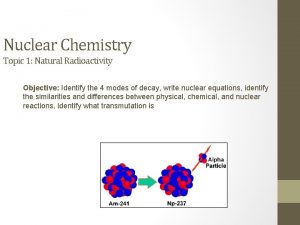
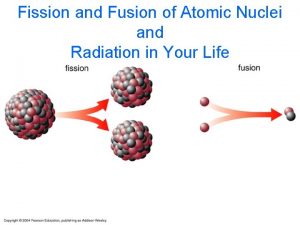
HALFLIFE NUCLEAR FISSION AND FUSION HALFLIFE AMOUNT OF












- Slides: 19

HALF-LIFE, NUCLEAR FISSION AND FUSION

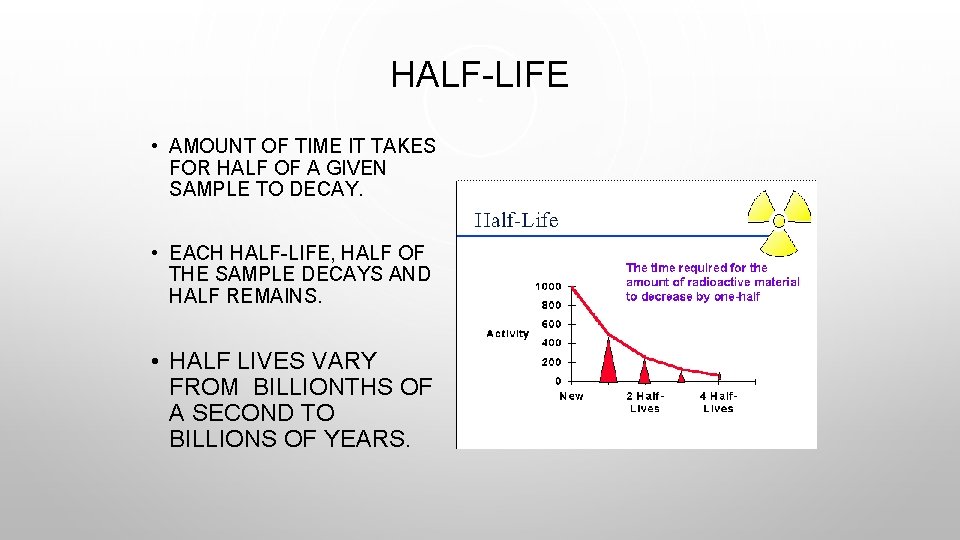
HALF-LIFE • AMOUNT OF TIME IT TAKES FOR HALF OF A GIVEN SAMPLE TO DECAY. • EACH HALF-LIFE, HALF OF THE SAMPLE DECAYS AND HALF REMAINS. • HALF LIVES VARY FROM BILLIONTHS OF A SECOND TO BILLIONS OF YEARS.

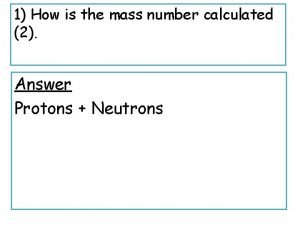
HALF-LIFE: EQUATION FORM • HOW DO WE PUT THIS INTO EQUATION FORM? • LET T 1/2 = THE HALF-LIFE OF THE ISOTOPE • ENDING AMOUNT=AMOUNT OF ISOTOPE THAT IS LEFT AT END • STARTING AMOUNT= THE AMOUNT OF ISOTOPE AT THE BEGINNING BEFORE IT STARTS TO DECAY • T= THE AMOUNT OF TIME THAT HAS PASSED LN (ENDING AMOUNT/STARTING AMOUNT) = -(0. 693/T 1/2)T

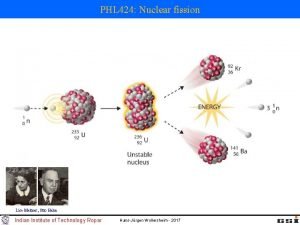
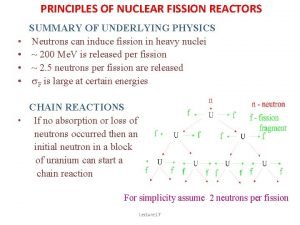
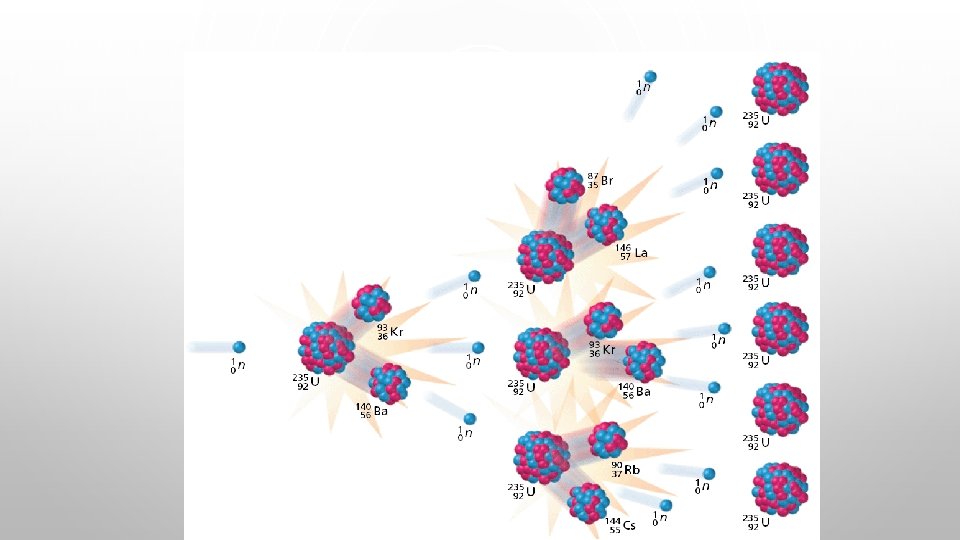
NUCLEAR REACTIONS - FISSION • IF NUCLEUS TOO BIG - NUCLEAR FISSION. • “FISSIONS” (BREAKS UP) INTO SEVERAL SMALLER NUCLEI ( AND USUALLY SOME EXTRA NEUTRONS AS WELL). • NOT EASILY PREDICTED. • OFTEN INITIATED BY ABSORBING A NEUTRON. • EXAMPLE: 92235 U + 01 N --> 56141 BA + 3692 KR + 3 01 N

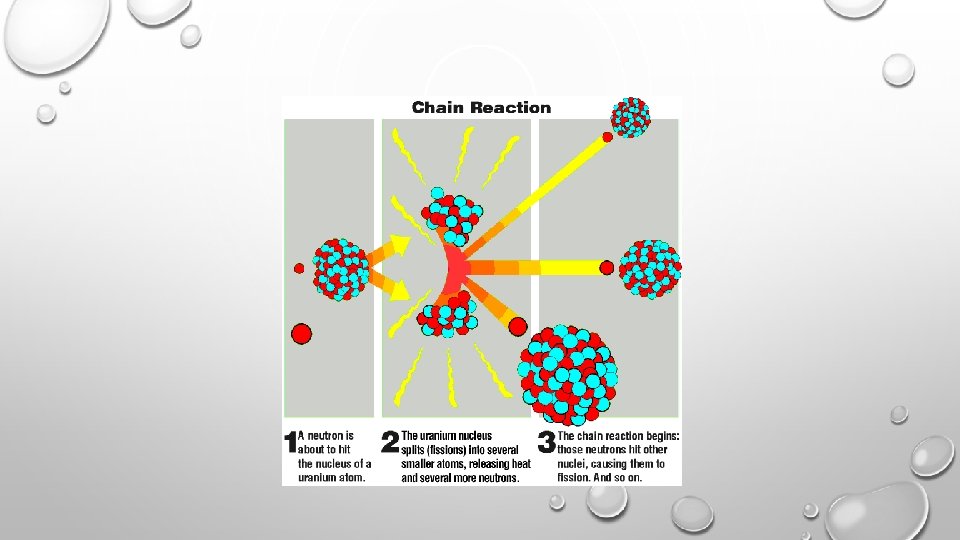
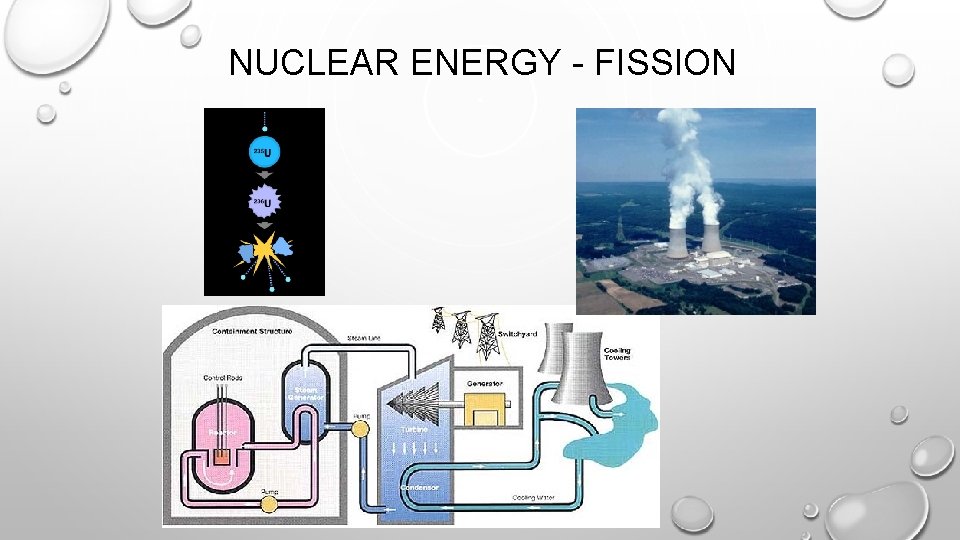
NUCLEAR ENERGY - FISSION • ENERGY CAN BE HARNESSED. 235 U + 1 N --> 141 BA + 92 KR + 3 1 N 92 0 56 36 0 THE NEUTRONS COLLIDE WITH OTHER ATOMS OF 235 U, SPLIT, PRODUCING MORE NEUTRONS. . . CAUSING A CHAIN REACTION. • THE ENERGY GIVEN OFF CAN BE HARNESSED TO PRODUCE ELECTRICITY (OR, UNFORTUNATELY, FOR MORE DESTRUCTIVE PURPOSES).



NUCLEAR ENERGY - FISSION

NUCLEAR WEAPONS

NUCLEAR WASTE This pool at the Areva Nuclear Plant near Cherbourg, France, cools spent nuclear fuel rods before they are moved underground. Francois Mori / AP

NUCLEAR FUSION • THE OPPOSITE OF NUCLEAR FISSION IS FUSION, WHEN SMALLER NUCLEI COME TOGETHER TO FORM LARGER NUCLEI. • EXAMPLE: 11 H + 13 H --> 24 HE • THE FUSION OF HYDROGEN TO FORM HELIUM IS THE SOURCE OF ENERGY FOR THE SUN AND MANY OTHER STARS.

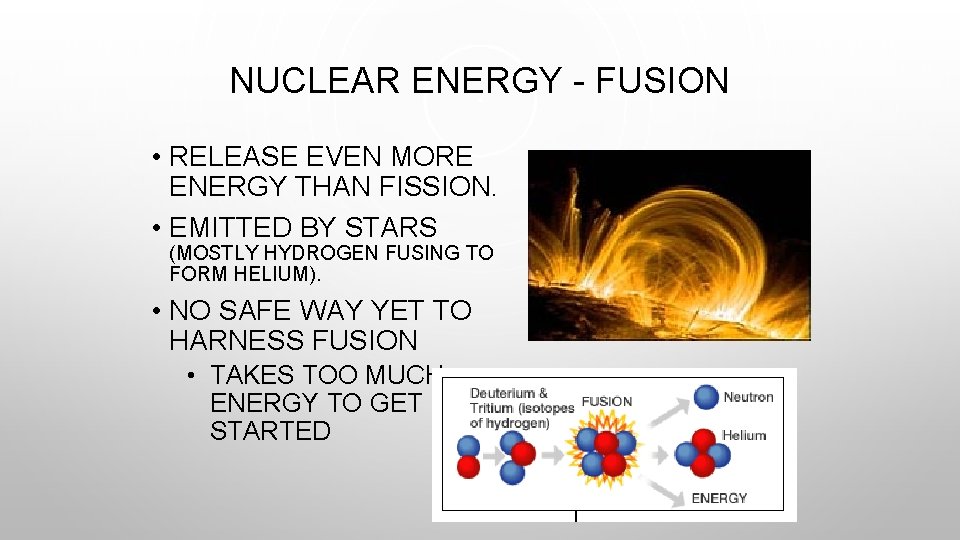
NUCLEAR ENERGY - FUSION • RELEASE EVEN MORE ENERGY THAN FISSION. • EMITTED BY STARS (MOSTLY HYDROGEN FUSING TO FORM HELIUM). • NO SAFE WAY YET TO HARNESS FUSION • TAKES TOO MUCH ENERGY TO GET STARTED

ENERGY IN RADIOACTIVE DECAY • RADIOACTIVE DECAY GENERALLY GIVES OFF LARGE AMOUNTS OF ENERGY. • WHERE DOES IT COME FROM? • THE ANSWER LIES IN SOMETHING CALLED “MASS DEFECT. ”

MASS DEFECT • LAW OF CONSERVATION OF MASS • MASS CANNOT BE CREATED OR DESTROYED. • LAW OF CONSERVATION OF ENERGY • ENERGY CANNOT BE CREATED OR DESTROYED. • BUT, DURING NUCLEAR REACTIONS: • MASS CAN BE CONVERTED INTO ENERGY AND ENERGY CAN BE CONVERTED INTO MASS.

MASS DEFECT • DURING NUCLEAR REACTION, SOME MASS IS EITHER LOST OR GAINED. THIS CHANGE IN MASS IS CALLED THE MASS DEFECT (DM). • DM = MASS OF PRODUCTS - MASS OF REACTANTS • THE RELATIONSHIP BETWEEN THE MASS DEFECT AND THE AMOUNT OF ENERGY GIVEN OFF OR ABSORBED (DE) IS DE = DMC 2 WHERE C = THE SPEED OF LIGHT = 3. 0 X 108 M/S.

MASS DEFECT DE = DMC 2 • EXAMPLE: IF 0. 01 G OF MASS IS CONVERTED INTO ENERGY, 9 X 10 11 J OF ENERGY IS GIVEN OFF. THAT’S ENOUGH TO HEAT 2. 7 MILLION LITERS OF WATER FROM ROOM TEMPERATURE TO BOILING! • BY COMPARISON: YOU WOULD NEED TO BURN 16 MILLION GRAMS OF METHANE TO GIVE OFF THE SAME AMOUNT OF HEAT.

Common Uses of Radioactivity Food Irradiation Archaeological Dating Medical Detection Medical Treatment

Daily Exposure to Radiation Cosmic rays Radon gas Smoke detectors

Detecting Radiation Geiger Counters (beta) Scintillation Counters (alpha, beta and gamma) Film (beta, gamma)
 Natural transmutation example
Natural transmutation example Nuclear fission and fusion similarities
Nuclear fission and fusion similarities Sun fusion or fission
Sun fusion or fission Fission vs fusion
Fission vs fusion Fission vs fusion
Fission vs fusion Fussion vs fission
Fussion vs fission Are nuclear power plants fission or fusion
Are nuclear power plants fission or fusion Fusion fission
Fusion fission Plutonium half life
Plutonium half life Difference between fission and fussion
Difference between fission and fussion Fission and fusion similarities
Fission and fusion similarities Fission vs fusion
Fission vs fusion Rds 37
Rds 37 Fission vs fusion
Fission vs fusion Fusion or fission
Fusion or fission Nuclear fission lise meitner
Nuclear fission lise meitner Applications of nuclear fission
Applications of nuclear fission Nuclear fission summary
Nuclear fission summary Nuclear fusion radiation
Nuclear fusion radiation Pluotnium
Pluotnium