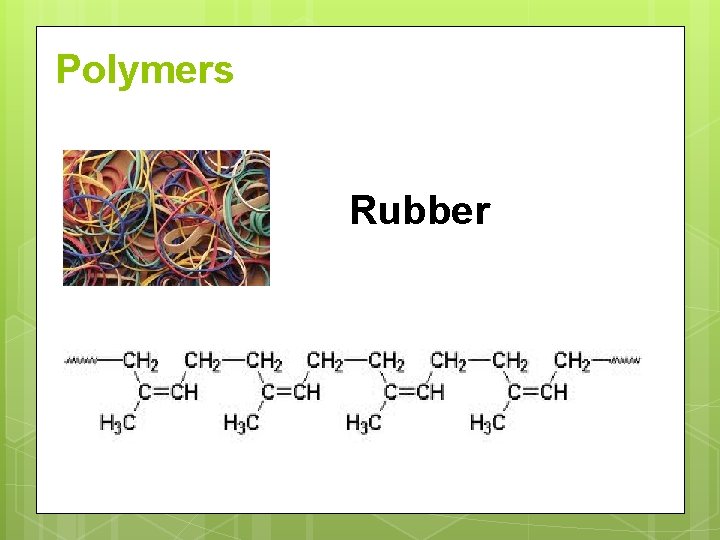
Polymers Rubber

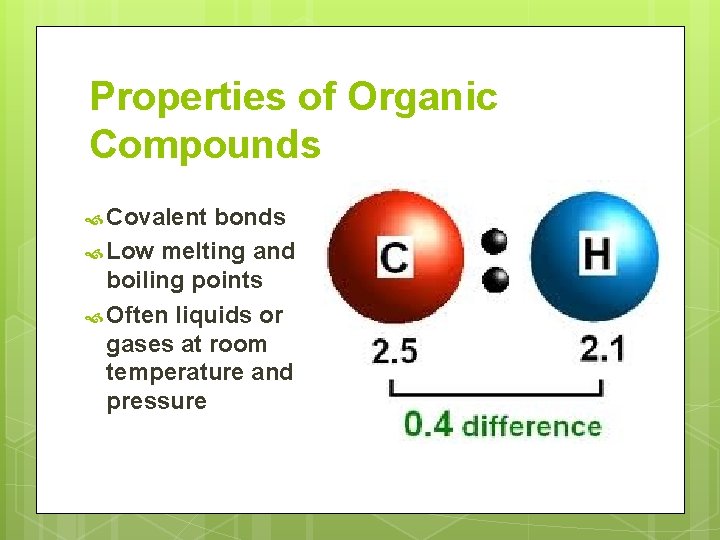
Properties of Organic Compounds Covalent bonds Low melting and boiling points Often liquids or gases at room temperature and pressure

Chemistry Joke Q: What did the bartender say when oxygen, hydrogen, sulfur, sodium, and phosphorus walked in? A: OH SNa. P!!!