To cause the state of matter to change




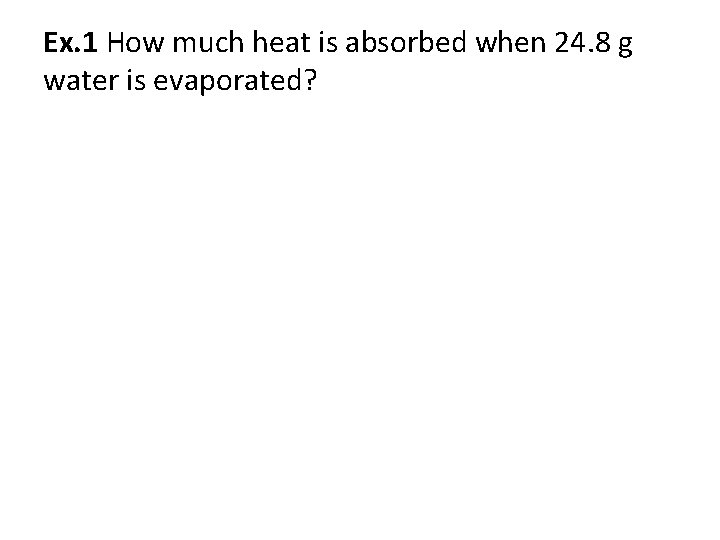



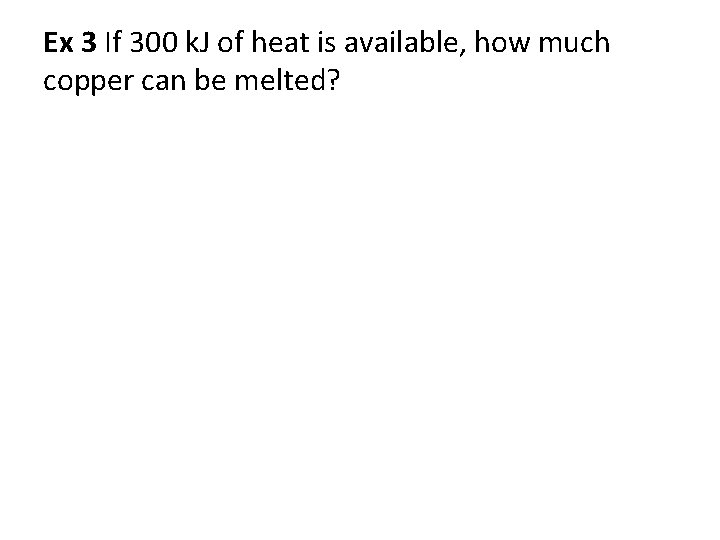

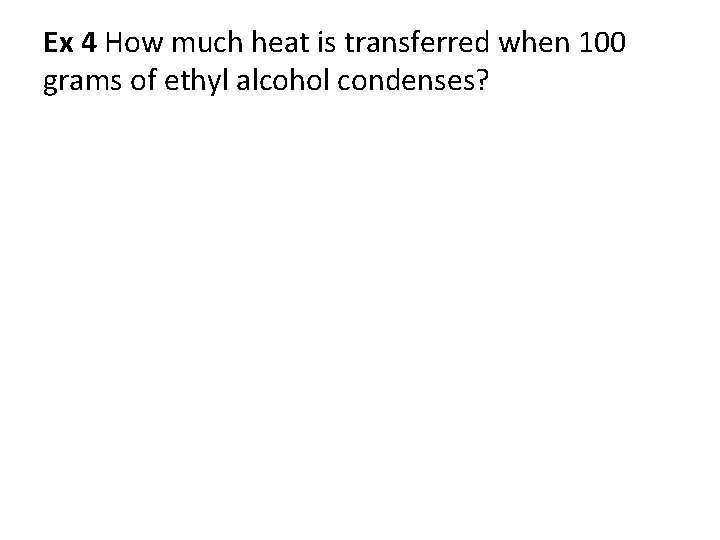












- Slides: 43

To cause the state of matter to change…. Heat/Energy needs to be…. . added or removed

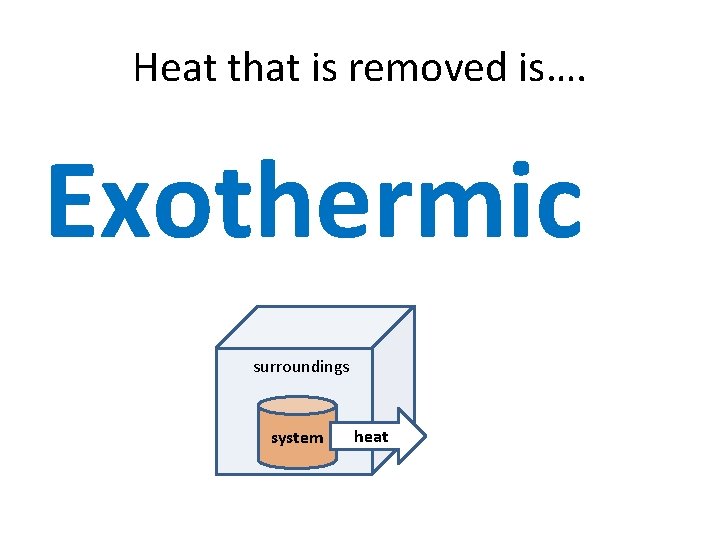
Heat that is removed is…. Exothermic surroundings system heat

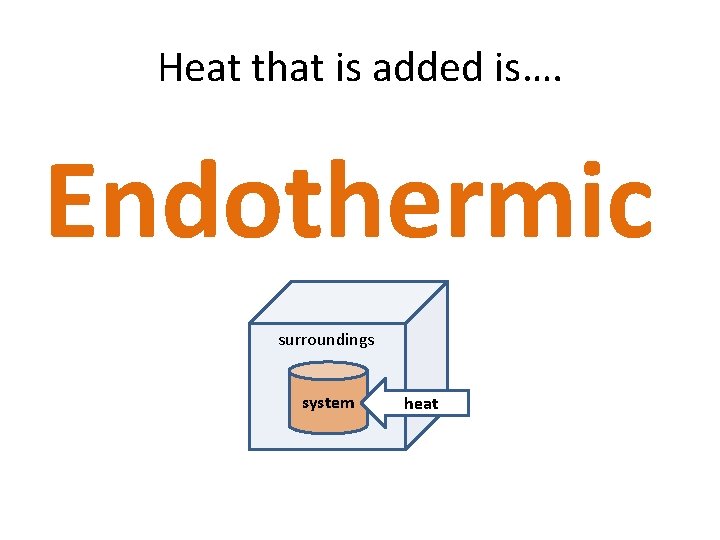
Heat that is added is…. Endothermic surroundings system heat

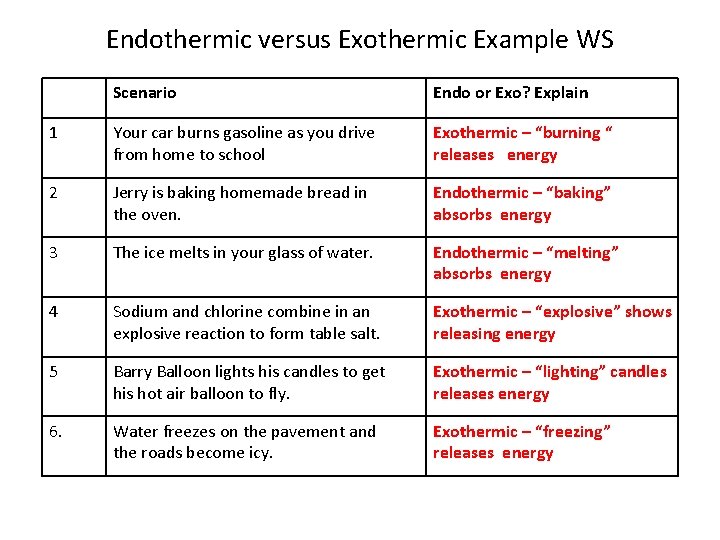
Endothermic versus Exothermic Example WS Scenario Endo or Exo? Explain 1 Your car burns gasoline as you drive from home to school Exothermic – “burning “ releases energy 2 Jerry is baking homemade bread in the oven. Endothermic – “baking” absorbs energy 3 The ice melts in your glass of water. Endothermic – “melting” absorbs energy 4 Sodium and chlorine combine in an explosive reaction to form table salt. Exothermic – “explosive” shows releasing energy 5 Barry Balloon lights his candles to get his hot air balloon to fly. Exothermic – “lighting” candles releases energy 6. Water freezes on the pavement and the roads become icy. Exothermic – “freezing” releases energy

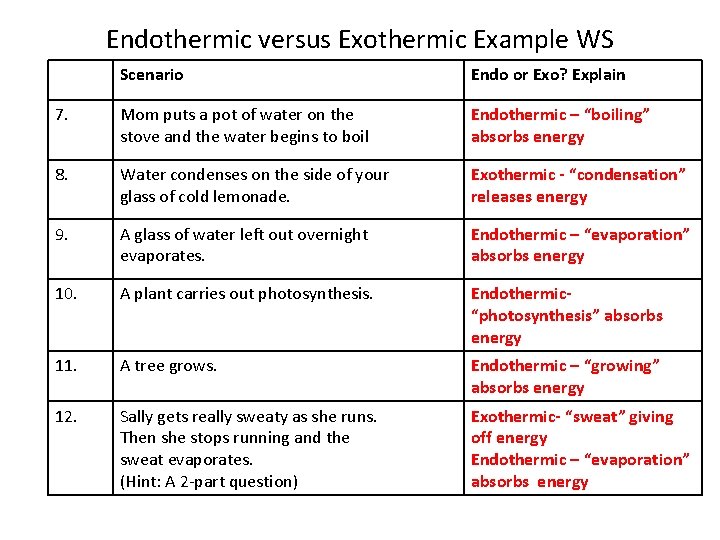
Endothermic versus Exothermic Example WS Scenario Endo or Exo? Explain 7. Mom puts a pot of water on the stove and the water begins to boil Endothermic – “boiling” absorbs energy 8. Water condenses on the side of your glass of cold lemonade. Exothermic - “condensation” releases energy 9. A glass of water left out overnight evaporates. Endothermic – “evaporation” absorbs energy 10. A plant carries out photosynthesis. Endothermic“photosynthesis” absorbs energy 11. A tree grows. Endothermic – “growing” absorbs energy 12. Sally gets really sweaty as she runs. Then she stops running and the sweat evaporates. (Hint: A 2 part question) Exothermic- “sweat” giving off energy Endothermic – “evaporation” absorbs energy

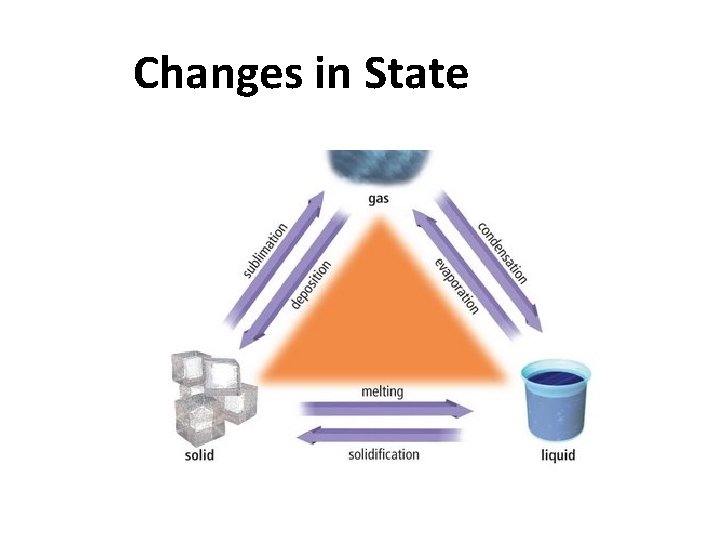
Changes in State

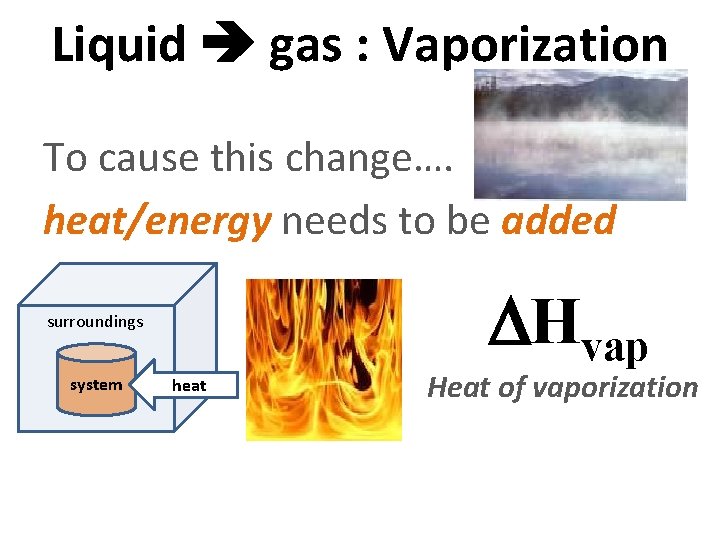
Liquid gas : Vaporization To cause this change…. heat/energy needs to be added surroundings system heat Hvap Heat of vaporization

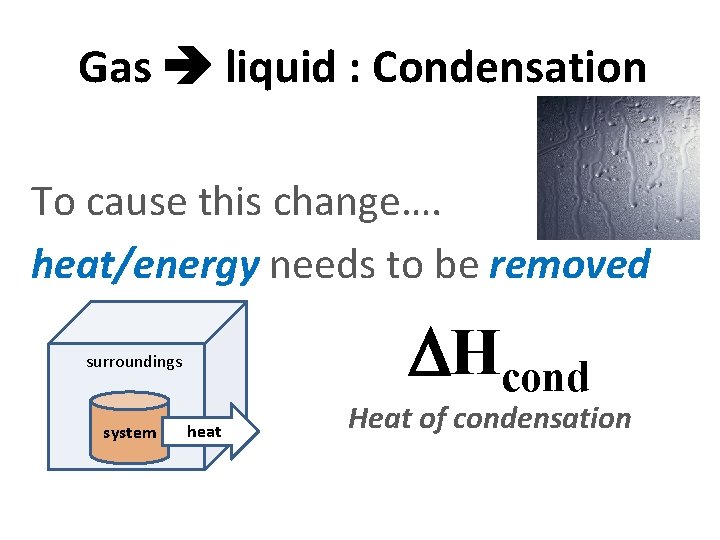
Gas liquid : Condensation To cause this change…. heat/energy needs to be removed surroundings system heat Hcond Heat of condensation

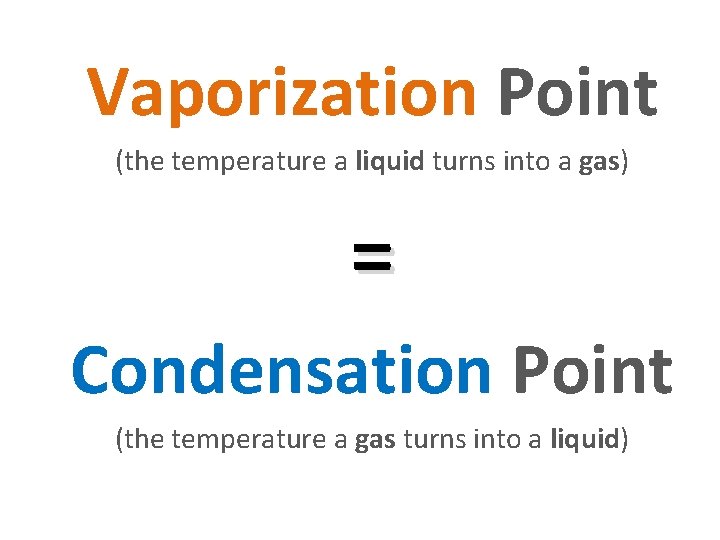
Vaporization Point (the temperature a liquid turns into a gas) = Condensation Point (the temperature a gas turns into a liquid)

Melting/Fusion is. . the vibrations in a solid are strong enough to… overcome attractions that keep solid atoms together

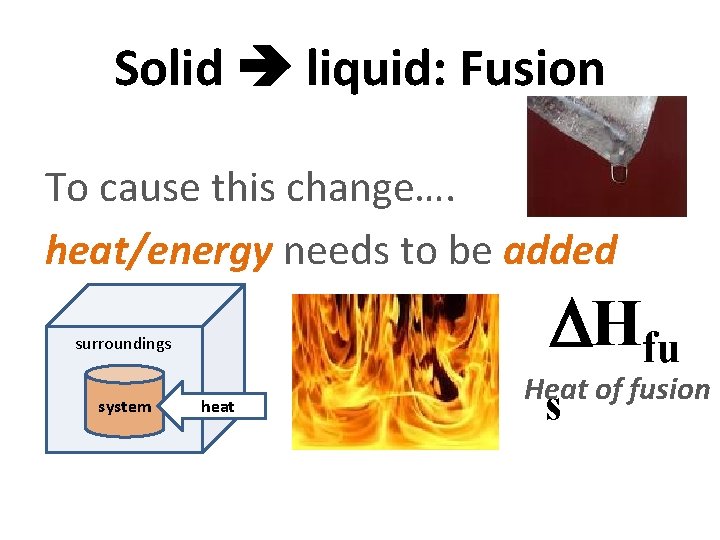
Solid liquid: Fusion To cause this change…. heat/energy needs to be added Hfu surroundings system heat Heat of fusion s

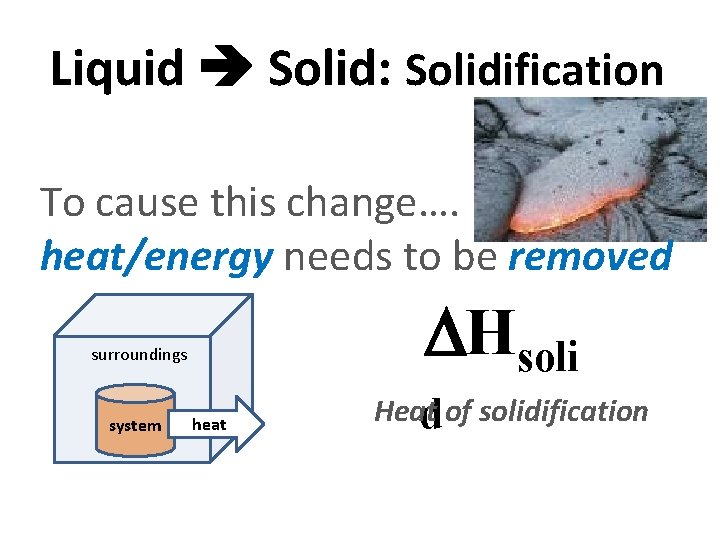
Liquid Solid: Solidification To cause this change…. heat/energy needs to be removed Hsoli surroundings system heat Heat d of solidification

Melting (Fusion) Point (the temperature a solid turns into a liquid) = Solidification Point (the temperature a liquid turns into a solid)

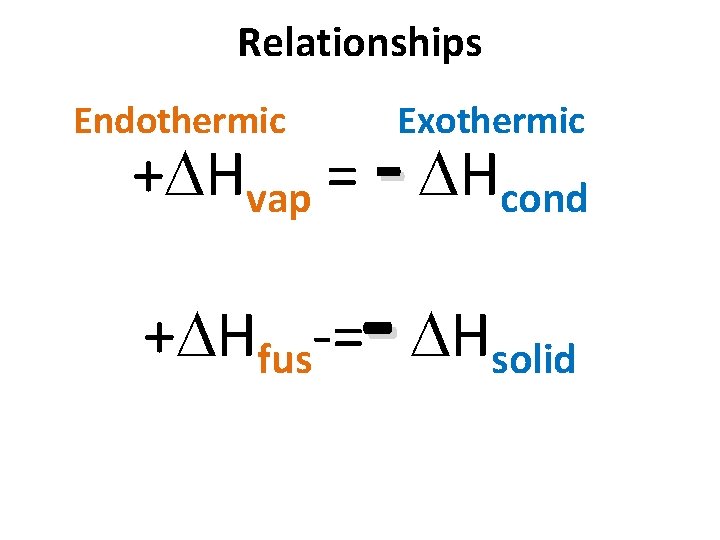
Relationships Endothermic Exothermic + Hvap = - Hcond - + Hfus = Hsolid

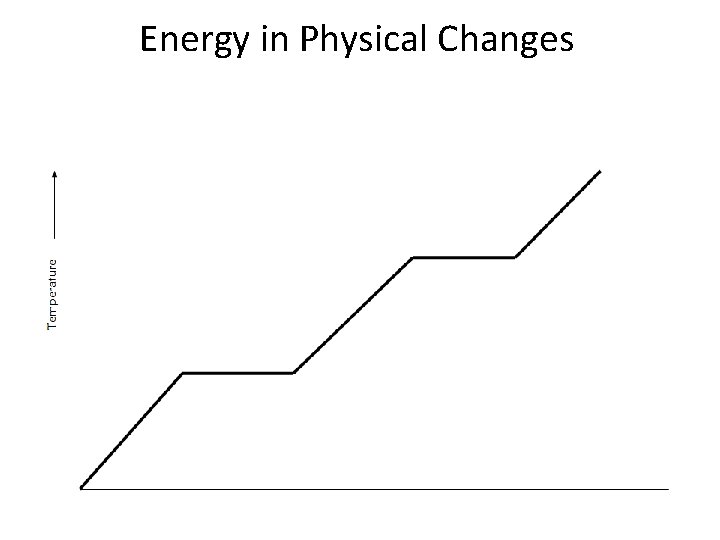
Energy in Physical Changes

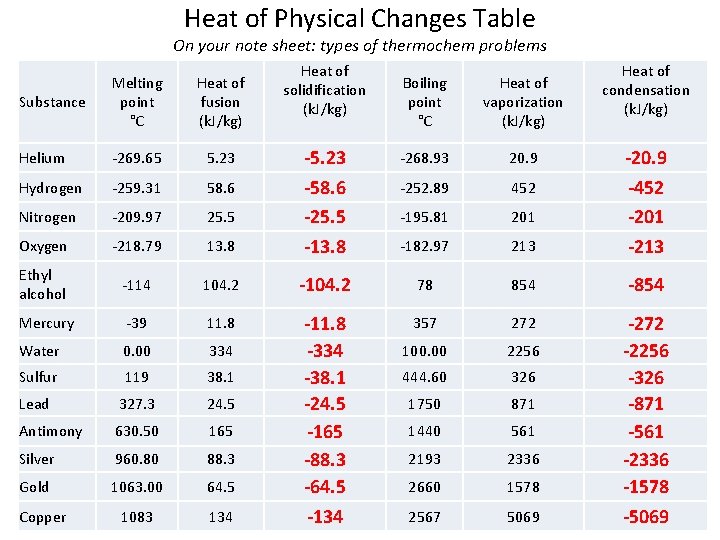
Heat of Physical Changes Table On your note sheet: types of thermochem problems Substance Melting point °C Heat of fusion (k. J/kg) Helium 269. 65 5. 23 Hydrogen 259. 31 58. 6 Nitrogen 209. 97 25. 5 Oxygen 218. 79 Ethyl alcohol Heat of solidification (k. J/kg) Heat of condensation (k. J/kg) Boiling point °C Heat of vaporization (k. J/kg) 268. 93 20. 9 252. 89 452 195. 81 201 13. 8 -5. 23 -58. 6 -25. 5 -13. 8 182. 97 213 -20. 9 -452 -201 -213 114 104. 2 -104. 2 78 854 -854 Mercury 39 11. 8 357 272 Water 0. 00 334 100. 00 2256 Sulfur 119 38. 1 444. 60 326 Lead 327. 3 24. 5 1750 871 Antimony 630. 50 165 1440 561 Silver 960. 80 88. 3 2193 2336 Gold 1063. 00 64. 5 2660 1578 1083 134 -11. 8 -334 -38. 1 -24. 5 -165 -88. 3 -64. 5 -134 2567 5069 -272 -2256 -326 -871 -561 -2336 -1578 -5069 Copper

When doing word problems…. 1. Find the question word: determine what you are looking for. WANT 2. What #s did the problem give you GIVEN 3. If only one # always start grid with that # 4. If Multiple #’s you NEED a formula
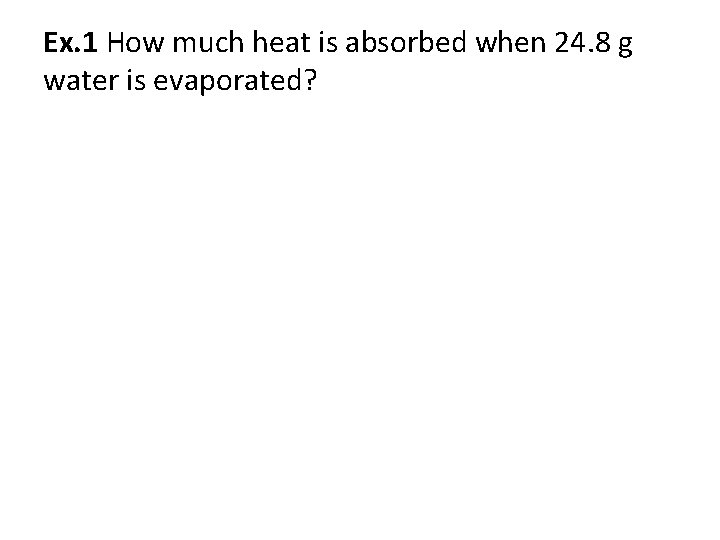
Ex. 1 How much heat is absorbed when 24. 8 g water is evaporated?


Ex 2 How much heat is transferred when 400 grams of mercury (Hg) is vaporized?

Ex 3 If 300 k. J of heat is available, how much copper can be melted?

Ex 4 How much heat is transferred when 100 grams of ethyl alcohol condenses?


Sublimation SOLID skips liquid stage goes straight into GAS stage

Demo 1: Sublimation

You forgot your glass of water outside. The next time you are outside, you realize your glass is empty. What happened?

Evaporation Vs Boiling • Both are Vaporization • Both allow liquid turn into a gas BUT…. • Evaporation is NOT Boiling

Evaporation In an open container Δ occurs @ the surface

Evaporation It’s a cooling process

Evaporation Explain how the following description is an analogy for evaporative cooling: If the fastest runner is removed from a race, the resulting average speed of the runners that remain will be lower.

Boiling Liquid has enough HEAT/ENERGY to overcome the External Pressure Vapor Pressure = External Pressure

to make something boil Energy/Heat is added Or the EXTERNAL pressure is changed EXTERNAL

Boiling

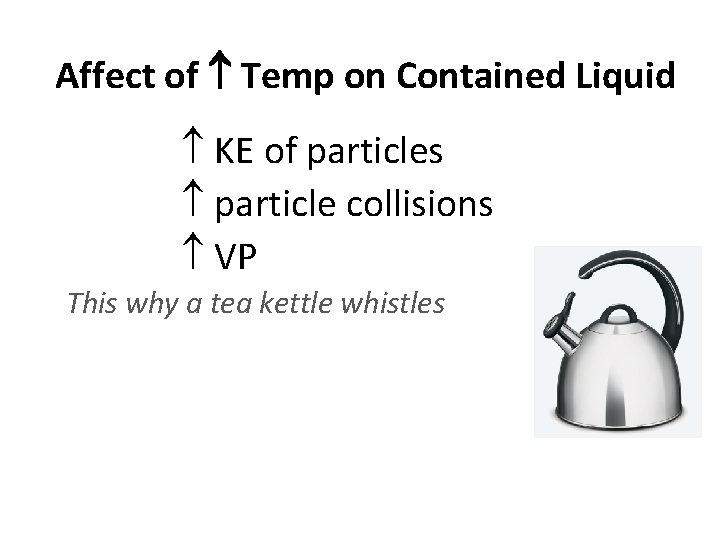
Affect of Temp on Contained Liquid KE of particles particle collisions VP This why a tea kettle whistles

Vacuum no gas particles = no collisions = NO PRESSURE

Demo 2: Boiling

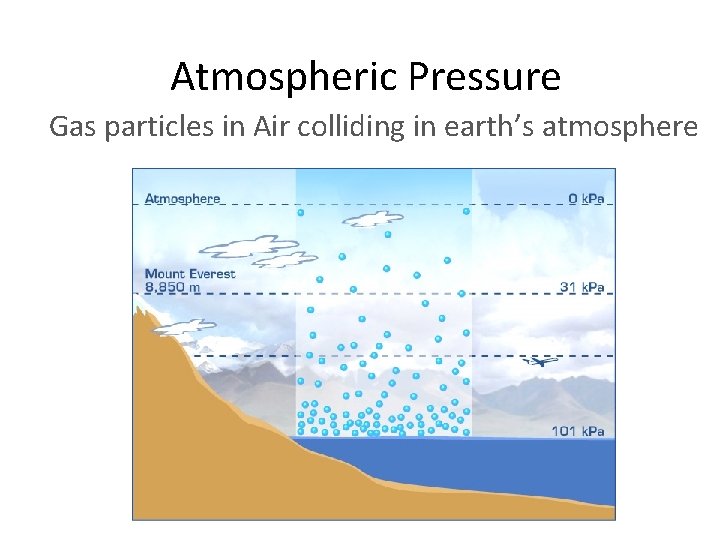
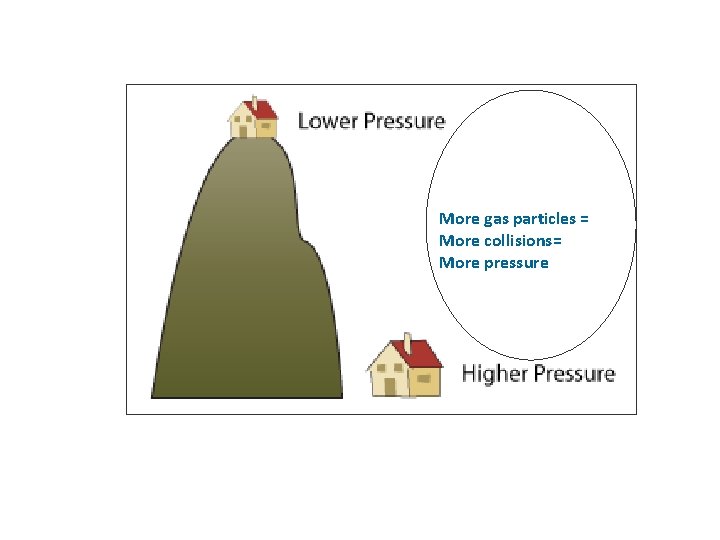
Atmospheric Pressure Gas particles in Air colliding in earth’s atmosphere

More gas particles = More collisions= More pressure

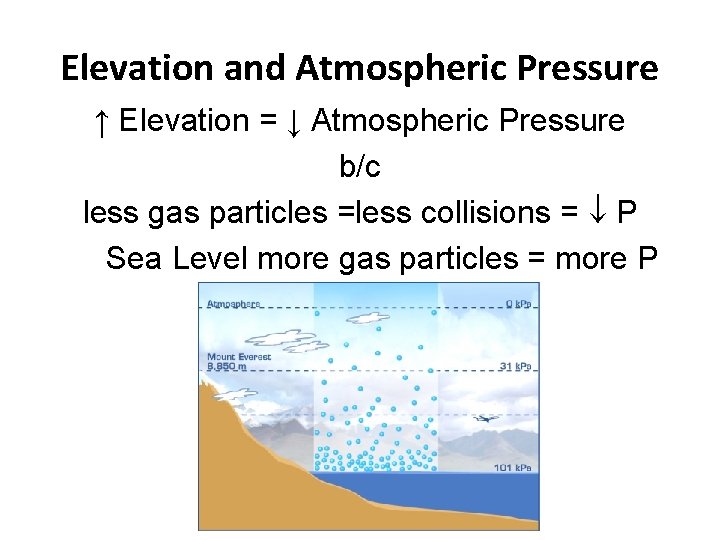
Elevation and Atmospheric Pressure ↑ Elevation = ↓ Atmospheric Pressure b/c less gas particles =less collisions = P Sea Level more gas particles = more P

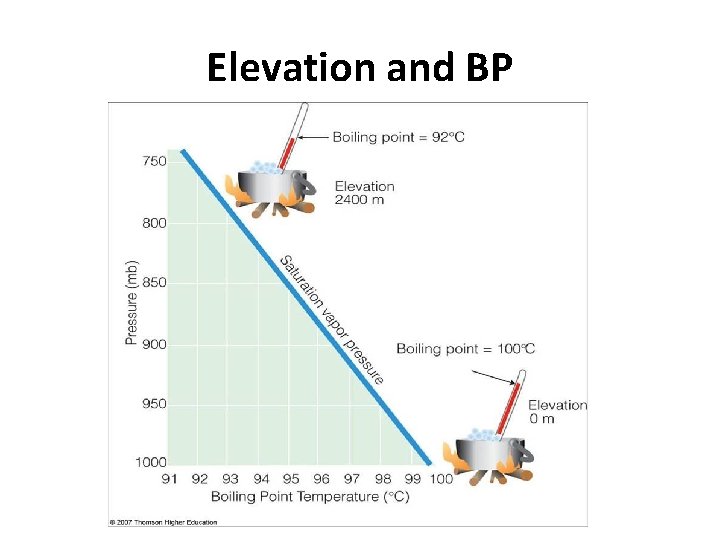
Elevation and BP

Pressure Cooker Creates a High External Pressure a bubble of vapor can’t form unless KE= T BP is = hotter liquid= shorter cooking time
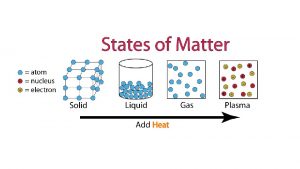
 Change of state of matter
Change of state of matter Underlying cause and immediate cause
Underlying cause and immediate cause Ultimate cause of behavior
Ultimate cause of behavior Proximate and ultimate causes of behaviour
Proximate and ultimate causes of behaviour Imprinting examples in animals
Imprinting examples in animals Section 1 composition of matter
Section 1 composition of matter Gray matter vs white matter
Gray matter vs white matter Composition of matter section 1
Composition of matter section 1 Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Primary taste cortex
Primary taste cortex Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Gray matter
Gray matter Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Energy is the ability to cause change
Energy is the ability to cause change The ability to do work
The ability to do work The ability to do work or cause a change.
The ability to do work or cause a change. The ability to do work or cause change
The ability to do work or cause change Temperature may cause leaves to change color hypothesis
Temperature may cause leaves to change color hypothesis Energy is the ability to cause change
Energy is the ability to cause change Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Chapter 1 review matter and change
Chapter 1 review matter and change Two types of changes
Two types of changes Changes in matter grade 5
Changes in matter grade 5 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change answer key chapter 2
Chemistry matter and change answer key chapter 2 Unit 2 matter and change
Unit 2 matter and change Circle the solute and underline the solvent
Circle the solute and underline the solvent The state or quality of being dedicated to a cause
The state or quality of being dedicated to a cause Plasma and bose-einstein condensate
Plasma and bose-einstein condensate Do
Do Thermal energy states of matter
Thermal energy states of matter Sixth state of matter
Sixth state of matter øis
øis Pepsi particles candy
Pepsi particles candy Physical state of matter
Physical state of matter Whats the fourth state of matter
Whats the fourth state of matter Solid particles diagram
Solid particles diagram Collidal state
Collidal state Particle theory of matter examples
Particle theory of matter examples Crystalline substances
Crystalline substances