Aim What is the halflife of a radioisotope





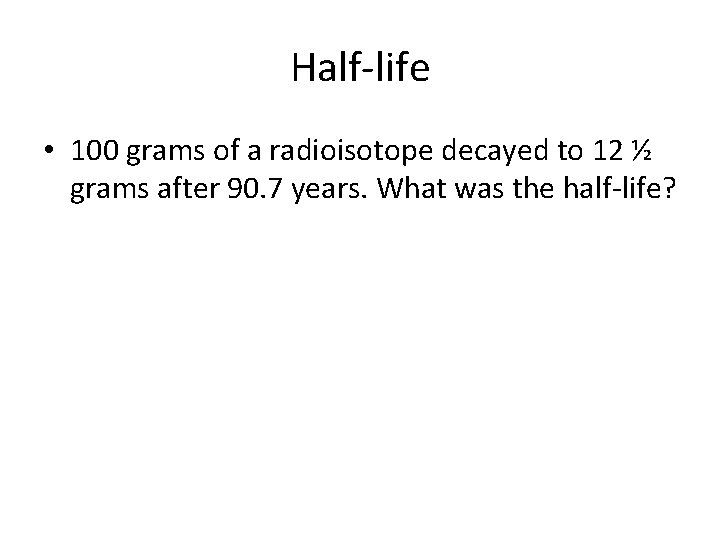
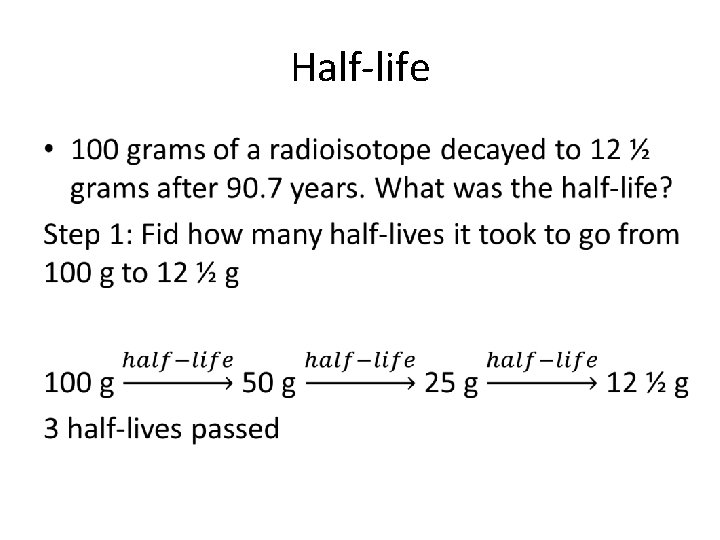
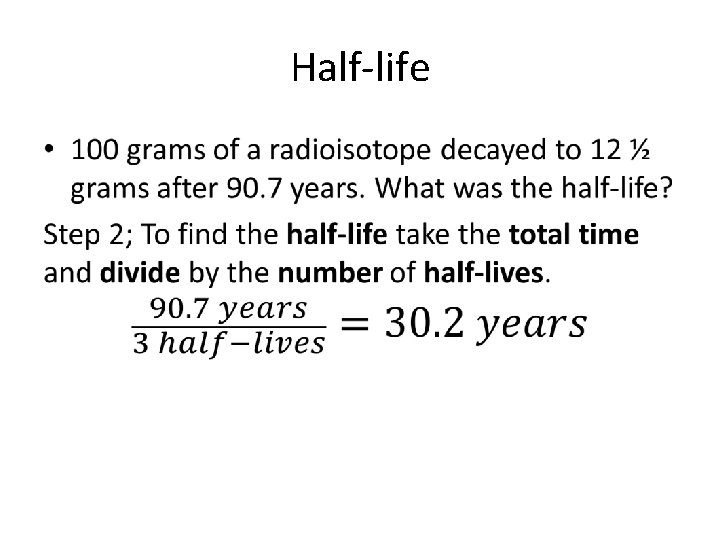






- Slides: 23
Aim: What is the half-life of a radioisotope • Do Now:

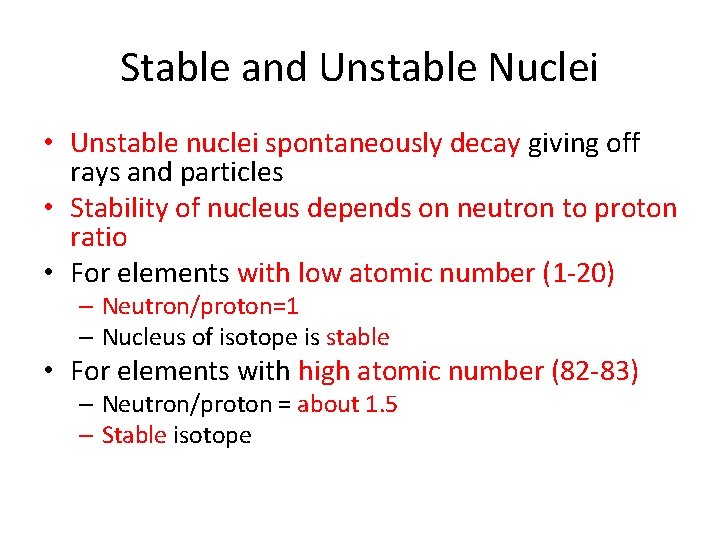
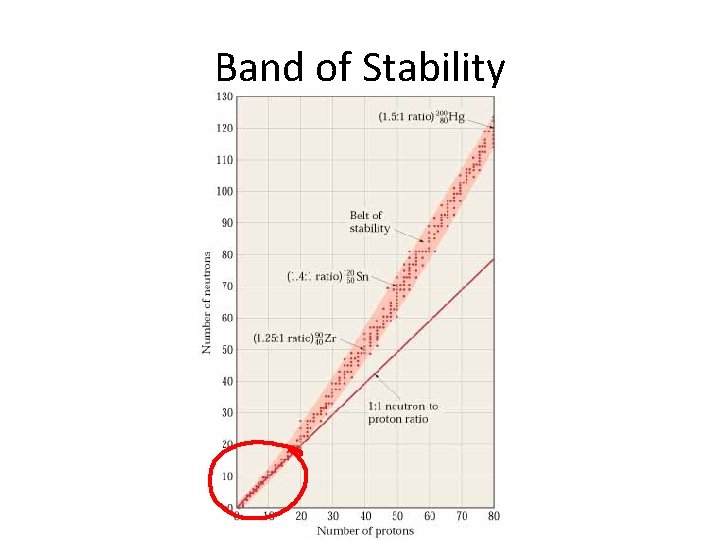
Stable and Unstable Nuclei • Unstable nuclei spontaneously decay giving off rays and particles • Stability of nucleus depends on neutron to proton ratio • For elements with low atomic number (1 -20) – Neutron/proton=1 – Nucleus of isotope is stable • For elements with high atomic number (82 -83) – Neutron/proton = about 1. 5 – Stable isotope

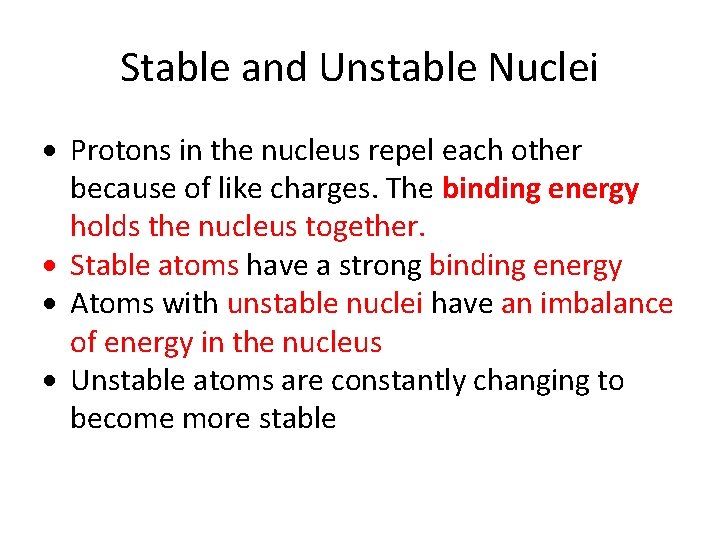
Stable and Unstable Nuclei Protons in the nucleus repel each other because of like charges. The binding energy holds the nucleus together. Stable atoms have a strong binding energy Atoms with unstable nuclei have an imbalance of energy in the nucleus Unstable atoms are constantly changing to become more stable

Band of Stability

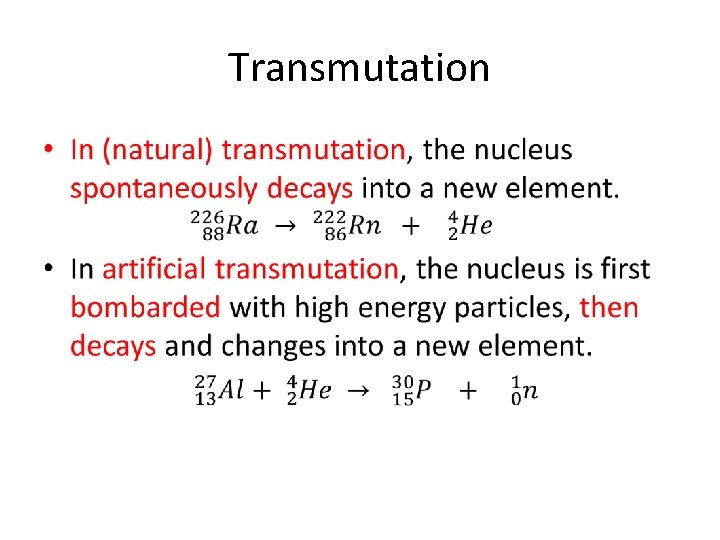
Transmutation •

Question

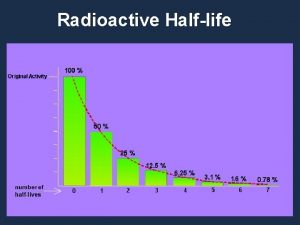
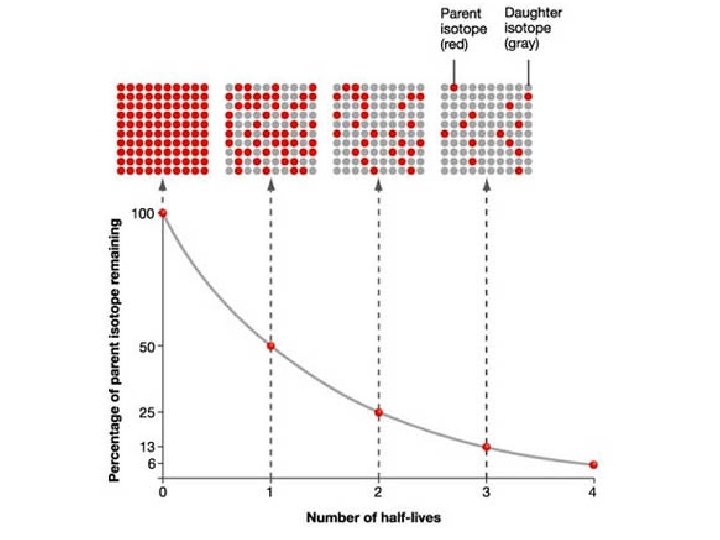
Half-Life • Every radioisotope has a rate of decay. • Half-life is the time it takes for half of the sample to decay into new elements.

Half-Life

Half-Life • Table N lists half-life, decay mode (particles emitted during decay), nuclide (radioisotope, and name of nuclide. • The half-life of Ra-226 is 1600 years; meaning, in 1600 years half of Ra-226 will decay, and in another 1600 years half of what was remaining will decay. • After 3200 years, how many half-lives has Ra 226 gone through?

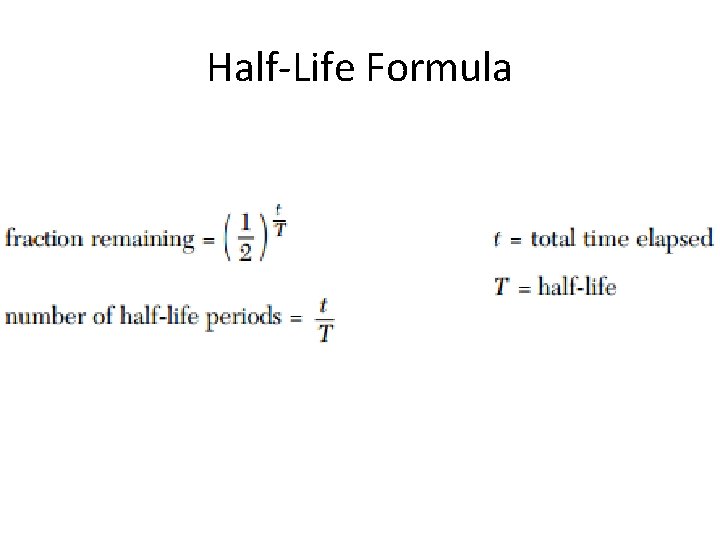
Half-Life Formula

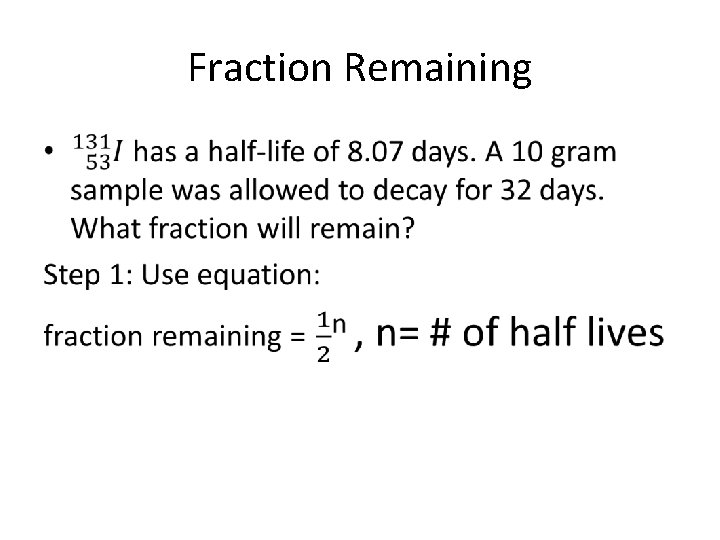
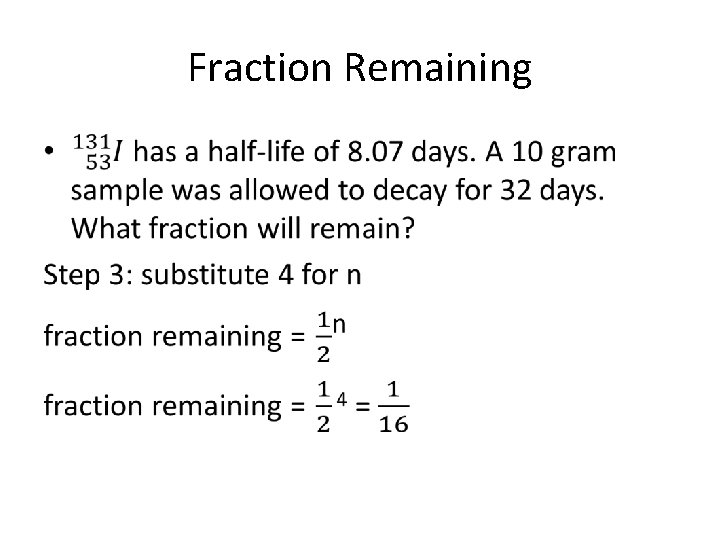
Fraction Remaining •

Fraction Remaining •

Fraction Remaining •

Fraction Remaining •
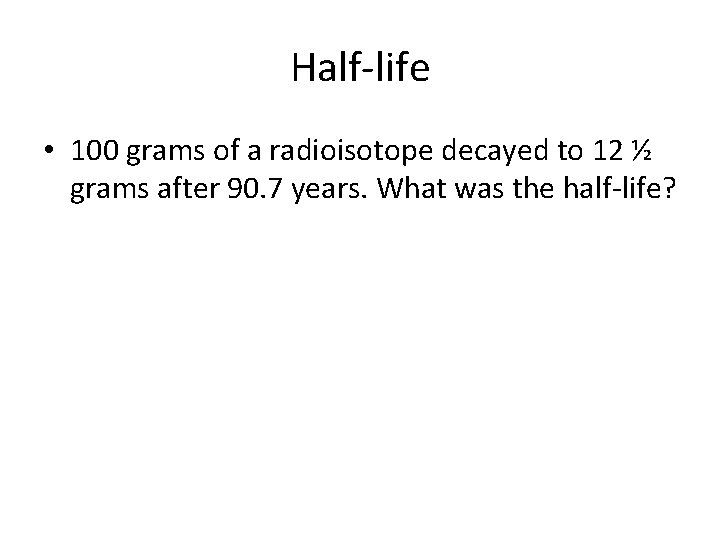
Half-life • 100 grams of a radioisotope decayed to 12 ½ grams after 90. 7 years. What was the half-life?
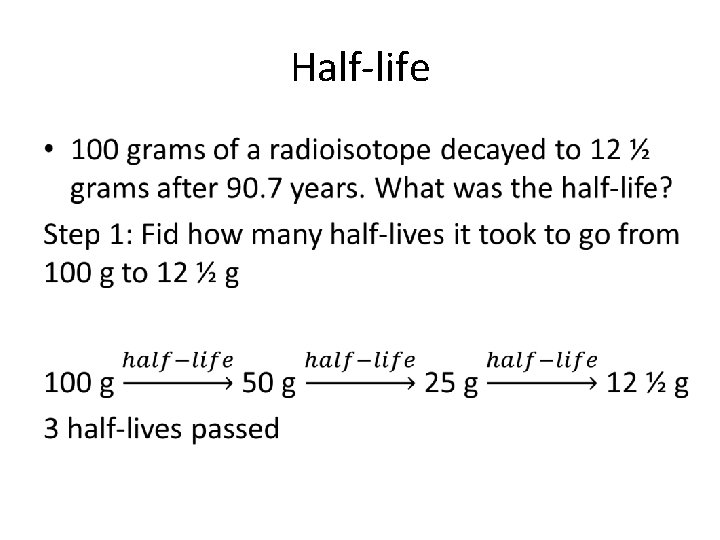
Half-life •
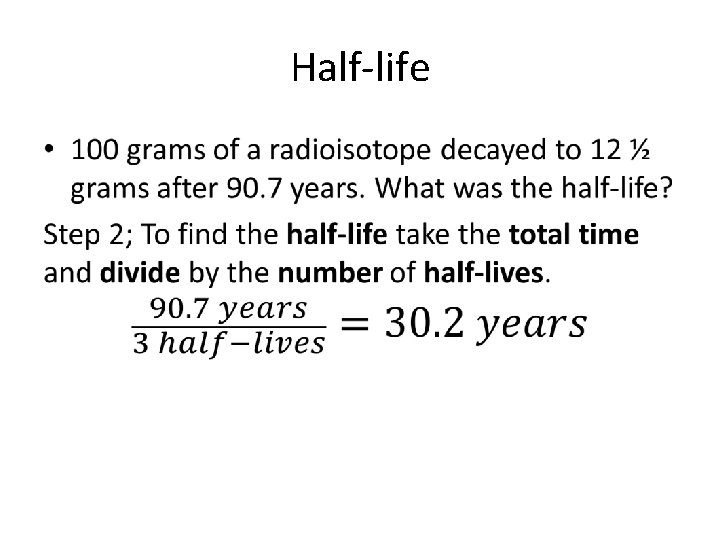
Half-life •

Initial Amount (Original Amount) • A radioisotope has a half-life of 10 days. 1 gram remains after 40 days. What was the initial amount of the radioisotope?

Initial Amount (Original Amount) •

Initial Amount (Original Amount) •

Initial Amount (Original Amount) • A radioisotope has a half-life of 10 days. 1 gram remains after 40 days. What was the initial amount of the radioisotope? Step 3: Find initial amount. 1/16 of the original amount remains 1 g = amount remaining 1 g = (1/16) x , x= initial amount

Questions 1. Which radioisotopes have the same decay mode and have half-lives greater than 1 hour? (1) Au-198 and N-16 (3) I-131 and P-32 (2) Ca-37 and Fe-53 (4) Tc-99 and U-233

Questions 2. After decaying for 48 hours, 1/16 of the original mass of a radioisotope sample remains unchanged. What is the half-life of this radioisotope? (1) 3. 0 h (3) 12 h (2) 9. 6 h (4) 24 h
 Lithium halflife
Lithium halflife Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Diễn thế sinh thái là
Diễn thế sinh thái là đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Gấu đi như thế nào
Gấu đi như thế nào Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Dot
Dot điện thế nghỉ
điện thế nghỉ Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Lp html
Lp html độ dài liên kết
độ dài liên kết Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Fecboak
Fecboak Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Một số thể thơ truyền thống
Một số thể thơ truyền thống