Halflife Engager n n To calculate halflife of
















- Slides: 27

Half-life

Engager n

n To calculate half-life of different radioactive isotopes. n Calculate count rate after a given number of half lives. n Explain what the term Half-life means Progress To know what half-life is

Radioactive decay is 1. Random an event that is described by a probability i. e. we can predict that so many decays will happen in the next minute, but we cannot say exactly when they will happen 2. Spontaneous without premeditation or external stimulus i. e. we cannot make an unstable nucleus decay (e. g by increasing the temperature or pressure).

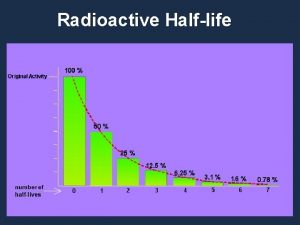
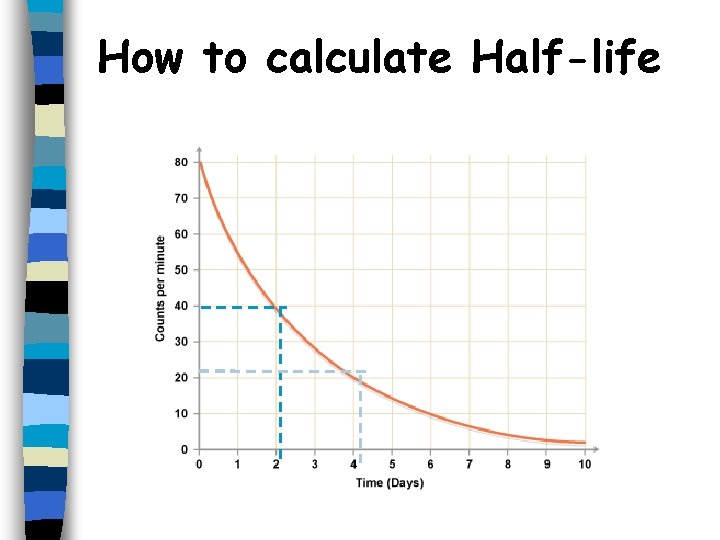
The half-life is the time taken for the number of radioactive nuclei in an isotope to half This can take hours, weeks, years, millions of years etc.


The time required for half of a sample of radioactive atoms to decay. Some isotopes have half-lives of fractions of a second but others have half lives of millions of years.

How to calculate Half-life

Calculating the half-life of Skittles Method • You have 50 skittles in your cup shake the cup and empty out • Remove the skittles with the ‘S’ showing, • Write down the number in table provided, • Repeat process until you removed all of your skittles, • Exchange your results with all groups. Draw a graph.

Half-life of skittles Number of shakes 0 1 2 3 4 5 6 7 8 9 10 Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Average skittle count

Graph for the Half-life of Skittles Count rate Number of shakes of the cup

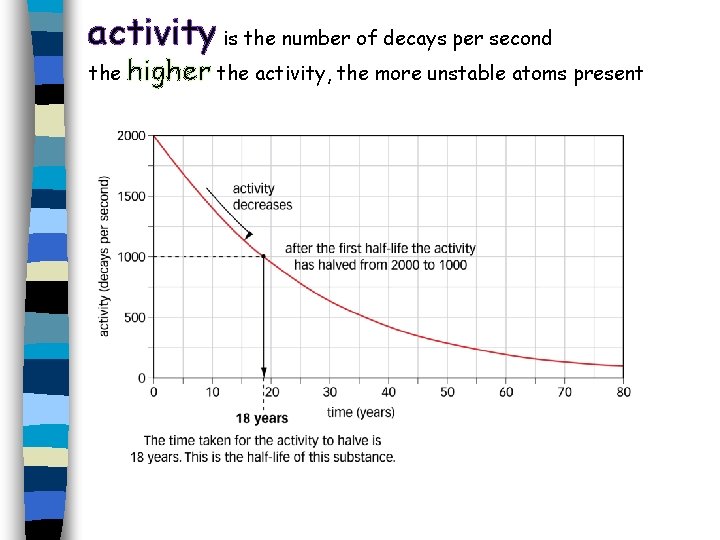
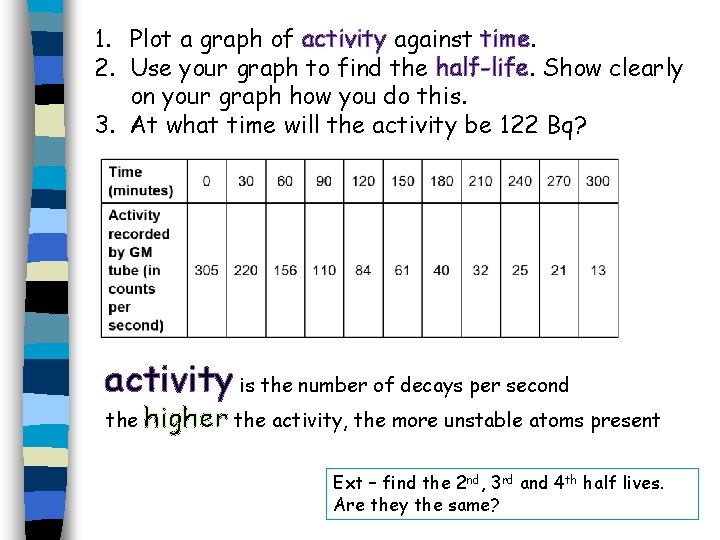
activity is the number of decays per second the higher the activity, the more unstable atoms present

1. Plot a graph of activity against time. 2. Use your graph to find the half-life. Show clearly on your graph how you do this. 3. At what time will the activity be 122 Bq? activity is the number of decays per second the higher the activity, the more unstable atoms present Ext – find the 2 nd, 3 rd and 4 th half lives. Are they the same?

Connector - Worksheet on Half-life

Answers To Worksheet Mark in Red! 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 5 seconds. 20 seconds. A 5 minutes. 50 atoms 8 hours 24 hours 100 16 hours 60 years

Higher tier
L. O. Homework explain how radioactivity is measured ü explain how half-life can be used to estimate the age of objects ü

Rocks don’t come with labels saying how old they are! But scientists can work out their age using radioactivity.

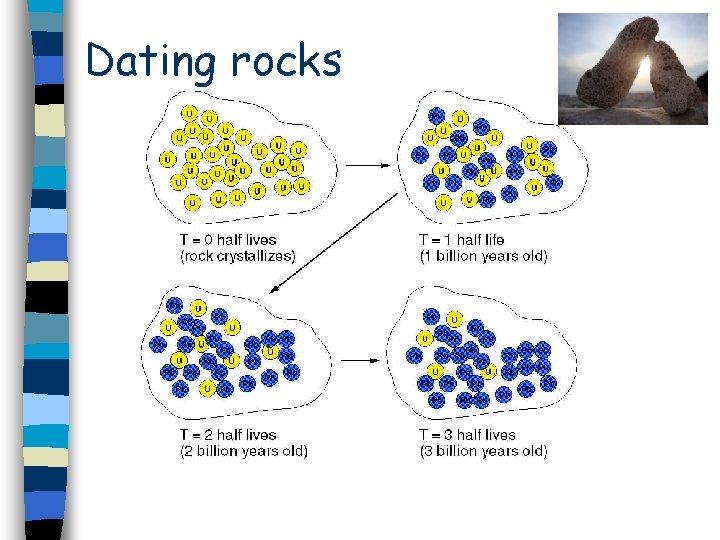
Dating rocks

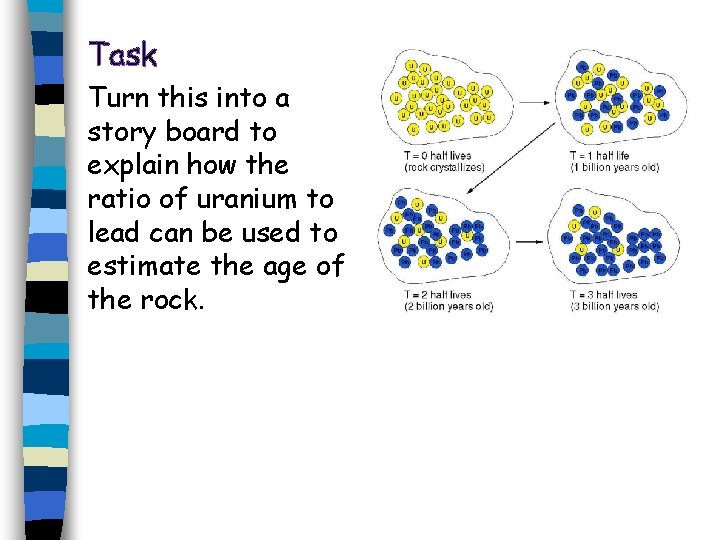
Task Turn this into a story board to explain how the ratio of uranium to lead can be used to estimate the age of the rock.

A rock formed and it contains no lead but does contain some radioactive uranium nuclei. n Over time, the uranium decays to form lead. n When half has decayed to lead, 1 billion years has passed (the half life). n If you measure the ratio of uranium to lead, you can work out the age of the rock from the number of half lives. n


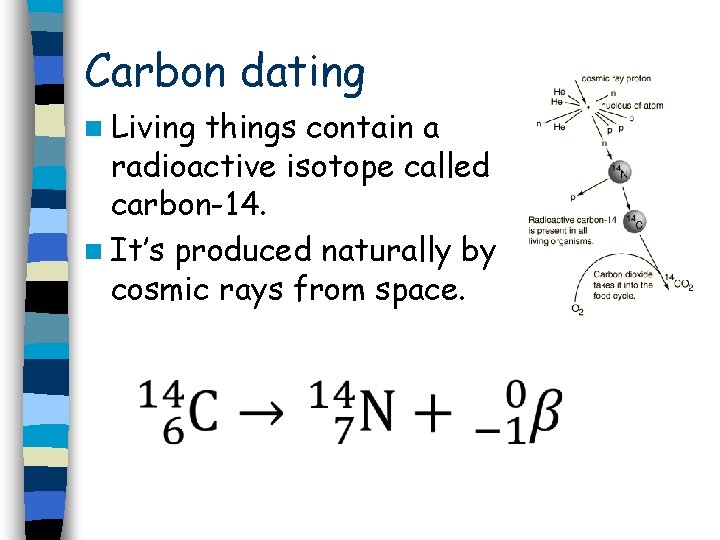
Carbon dating n Living things contain a radioactive isotope called carbon-14. n It’s produced naturally by cosmic rays from space.

n Animals absorb carbon-14 when they eat or breathe. n Plants absorb carbon-14 when they exchanges gases during photosynthesis and respiration. n When something is alive, the activity of the carbon-14 inside it is the same as that in the air.

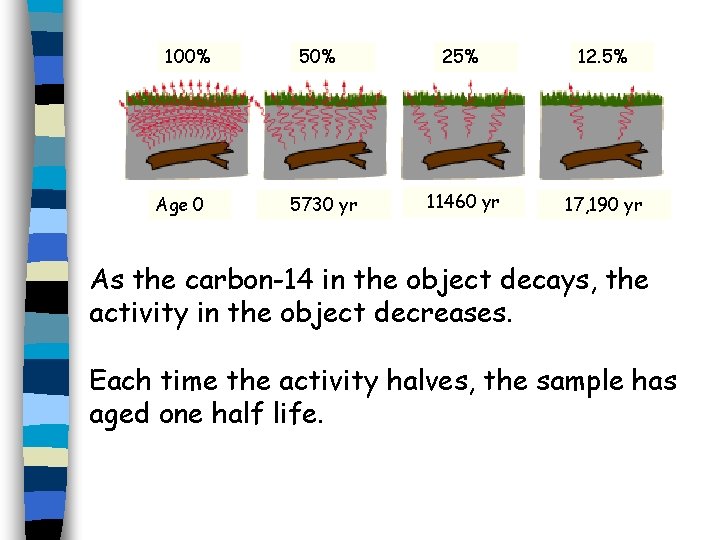
When a living thing dies, it no longer takes in any carbon-14. n So the amount present gets smaller and smaller over time. n Eventually there is none left. n 100% Age 0 50% 5730 yr 25% 12. 5% 11460 yr 17, 190 yr

100% Age 0 50% 5730 yr 25% 12. 5% 11460 yr 17, 190 yr As the carbon-14 in the object decays, the activity in the object decreases. Each time the activity halves, the sample has aged one half life.

the amount of Carbon-14 in the air has not changed for thousands of years n living things absorb Carbon-14 by gas exchange but this process stops as soon as they die n as the Carbon-14 in the object decays the activity of the object decreases n the ratio of activity from living matter to the activity of the sample is used to calculate the age n
 Student worksheet modeling nuclear changes answer key
Student worksheet modeling nuclear changes answer key How to calculate initial rate of reaction
How to calculate initial rate of reaction Calculate the number of grams of al in 371g of al2o3
Calculate the number of grams of al in 371g of al2o3 Pulmonary vascular resistance normal range
Pulmonary vascular resistance normal range Clearly trivial threshold
Clearly trivial threshold Residual earnings valuation model
Residual earnings valuation model How to calculate experimental probability
How to calculate experimental probability How to calculate heat absorbed
How to calculate heat absorbed Formula of effort
Formula of effort How to calculate excess reserves
How to calculate excess reserves How jlpt score is calculated
How jlpt score is calculated How to calculate approach velocity
How to calculate approach velocity Durbin-watson test excel
Durbin-watson test excel Net reproductive rate
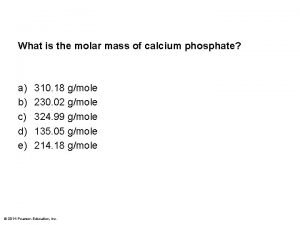
Net reproductive rate Calcium phosphate molar mass
Calcium phosphate molar mass Real per capita gdp formula
Real per capita gdp formula Calculate the density of the cube.
Calculate the density of the cube. How do you find the volume of a trapezoidal prism
How do you find the volume of a trapezoidal prism Margin of safety formula units
Margin of safety formula units How to calculate empirical formula
How to calculate empirical formula How to calculate theoretical yield
How to calculate theoretical yield What is meant by empirical formula
What is meant by empirical formula Reorder point formula
Reorder point formula Days sales uncollected
Days sales uncollected Aggregate demand and aggregate supply
Aggregate demand and aggregate supply Coefficient of friction formula
Coefficient of friction formula How to calculate lod score
How to calculate lod score Calculate the empirical formula
Calculate the empirical formula