Halflife speed of radioactive decay Halflife amount of













- Slides: 19

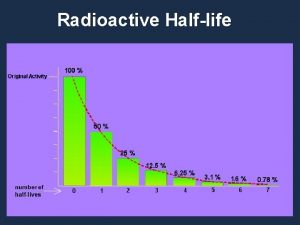
Half-life speed of radioactive decay

Half-life amount of time parent isotope 226 88 Ra 222 86 Rn + Parent isotope ½ of the amount daughter product 4 2 a Daughter product Half-life would equal the amount of time it takes for 10 g of Ra to decay to 5 grams of Ra

Why do we care? It can be difficult to determine the ages of objects by sight alone e. g. It can be difficult to tell which rock is the oldest. Radioactivity provides a method to determine age by comparing the relative amount of remaining radioactive material to the amount of stable products formed called radio-dating

The Importance of Carbon - All life is Carbon Based - Carbon has two useful isotopes - C-14 = radioactive - C-12 = stable (makes up 98. 9% of Carbon atoms)

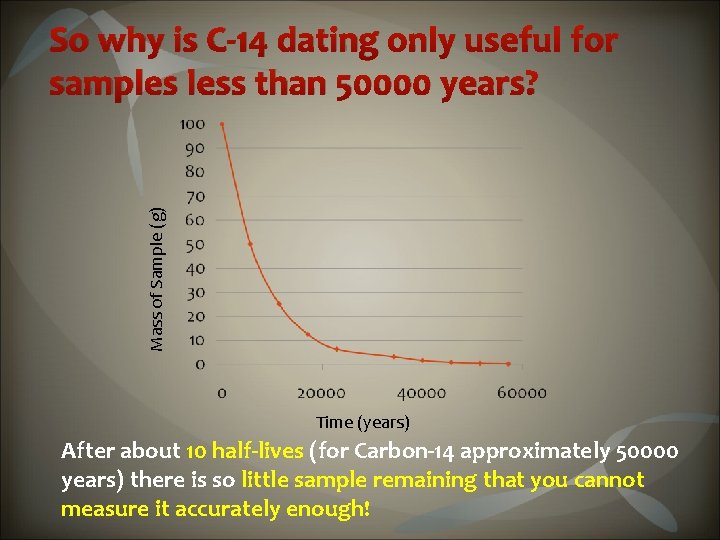
Carbon dating measures the ratio of carbon-12 and carbon-14. When an organism dies, carbon-14 stops being produced as the organism slowly decays. Measuring the relative amounts of carbon-12 : carbon-14 is called radiocarbon dating. C-14 has a Half-life of 5730 years We can use radio dating for up to 10 half-lives of a radio active isotope So radiocarbon dating can be used to provide the age of any organism or organic material… less than 50 000 years old Using radiocarbon dating, these cave paintings of horses, from France, were determined to have been drawn 30 000 years ago.

Half-life measures the rate of radioactive decay Half-life = time required for half of a radioactive sample to decay. The half life for a radioactive element is a constant rate of decay. e. g. Strontium-90 has a half-life of 29 years. If you have 10 g of strontium-90 today, there will be 5 g remaining in 29 years. Half-lives of many common radioisotopes are listed on pg. 4 of your data booklet.

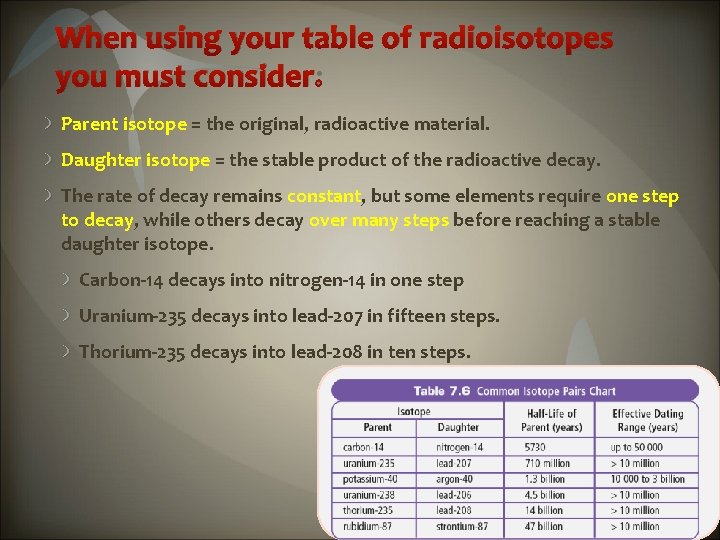
When using your table of radioisotopes you must consider: Parent isotope = the original, radioactive material. Daughter isotope = the stable product of the radioactive decay. The rate of decay remains constant, but some elements require one step to decay, while others decay over many steps before reaching a stable daughter isotope. Carbon-14 decays into nitrogen-14 in one step Uranium-235 decays into lead-207 in fifteen steps. Thorium-235 decays into lead-208 in ten steps.

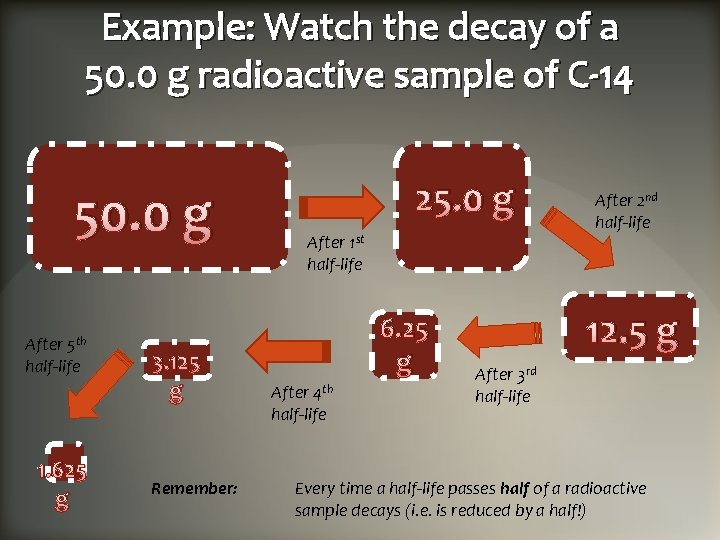
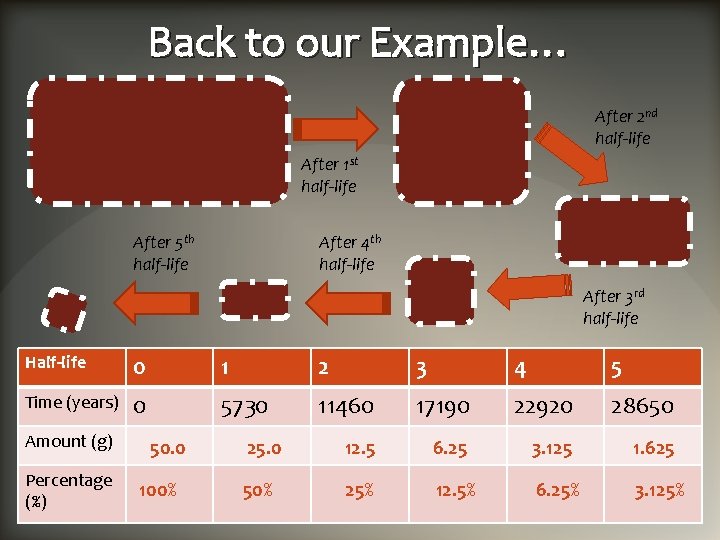
Example: Watch the decay of a 50. 0 g radioactive sample of C-14 50. 0 g After 5 th half-life 1. 625 g 3. 125 g Remember: 25. 0 g After 1 st half-life 6. 25 g After 4 th half-life After 2 nd half-life 12. 5 g After 3 rd half-life Every time a half-life passes half of a radioactive sample decays (i. e. is reduced by a half!)

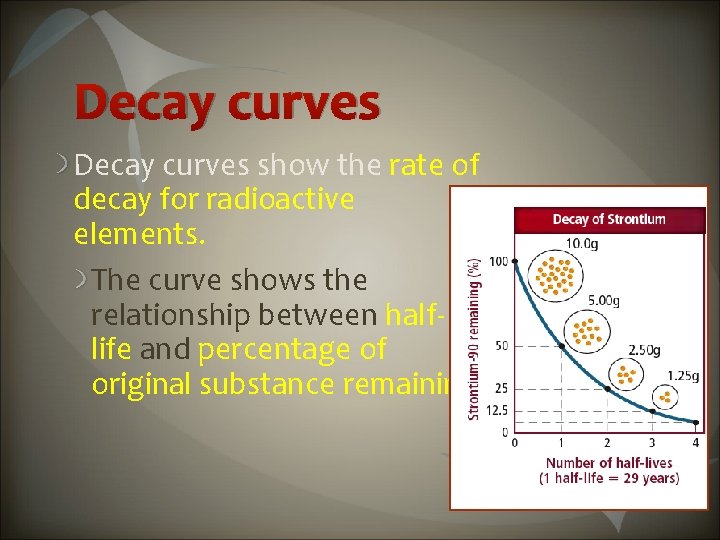
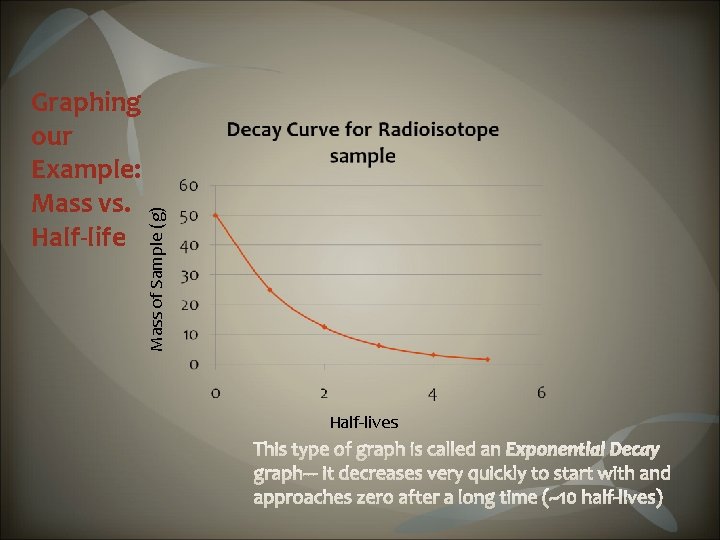
Decay curves show the rate of decay for radioactive elements. The curve shows the relationship between halflife and percentage of original substance remaining.

Back to our Example… After 2 nd half-life After 1 st half-life After 5 th half-life After 4 th half-life After 3 rd half-life Half-life 0 1 2 3 4 5 Time (years) 0 5730 11460 17190 22920 28650 25. 0 12. 5 6. 25 3. 125 1. 625 50% 25% 12. 5% 6. 25% 3. 125% Amount (g) Percentage (%) 50. 0 100%

Mass of Sample (g) Graphing our Example: Mass vs. Half-life Half-lives

Percentage of Sample (%) Graphing our Example: % Remaining vs. Time (years)

Mass of Sample (g) So why is C-14 dating only useful for samples less than 50000 years? Time (years) After about 10 half-lives (for Carbon-14 approximately 50000 years) there is so little sample remaining that you cannot measure it accurately enough!

The Potassium-40 Clock very old things The potassium-40/argon-40 clock has a half-life of 1. 3 billion years. Argon-40 produced by the decay of potassium-40 becomes trapped in rock. Ratio of argon-40 : potassium-40 shows age of rock.

The Potassium-40 Clock 1. A rock is found to contain 25% potassium-40 and 75% argon-40. A) How many half-lives old is the rock? 2 half lives old B) How many years old is the rock? 2. 6 billions years old 2. What is the ratio of argon-40 to potassium-40 after 5. 2 billion years? 15: 1 (93. 75% argon-40 : 6. 25% potassium-40)

PRACTICE HALF-LIFE 0 1 2 3 4 Percent of Parent Isotope Percent of Daughter Isotope 100 0 50 50 25 75 12. 5 87. 5 6. 25 93. 75

PRACTICE HALF-LIFE Fraction of Parent Isotope Fraction of Daughter Isotope 0 1 1/2 2 1/4 3 1/8 7/8 4 1/16 15/16

HALF-LIFE Time (years) Mass (grams) 0 0 120 1 5 60 2 10 30 3 15 15 4 20 7. 5 5 25 3. 75

3. 75 3 half-lives 20 years
 Student worksheet modeling nuclear changes answer key
Student worksheet modeling nuclear changes answer key How to calculate radioactive decay
How to calculate radioactive decay Unstable nuclei and radioactive decay
Unstable nuclei and radioactive decay Radioactive decay formula
Radioactive decay formula Natural vs artificial radioactivity
Natural vs artificial radioactivity Radioactive decay law
Radioactive decay law Type of radioactive decay
Type of radioactive decay Half life example
Half life example Chemistry
Chemistry Radioactive decay law
Radioactive decay law How to calculate half life physics
How to calculate half life physics Radioactive decay formula
Radioactive decay formula What is decay factor
What is decay factor Beta minus decay vs beta plus decay
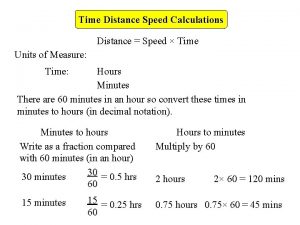
Beta minus decay vs beta plus decay Time per distance
Time per distance Distance formula speed time
Distance formula speed time Passengers can help a driver better manage emotions by
Passengers can help a driver better manage emotions by Speed detection of moving vehicle using speed cameras
Speed detection of moving vehicle using speed cameras Chapter 24: nuclear chemistry answer key
Chapter 24: nuclear chemistry answer key Radioactive waste
Radioactive waste