Half Life HalfLife HalfLife is the time it






- Slides: 11

Half - Life

Half-Life • Half-Life is the time it takes for ½ of a radioactive sample to decay or change into something else. • For example, if you had 20 grams of carbon-14 and it has a half-life of 5000 years, how much Carbon-14 would remain after 5000 years? 10, 000 years?
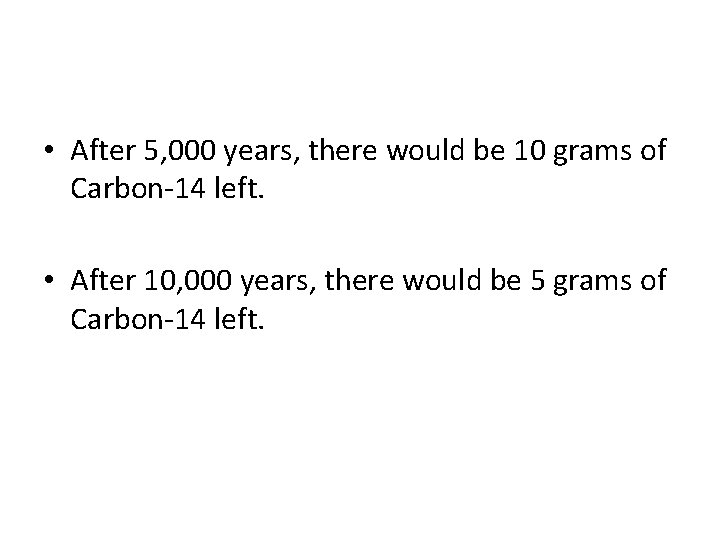
• After 5, 000 years, there would be 10 grams of Carbon-14 left. • After 10, 000 years, there would be 5 grams of Carbon-14 left.

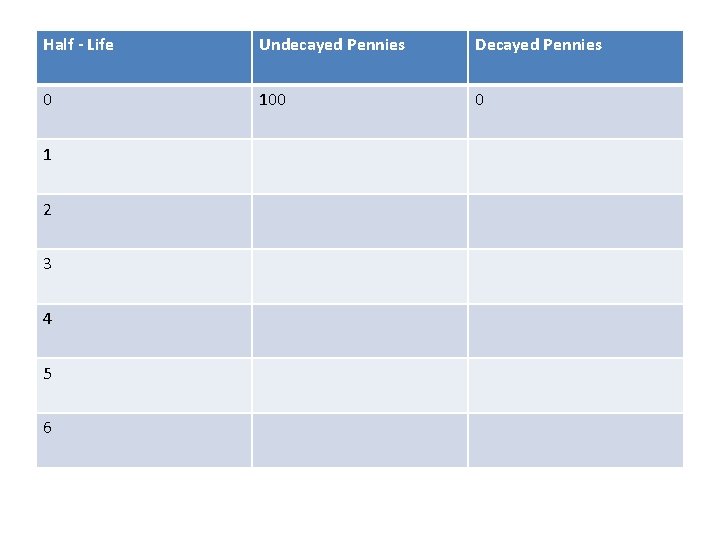
Activity Background • At your lab table there is a container with 100 pennies • These represent 100 radioactive atoms • We will use these pennies to demonstrate half -life, the time it takes for ½ of a radioactive isotope to change

Lab Directions • 1. Confirm that you have 100 pennies in your container • 2. Empty the container onto the lab table • 3. Pennies that are “tails” have decayed and should be set aside for the remainder of the exercise. Record the number of decayed pennies. • 4. Pennies that are “heads” have not decayed and should be returned to the container

Lab Directions • 5. Continue this until all of the pennies have decayed. • Make sure to record all data accurately.

Half - Life Undecayed Pennies Decayed Pennies 0 100 0 1 2 3 4 5 6


Analyzing the Data • Prepare a graph using your group’s data and the pooled class data by plotting the number of half-lives on the x-axis and the number of undecayed atoms remaining after each halflife on the y-axis. Plot and label 2 graph lines – one for your group and one for the class data. Using different colors for each of these lines.

Questions • 1. Describe the appearance of your 2 graph lines. Straight or curved? Which set of data provides a more convincing demonstration of half-life? Why? • 2. About how many undecayed atoms (heads) would remain after 3 half-lives if the initial sample had 600 atoms? • 3. If 190 heads remain from an original sample of 3000 atoms, about how many half-lives must have passed?

• 4. Describe one similarity and one difference between this model based on pennies and actual radioactive decay? • 5. How could you modify this “decay” model to demonstrate that different isotopes have different half-lives? • 6. How many half-lives would it take for one mole of a radioactive isotope to decay to 6. 25% of the original number of atoms? Is it likely any of the original isotopes still remain after 10 half-lives? 100 half-lives? • 7. Can you predict when a particular penny will “decay”? If you could follow one atom in a sample of radioactive material, could you predict when it would decay? Why or why not?