MALIGNANT CATARRHAL FEVER Synonyms Malignant Catarrhal fever Malignant

















- Slides: 22

MALIGNANT CATARRHAL FEVER

Synonyms Malignant Catarrhal fever Malignant Head Catarrh Gangrenous Coryza Bovine herpes virus-3 infection

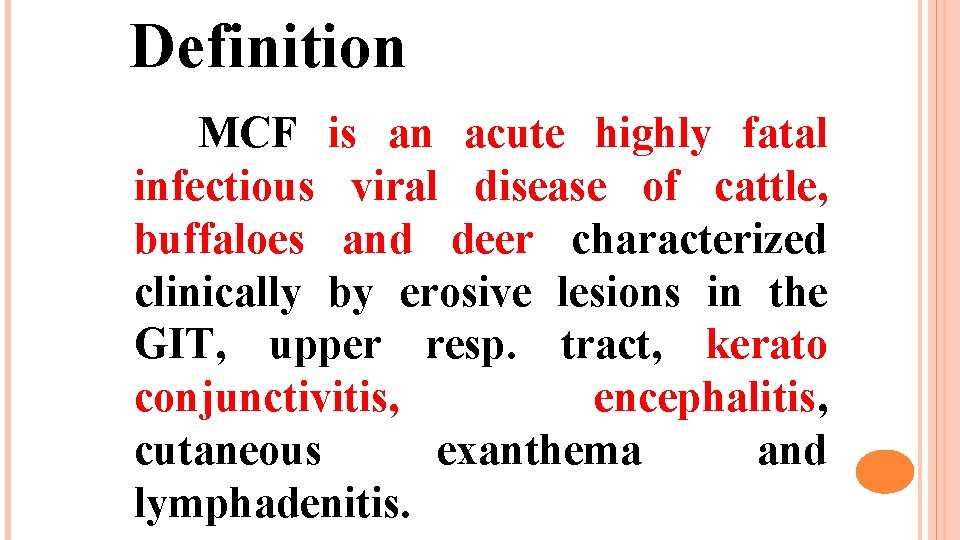
Definition MCF is an acute highly fatal infectious viral disease of cattle, buffaloes and deer characterized clinically by erosive lesions in the GIT, upper resp. tract, kerato conjunctivitis, encephalitis, cutaneous exanthema and lymphadenitis.

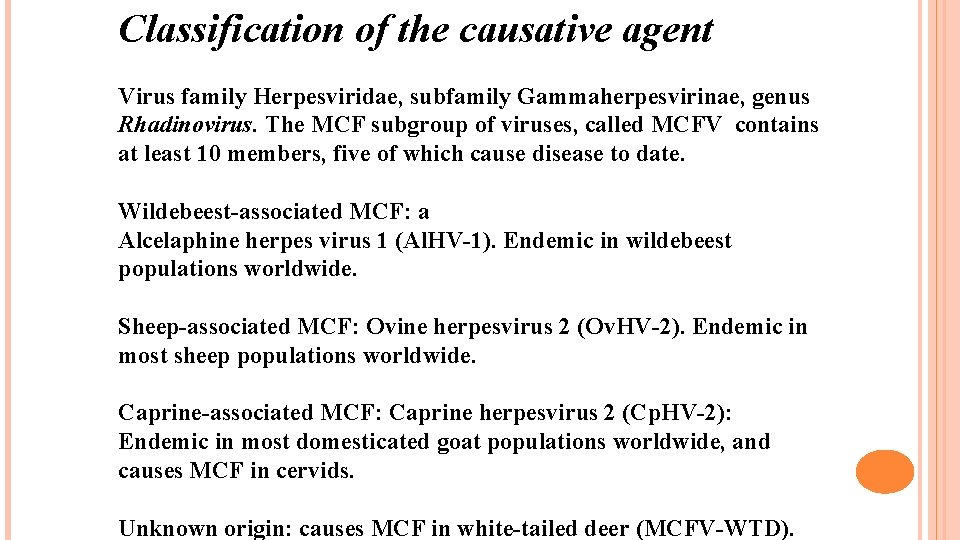
Classification of the causative agent Virus family Herpesviridae, subfamily Gammaherpesvirinae, genus Rhadinovirus. The MCF subgroup of viruses, called MCFV contains at least 10 members, five of which cause disease to date. Wildebeest-associated MCF: a Alcelaphine herpes virus 1 (Al. HV-1). Endemic in wildebeest populations worldwide. Sheep-associated MCF: Ovine herpesvirus 2 (Ov. HV-2). Endemic in most sheep populations worldwide. Caprine-associated MCF: Caprine herpesvirus 2 (Cp. HV-2): Endemic in most domesticated goat populations worldwide, and causes MCF in cervids. Unknown origin: causes MCF in white-tailed deer (MCFV-WTD).

Susceptible animals Cattle, bison, water buffalo Deer, reindeer Giraffes and pigs MCF viruses are carried asymptomatically by their reservoir hosts (Wildebeest, sheep and Goats).

Resistance to physical and chemical action Temperature: No data available, but virus is very labile. p. H: Mostly stable between p. H 5. 5 and 8. 5 Disinfectants/chemicals: Inactivated by common disinfectants including sodium hypochlorite (3% solution if heavy organic debris present) Survival: Inactivated rapidly by sunlight. Cell-associated virus survives 72 hours Outside the host; cell-free virus is inactivated quickly in dry environments but may survive over 13 days in humid environment

Sources of virus Nasal and ocular secretions mainly, but also reported in faeces and semen (Ov. HV-2 DNA detected in semen of domestic rams)

Distribution: Ø OHV-2 and Cp. HV-2 can be seen throughout the world. Ø AHV-1 associated disease mainly occurs in cattle in areas of Africa south of the Sahara desert where wildebeest are found.

Animal Transmission AHV 1 In utero Aerosols Contamination of pastures may also contribute to transmission. OHV-2 and Cp. HV-2 are the same but in utero transmission is not recorded.

Clinical Signs Incubation period 9 to 77 days experimentally Unknown in natural infections

Clinical Signs Peracute form: sudden death Head and eye form: Majority of cattle cases Intestinal form: Initially like head and eye form, but death occurs from severe diarrhea Mild form: Recovery expected

Head and Eye Form: Early Stages Reddened eyelids Bilateral corneal opacity Cattle often have bilateral corneal opacity that begins at the corneoscleral junction and progresses inward. Crusty muzzle, nares Nasal discharge Salivation

Head and Eye Form: Later Stages Erosions on the tongue Erosions on the buccal mucosa

Clinical Signs Joints, superficial lymph nodes swell Nervous signs Incoordination, head pressing, nystagmus, hyperesthesia

Differential Diagnosis BVD mucosal disease Rinderpest FMD

Sampling Before collecting or sending any samples, the proper authorities should be contacted Samples should only be sent under secure conditions and to authorized laboratories to prevent the spread of the disease

Clinical Diagnosis Any susceptible animal with sudden death, fever, erosions of the mucosa, nasal/lacrimal discharge, or bilateral corneal opacity should be tested for MCF Particularly with a history of exposure to sheep, goats, or wildebeest

Laboratory Diagnosis PCR Virus isolation Serology

Treatment Survival is rare if clinically ill Mortality reaches 100% Supportive therapy, antibiotics for secondary bacterial infection Recovered animals will remain virus carriers

Recommended actions Immediately notify authorities State veterinarian Quarantine

Prevention and Control Separate infected and carrier animals from susceptible species Zoological parks Introduce seronegative animals only No vaccine available

Vaccine Unfortunately a vaccine for malignant catarrhal fever (MCF) has not yet been developed. Developing a vaccine has been difficult because the virus will not grow in cell culture and until recently it was not known why. Researchers at the Agricultural Research Service (ARS) found that the virus undergoes changes within the animal’s body, a process known as ( In cell tropism switching) the virus targets different cells at different points in its life cycle, this phenomenon explains why it has been impossible to grow the virus on any one particular cell culture.
 Regurgitation test
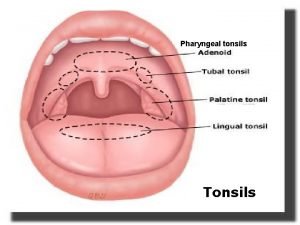
Regurgitation test Pharyngeal tonsils
Pharyngeal tonsils Bradykinin nitric oxide
Bradykinin nitric oxide Crohn's disease pathology outlines
Crohn's disease pathology outlines Neuroleptic malignant syndrome
Neuroleptic malignant syndrome Malignant hypertension ppt
Malignant hypertension ppt Perdida de polaridad celular
Perdida de polaridad celular Malignant mesothelioma
Malignant mesothelioma Malignant hypertension management
Malignant hypertension management Nursing care plan for schizophrenia pdf
Nursing care plan for schizophrenia pdf Malignant hypertension treatment
Malignant hypertension treatment Hypertensive urgency vs emergency
Hypertensive urgency vs emergency Benign vs malignant
Benign vs malignant Liposarcoma
Liposarcoma Peter hino md
Peter hino md Hypertensive emergency
Hypertensive emergency Hypertensive emergency vs urgency
Hypertensive emergency vs urgency Malignant neuroleptic syndrome
Malignant neuroleptic syndrome Carcinoma in situ
Carcinoma in situ Kitwood malignant social psychology
Kitwood malignant social psychology Local invasion
Local invasion Malignant neoplasm of the blood-forming organs
Malignant neoplasm of the blood-forming organs Carotonemia
Carotonemia