ACUTE LEUKEMIA 2012 2013 Leukemia Group of malignant




































- Slides: 61

ACUTE LEUKEMIA 2012 -2013

Leukemia Group of malignant disorders of the hematopoietic tissues characteristically associated with increased numbers of white cells in the bone marrow and / or peripheral blood

Classification • Classified based on cell type involved and the clinical course – Acute : • ALL • AML – Chronic : • CLL • CML

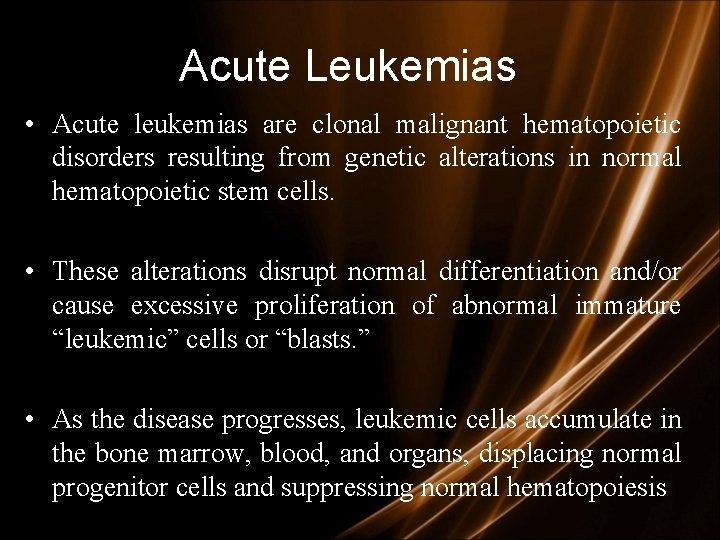
Acute Leukemias • Acute leukemias are clonal malignant hematopoietic disorders resulting from genetic alterations in normal hematopoietic stem cells. • These alterations disrupt normal differentiation and/or cause excessive proliferation of abnormal immature “leukemic” cells or “blasts. ” • As the disease progresses, leukemic cells accumulate in the bone marrow, blood, and organs, displacing normal progenitor cells and suppressing normal hematopoiesis

Acute Leukemia - Essentials • Short course of symptoms • Fatigue, fever, easy bruising, bleeding • Cytopenias - or pancytopenia • More than 20% blasts in bone marrow • Blasts in peripheral blood in 90% cases

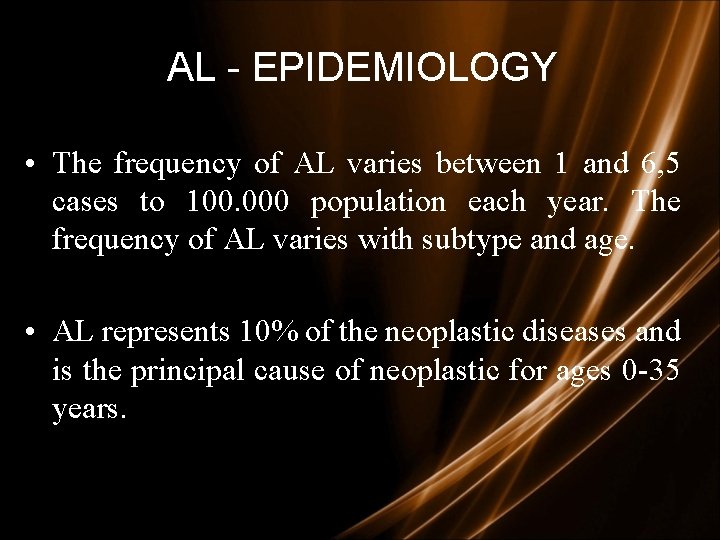
AL - EPIDEMIOLOGY • The frequency of AL varies between 1 and 6, 5 cases to 100. 000 population each year. The frequency of AL varies with subtype and age. • AL represents 10% of the neoplastic diseases and is the principal cause of neoplastic for ages 0 -35 years.

ALL - Epidemiology • Approximately 3, 000 new cases per year • Mostly affects children, accounts for 2/3 of childhood children leukemia (peak age 4 years) years • Comprises less than 20% of leukemia in young adults • May be B-cell, T-cell, or null-type (non-B, non-T cell) • Only 20 -40% of adults with ALL are cured with current regimens.

AML - Epidemiology • 2. 3 per 100, 000 people per year • Higher among men than women (2. 9 vs 1. 9). The difference is even more apparent in older patients. • Most common leukemia in adults (80% of cases) • Vast majority of patients 65 years or older • AML is more common in whites than in other populations.

ALL - Etiology • Uncertain, but several proposed linkages: · Genetic - Philadelphia chromosome · Viral infection (EBV, HIV) · Exposure to high energy radiation (T-cell ALL) · Toxic chemical exposure · Smoking

AML - Etiology • Primary AML – Increased incidence • Genetic fragility – Bloom syndrome – Faconi anemia – Wiskott Aldrich – Down, Klinefelter, Patau syndromes • tobacco use? • herbicides? , pesticides? • benzene exposure • Secondary AML – XRT – Topoisomerase II inhibitors (e. g etopisode), alkylating agents – MDS – other cell proliferation disorders • CML, polycythemia vera, primary thrombocytosis, PNH

Clinical features General : Onset is abrupt & stormy (usually present within 3 months) – Bone marrow failure (anemia, infection , bleeding) – Bone pain & tenderness

Clinical presentation: • Will present with sign or symptoms related to : – Pancytopenia: • WBC infection infectious syndrome • Hb anemia anemic syndrome • Platelets bleeding – hemorragic syndrome – Organ infiltration tumoral syndrome • • Lymphadenopathy. More common with ALL than AML. Splenomegally. Hepatomegally. CNS: 5 -10% of patient with ALL

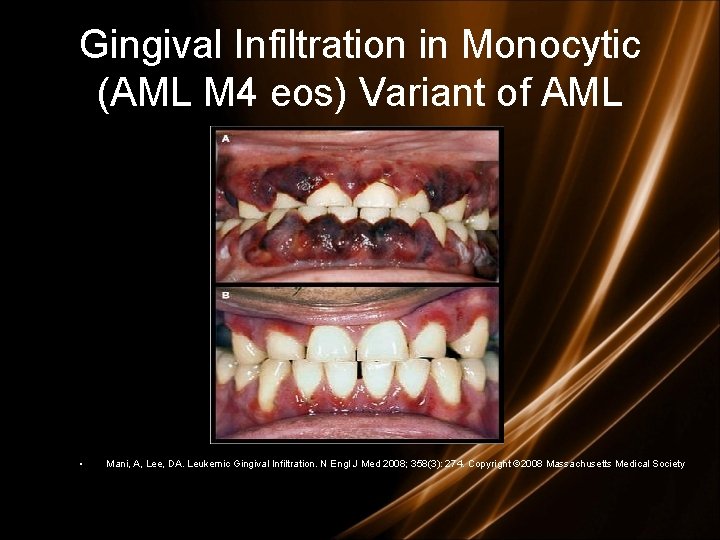
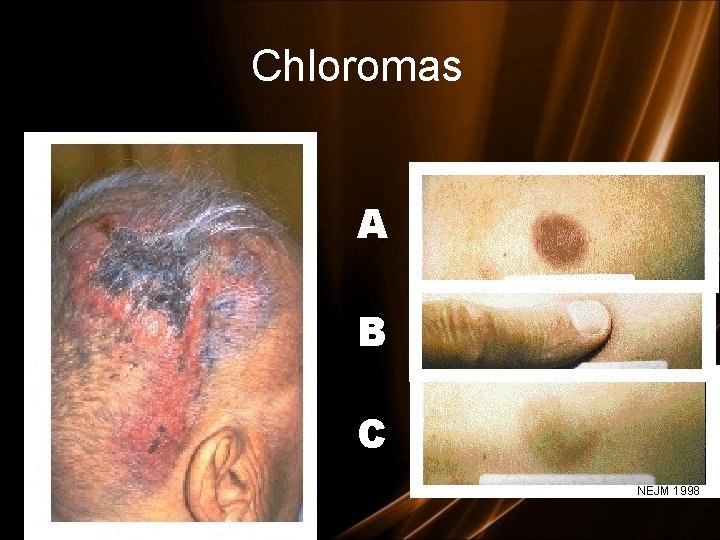
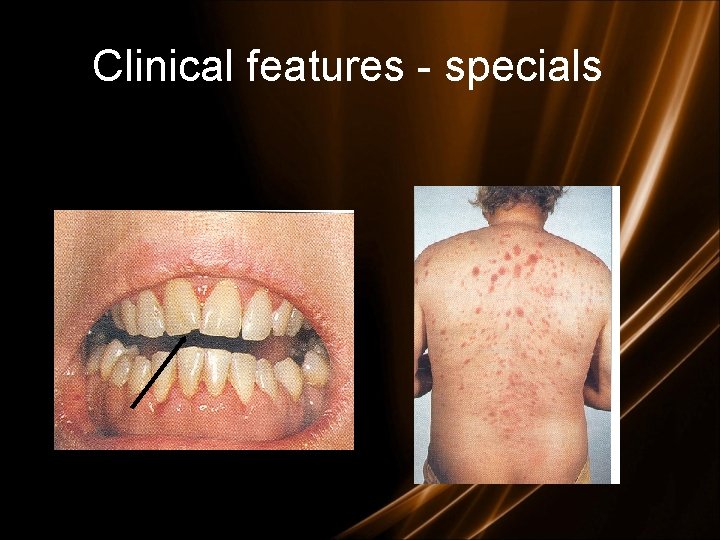
Clinical features - specials • L mediastinal tumoral mass • L CNS infiltration • M 2 : Chloroma: -presents as a mass lesion ‘tumor of leukemic cells’ • M 3 : DIC • M 4/M 5 : Infiltration of soft tissues, gum infiltration, skin deposits , Meningeal involvement-headache, vomiting, eye symptoms

Gingival Infiltration in Monocytic (AML M 4 eos) Variant of AML • Mani, A, Lee, DA. Leukemic Gingival Infiltration. N Engl J Med 2008; 358(3): 274. Copyright © 2008 Massachusetts Medical Society

Gum hypertrophy

Chloromas A B C NEJM 1998

Clinical symptoms/Physical Findings • Extramedullary disease (ie, myeloid sarcoma) – Can also have involvement of lymph nodes, intestine, mediastinum, ovaries, uterus

Clinical features - specials

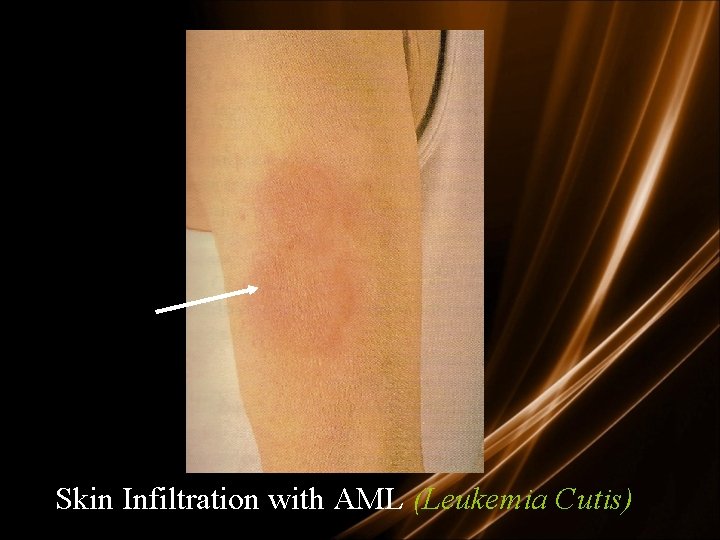
Skin Infiltration with AML (Leukemia Cutis)

Leukostasis • Leukostasis – predominantly in those with WBC counts > 100, 000 (10% of patients); can also be seen in patients with WBC > 50, 000 – Most common in those with M 4 or M 5 leukemia – Function of the blast cells being less deformable than mature myeloid cells. As a result, intravascular plugs develop. – High metabolic activity of blast cells and local production of various cytokines contribute to underlying hypoxia

Leukostasis Thornton, KA, Levis, M. FLT 3 Mutation and Acute Myelogenous Leukemia with Leukostasis. N Engl J Med 2007; 357: 1639. Copyright © 2007 Massachusetts Medical Society

Leukostasis • Common symptoms – Pulmonary: dyspnea, chest pain – CNS: headaches, altered mentation, CN palsies, ocular symptoms – Priapism – Myocardial Infarction

Acute leukemia - Diagnosis – Lab evaluation • The lab diagnosis is based on two things – Finding a significant increase in the number of immature cells in the bone marrow including blasts, promyelocytes, promonocytes (>30% blasts is diagnostic) – Identification of the cell lineage of the leukemic cells

Investigations • • • CBC: – 60% of pts have an elevated WBC. – Most are anemic – Most are thrombocytopenic – 90%have blast in the periphral blood film. electrolytes: – Hypo/hyper kalemia – Hypomagnesimia – hyperphosphatemia Hypermetabolism: – LDH. – uric acid. DIC: – Most common with promyelocytic leukemia, small% monocytic leukemia&ALL Bone marrow biopsy and aspirate: – 30%or more of all nucleated cells are blast. Radiology: – CXR: mediastinal mass(T-cell ALL) – Osteopenia or lytic lesion 50% of patients with ALL. (itractable pain).

Acute leukemia - Diagnosis – Peripheral blood: • Anemia (normochromic, normocytic) • Decreased platlets • Variable WBC count – The degree of peripheral blood involvement determines classification: » Leukemic – increased WBCs due to blasts » Subleukemic – blasts without increased WBCs » Aleukemic – decreased WBCs with no blasts

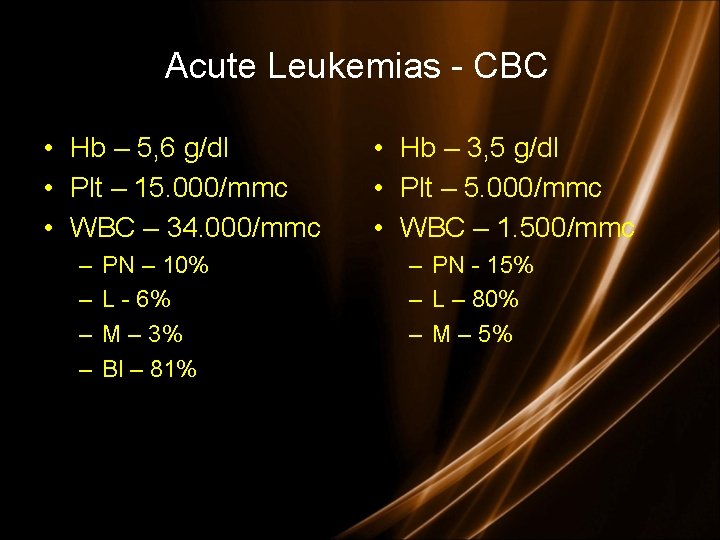
Acute Leukemias - CBC • Hb – 5, 6 g/dl • Plt – 15. 000/mmc • WBC – 34. 000/mmc – – PN – 10% L - 6% M – 3% Bl – 81% • Hb – 3, 5 g/dl • Plt – 5. 000/mmc • WBC – 1. 500/mmc – PN - 15% – L – 80% – M – 5%

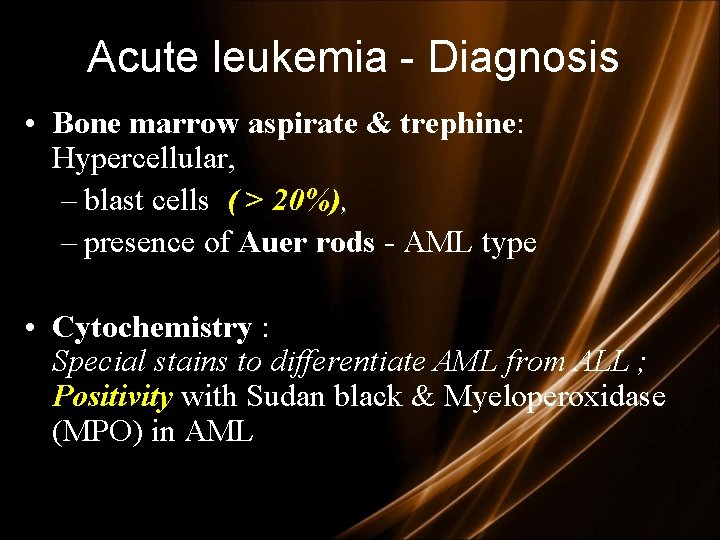
Acute leukemia - Diagnosis • Bone marrow aspirate & trephine: Hypercellular, – blast cells ( > 20%), – presence of Auer rods - AML type • Cytochemistry : Special stains to differentiate AML from ALL ; Positivity with Sudan black & Myeloperoxidase (MPO) in AML

Jemshidi trephine & Salah aspiration needle

Acute leukemia - Diagnosis • Diagnosis and classification of the immature cells involved may be done by : – Morphology – Cytochemistry – Immunophenotyping – Cytogenetic

Classification • Criteria: - Morphology : apperance of cell under microscope. - Cytochemistry: chemical activity of the cell. (myeloperoxidase , Sudan Black B) - immunophenotyping: antigen pressent in the cell membrane - Cytogenetics: chromosome of the cell - Molecular biology: • Classification: 3 groups of acute leukemias: - acute myeloid leukemias AML(M 1 –M 6). - acute lymphoblastic leukemias ALL (L 1 -L 3). - Biphenotypic leukemias or Acute undifferentiated leukemia

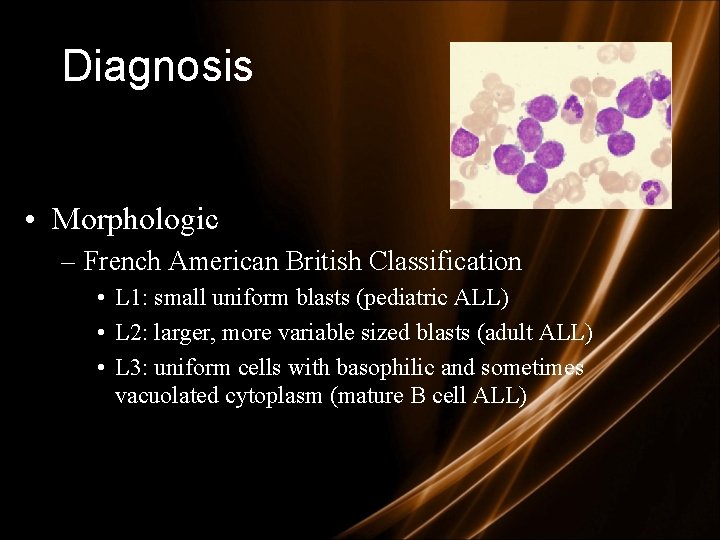
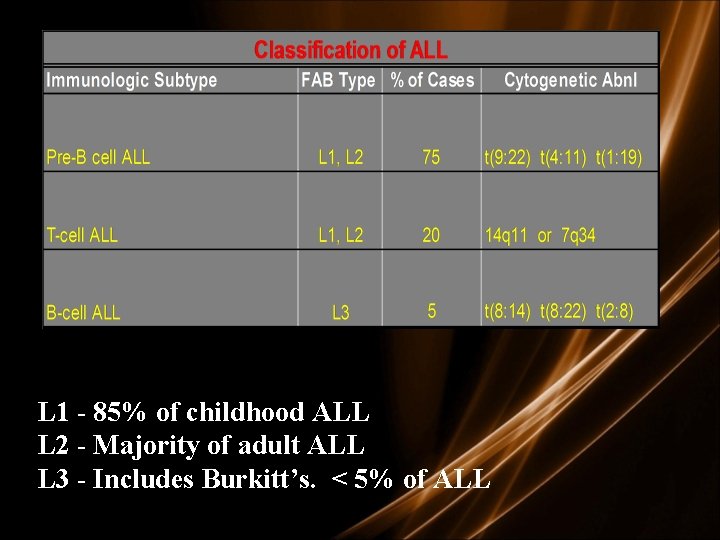
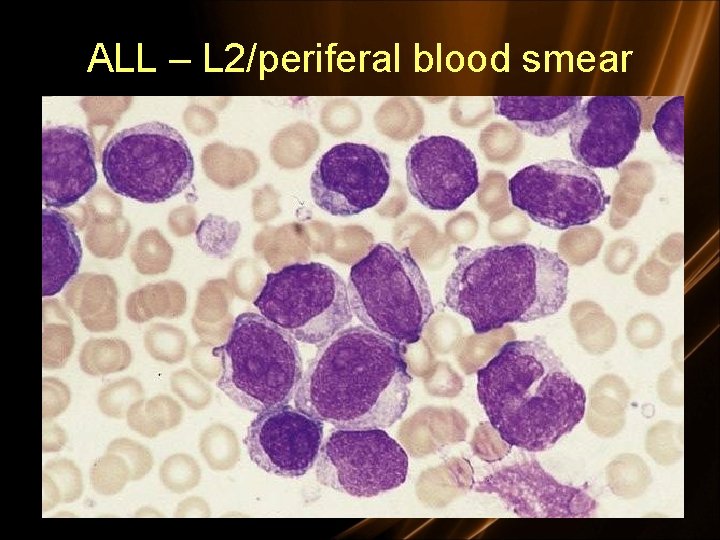
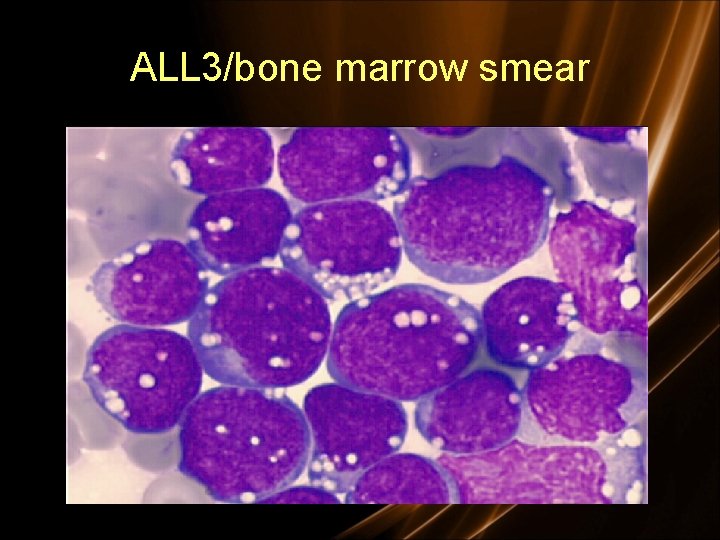
Diagnosis • Morphologic – French American British Classification • L 1: small uniform blasts (pediatric ALL) • L 2: larger, more variable sized blasts (adult ALL) • L 3: uniform cells with basophilic and sometimes vacuolated cytoplasm (mature B cell ALL)

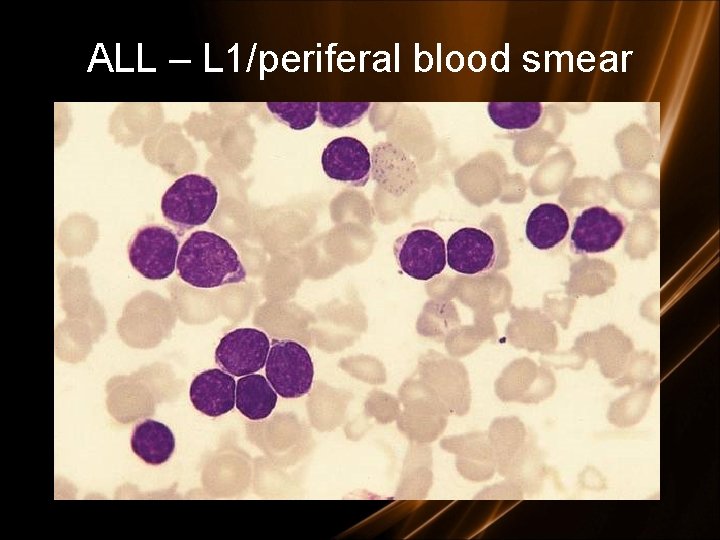
L 1 - 85% of childhood ALL L 2 - Majority of adult ALL L 3 - Includes Burkitt’s. < 5% of ALL

ALL – L 1/periferal blood smear

ALL – L 2/periferal blood smear

ALL 3/bone marrow smear

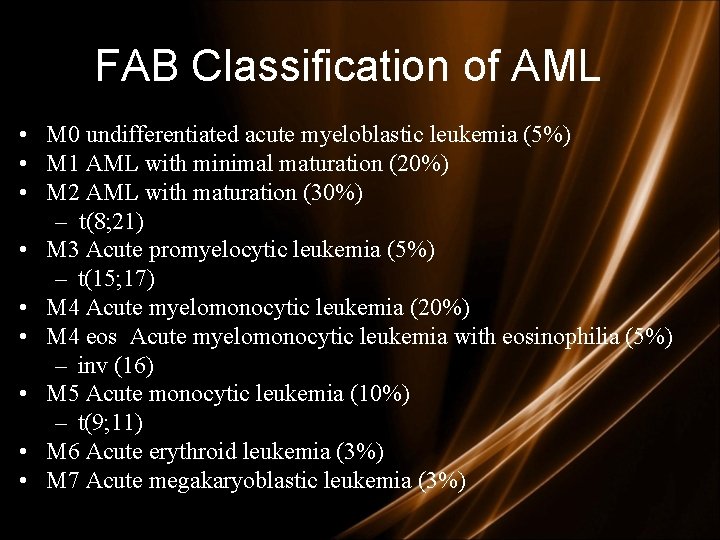
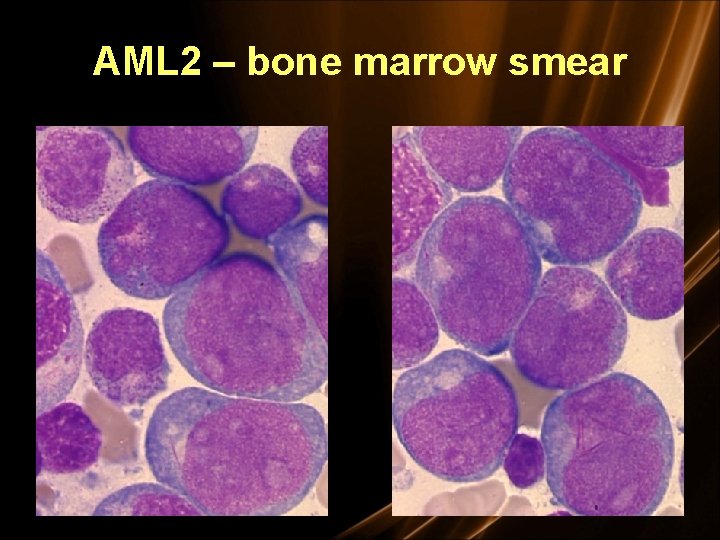
FAB Classification of AML • M 0 undifferentiated acute myeloblastic leukemia (5%) • M 1 AML with minimal maturation (20%) • M 2 AML with maturation (30%) – t(8; 21) • M 3 Acute promyelocytic leukemia (5%) – t(15; 17) • M 4 Acute myelomonocytic leukemia (20%) • M 4 eos Acute myelomonocytic leukemia with eosinophilia (5%) – inv (16) • M 5 Acute monocytic leukemia (10%) – t(9; 11) • M 6 Acute erythroid leukemia (3%) • M 7 Acute megakaryoblastic leukemia (3%)

WHO Classification • • • AML with certain genetic abnormalities – t(8; 21), t(16), inv(16), chromosome 11 changes – t(15; 17) as usually seen with AML M 3 AML with multilineage dysplasia (more than one abnormal myeloid cell type is involved) AML related to previous chemotherapy or radiation AML not otherwise specified – undifferentiated AML (M 0) – AML with minimal maturation (M 1) – AML with maturation (M 2) – acute myelomonocytic leukemia (M 4) – acute monocytic leukemia (M 5) – acute erythroid leukemia (M 6) – acute megakaryoblastic leukemia (M 7) – acute basophilic leukemia – acute panmyelosis with fibrosis – myeloid sarcoma (also known as granulocytic sarcoma or chloroma) Undifferentiated or biphenotypic acute leukemias (leukemias that have both lymphocytic and myeloid features. Sometimes called ALL with myeloid markers, AML with lymphoid markers, or mixed lineage leukemias. )

AML 2 – bone marrow smear

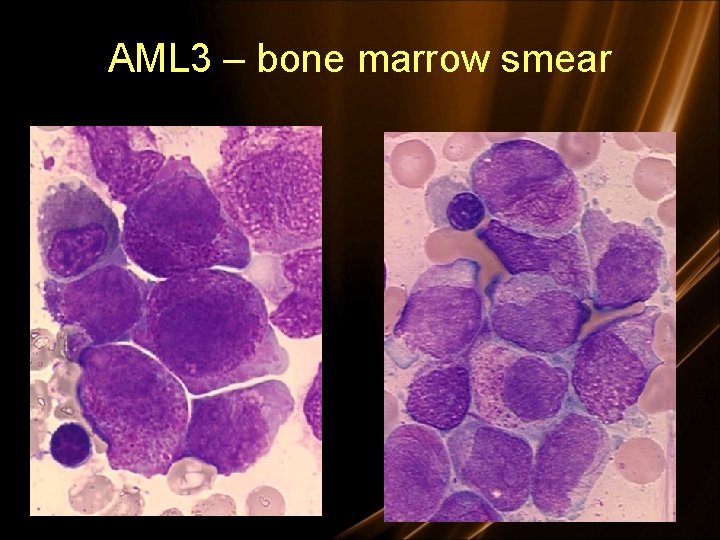
AML 3 – bone marrow smear

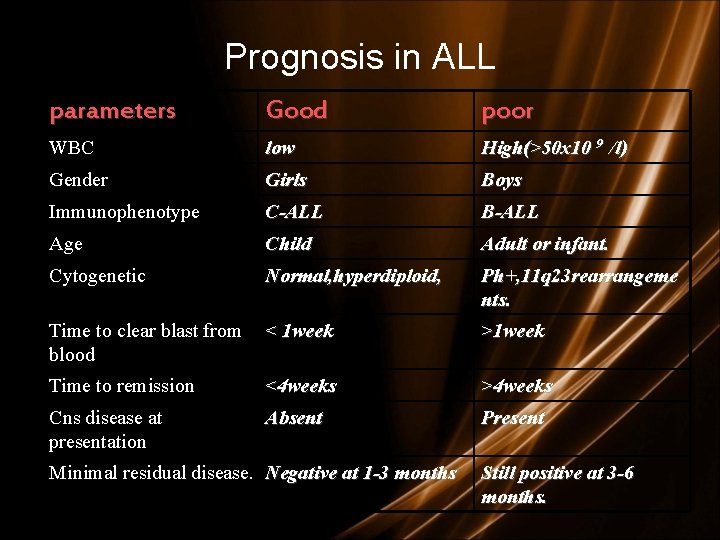
Prognosis in ALL parameters Good poor WBC low High(>50 x 10 9 /l) Gender Girls Boys Immunophenotype C-ALL B-ALL Age Child Adult or infant. Cytogenetic Normal, hyperdiploid, Ph+, 11 q 23 rearrangeme nts. Time to clear blast from < 1 week blood >1 week Time to remission <4 weeks >4 weeks Cns disease at presentation Absent Present Minimal residual disease. Negative at 1 -3 months Still positive at 3 -6 months.

Prognosis in ALL • Good risk includes – (1) no adverse cytogenetics, – (2) age younger than 30 years – (3) WBC count of less than 30, 000/m. L, and – (4) complete remission within 4 weeks. • Intermediate risk – does not meet the criteria for either good risk or poor risk. • Poor risk includes – (1) adverse cytogenetics [(t 9; 22), (4; 11)], – (2) age older than 60 years, – (3) precursor B-cell WBCs with WBC count greater than 100, 000/m. L, or – (4) failure to achieve complete remission within 4 weeks.

Prognosis in AML Prognostic Factors: • Age at diagnosis • Comorbidities (acute vs chronic) • Chromosomal findings • Symptomatic interval preceding diagnosis • Presenting Leukocyte count • Circulating myeloblast count • FAB classification • Morphologic characteristics of the leukemic cell

Treatment of acute leukemias Choice of Rx is influenced by: • type (AML vs ALL) • age • curative vs palliative intent

Principles of treatment • combination chemotherapy – first goal is complete remission – further Rx to prevent relapse • supportive medical care – transfusions, antibiotics, nutrition • psychosocial support – patient and family

Supportive measures: -isolation in positive laminer flux room -insertion of central line -family and patient support by permanent social worker -Alkaline diuresis to prevent tumor lysis syndrome -oropharynx/GIT decontamination to prevent fungal infection -IV antibiotics for infection -Blood transfusion if anemia and thrombocytopenia.


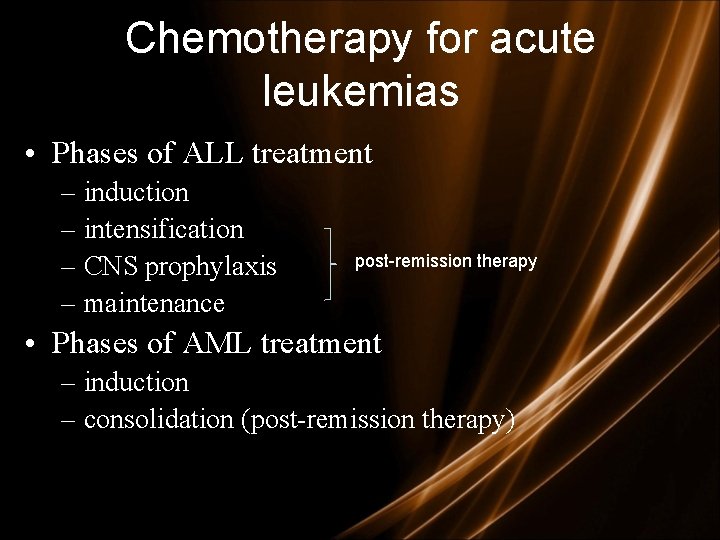
Chemotherapy for acute leukemias • Phases of ALL treatment – induction – intensification – CNS prophylaxis – maintenance post-remission therapy • Phases of AML treatment – induction – consolidation (post-remission therapy)

ALL Treatment • 1 – Remission Induction • 2 – Intensification (Consolidation) Therapy • 3 – Maintenance Therapy • 4 – CNS Prophylaxis • 5 – Allogeneic Stem Cell Transplant

ALL Treatment • Remission Induction – Goals: restore normal hematopoiesis, induce a complete remission rapidly in order to prevent resistance to drugs – Standard induction regimen • 4 or 5 drugs: vincristine, prednisone, anthracycline, L-asparaginase, +/- cyclophosphamide – 80 -90% complete remission • Intensification – High doses of multiple agents not used during induction or readministration of the induction regimen

ALL Treatment • Maintenance Therapy – Daily po 6 MP, weekly MTX, monthly pulses of vincristine and prednisone for 2 -3 yrs • CNS Prophylaxis – – Given during induction and intensification Intrathecal: MTX, Cytarabine, corticosteroids Systemic: high dose mtx, cytarabine, L-asparaginase +/- Cranial Irradiation

ALL Treatment • Stem Cell Transplant – Done during first CR – Indications: • Ph Chromosome • t(4; 11) mutation • Poor initial response to induction therapy • Other – Adolescents benefit significantly from pediatric ALL regimens vs. adult regimens

AML Treatment • Remission induction therapy – Commonly anthracycline (ie, daunorubicin, idarubicin) and cytarabine → (“ 3+7 regimen”) • • • Cytarabine has ample CNS penetration so no need for prophylactic intrathecal chemotx (also, ↓ risk in patients with AML compared to ALL) 60 -80% achieve complete remission Postremission therapy 1. Consolidation – – 2. longer survival than maintence alone typically high dose cytarabine Maintenance – continue chemotx monthly for 4 -12 months – nonmyelosuppressive doses

COMPLICATIONS TREATMENT

Tumor Lysis Syndrome • Characterized by metabolic derangements caused by massive release of cellular components following lysis of malignant cells • Commonly seen in malignancies with high rates of cell proliferation (esp. ALL, Burkitt’s lymphoma); also can be seen with AML

Tumor Lysis Syndrome • Tumor lysis syndrome - hyperphosphatemia, hyperkalemia, hyperuricemia, hypocalcemia and uremia – Retrospective study of 788 patients (433 adults) found incidence of hyperuricemia and TLS to be 14. 7%/3. 4% in patients with AML compared to 21. 4%/5. 2% in patients with ALL and 19. 6%/6. 1% in patients with NHL • Electrolyte abnormalities can occur without the entire spectrum of TLS or even before tx is initiated – Hyperuricemia – Lactic acidosis

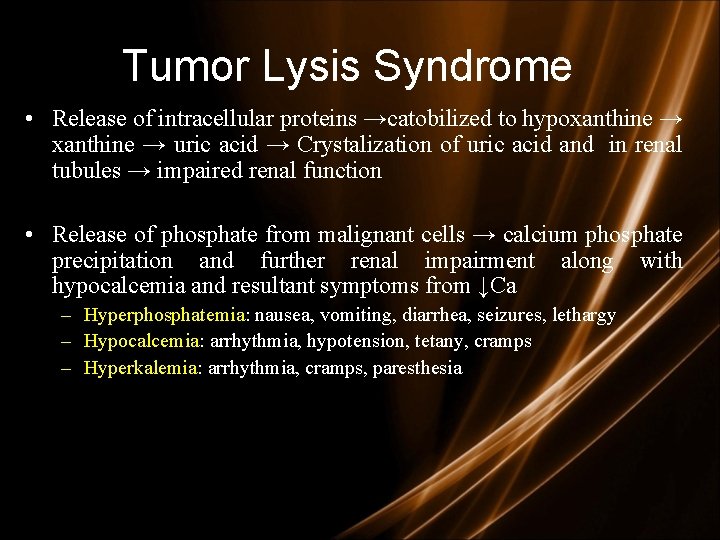
Tumor Lysis Syndrome • Release of intracellular proteins →catobilized to hypoxanthine → uric acid → Crystalization of uric acid and in renal tubules → impaired renal function • Release of phosphate from malignant cells → calcium phosphate precipitation and further renal impairment along with hypocalcemia and resultant symptoms from ↓Ca – Hyperphosphatemia: nausea, vomiting, diarrhea, seizures, lethargy – Hypocalcemia: arrhythmia, hypotension, tetany, cramps – Hyperkalemia: arrhythmia, cramps, paresthesia

Tumor Lysis Syndrome • Prevention and management – IV hydration : promotes excretion of uric acid and phosphate; improves renal blood flow/GFR – Allopurinol → competitive inhibitor for xanthine oxidase. Therefore, ↓ conversion of purine metabolites to uric acid • However, must consider buildup of xanthine crystals → acute obstructive uropathy (HYDRATE!!!) – Recominant urate oxidase (rasburicase) • Promotes conversion of uric acid to allantoin (highly soluble; urinary excretion) • Indicated in patients at high risk of TLS (Burkitt’s Lymphoma, B-ALL, ALL (WBC >100, 000), AML (WBC >50, 000) • Also indicated in patients that develop hyperuricemia despite allopurinol – Dialysis can be used in severe cases – Urine alkalization is NOT recommended – does not increase solubility of xanthine/hypoxanthine with an increased propensity to develop xanthineobstructive uropathies (esp with allopurinol use)

DIC • Common symptoms/findings – – – in addition to weakness (anemia), infections/fever (malfunctioning WBCs) petechiae, ecchymoses, hematuria, bleeding from venipuncture sites migratory thrombophlebitis (Trousseau’s syndrome) nonbacterial thrombotic (marantic) endocarditis DVT/PE • Lab findings – – – Prolonged PT/INR, PTT microangiopathic anemia (schistocytes) thrombocytopenia elevated fibrin split products elevated D-dimer low fibrinogen

• Treatment DIC 1. Supportive therapy • • • Platelets Cryoprecipitate (fibrinogen) FFP 2. Treatment for obvious thrombosis (e. g thrombophlebitis, mural thrombus) • • UFH or LMWH; often resistant to coumadin activated protein C 3. Treatment of underlying malignancy • In the case of AML M 3 → All-Trans Retinoic Acid (PML-RARα) – – • • Induces differentiation beyond promyelocyte phase Only with the more common t(15; 17) translocation; t(11; 17) and t(5; 17) do not respond to ATRA Remission rates of greater than 90% with AML M 3 patient treated with ATRA and chemotx (eg, anthracyclines (idarubicin)) with 60 -70% disease free survival Arsenic trioxide in those that relapse – achieves complete remission in >90%

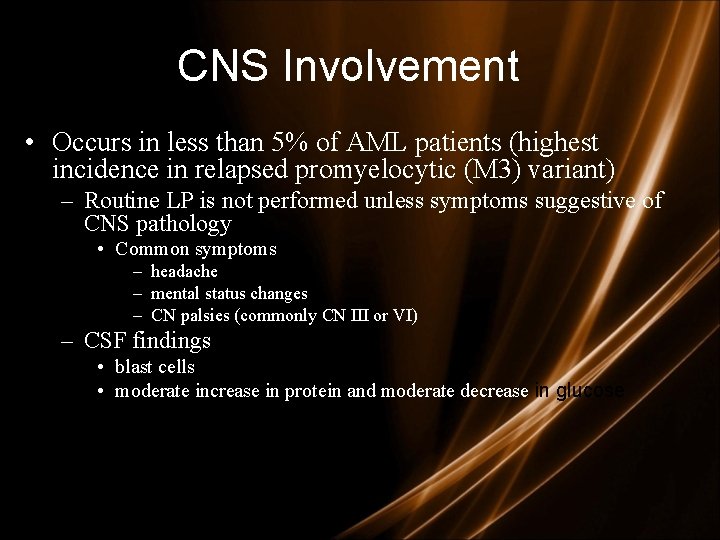
CNS Involvement • Occurs in less than 5% of AML patients (highest incidence in relapsed promyelocytic (M 3) variant) – Routine LP is not performed unless symptoms suggestive of CNS pathology • Common symptoms – headache – mental status changes – CN palsies (commonly CN III or VI) – CSF findings • blast cells • moderate increase in protein and moderate decrease in glucose

CNS Involvement • Treatment – Intrathecal chemotherapy (methotrexate or cytarabine) +/- whole brain XRT • addition of XRT depends on response to intrathecal chemotx and whethere is cranial nerve involvement • high relapse rate – Commonly administer prophylactic intrathecal chemotx in relapsed promyelocytic disease
 Differentiation syndrome
Differentiation syndrome Promyelocytic
Promyelocytic Acute mylogenous leukemia
Acute mylogenous leukemia Benign or malignant
Benign or malignant Malignant neuroleptic syndrome
Malignant neuroleptic syndrome Stratum basale
Stratum basale Malignant hypertension management
Malignant hypertension management Peter hino md
Peter hino md Kitwood flower of needs
Kitwood flower of needs Neuroleptic malignant syndrome
Neuroleptic malignant syndrome Malignant hypertension treatment
Malignant hypertension treatment Norepinephrine computation
Norepinephrine computation Malignant neoplasm of the blood-forming organs
Malignant neoplasm of the blood-forming organs Malignant mesothelioma
Malignant mesothelioma Perdida de polaridad celular
Perdida de polaridad celular Prostatic adenocarcinoma
Prostatic adenocarcinoma Md frcpc definition
Md frcpc definition Benign and malignant tumor
Benign and malignant tumor Nursing care plan for schizophrenia pdf
Nursing care plan for schizophrenia pdf Hypertensive emergency
Hypertensive emergency Local invasion
Local invasion Essential hypertension
Essential hypertension Hypertensive emergency vs urgency
Hypertensive emergency vs urgency Leukemia vs lymphoma
Leukemia vs lymphoma Myelobast
Myelobast Cml stages
Cml stages Leukemia
Leukemia Leukemia statics
Leukemia statics Leukemia
Leukemia Suffix -ion medical terminology
Suffix -ion medical terminology Zhang wang leukemia
Zhang wang leukemia Leukemoid reaction
Leukemoid reaction Limfoblast
Limfoblast Funkcie krvi
Funkcie krvi Leukemia conclusion
Leukemia conclusion Hairy cell leukemia
Hairy cell leukemia Lll leukemia
Lll leukemia Specific esterase stain principle
Specific esterase stain principle Nk leukemia
Nk leukemia Ostomy medical term suffix
Ostomy medical term suffix Chronic myeloid leukemia
Chronic myeloid leukemia Chronic myeloid leukemia
Chronic myeloid leukemia Leukemia statics
Leukemia statics Mark juckett md
Mark juckett md Social facilitation psychology
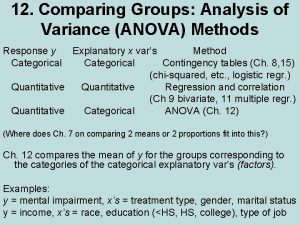
Social facilitation psychology Within group variance vs between group
Within group variance vs between group Anova within group and between group
Anova within group and between group Primary group in sociology
Primary group in sociology Joint royal college of physicians training board
Joint royal college of physicians training board Thermal stability of group 2 carbonates and nitrates
Thermal stability of group 2 carbonates and nitrates Amino group and carboxyl group
Amino group and carboxyl group Amino group and carboxyl group
Amino group and carboxyl group In group out group
In group out group Group yourselves
Group yourselves Sumner's classification of social groups
Sumner's classification of social groups Joining together group theory and group skills
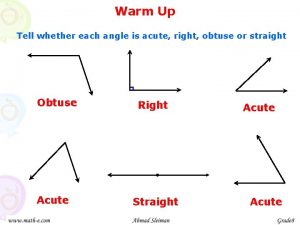
Joining together group theory and group skills Name 2 objects with acute angles
Name 2 objects with acute angles Angioectasia icd 10
Angioectasia icd 10 Complementary linear vertical adjacent
Complementary linear vertical adjacent Iga nephropathy vs psgn
Iga nephropathy vs psgn Classifying triangles and quadrilaterals
Classifying triangles and quadrilaterals Paradoxical bronchospasm
Paradoxical bronchospasm