SOLID STATE CHEMISTRY contents Introduction Types of solids














- Slides: 65

SOLID STATE CHEMISTRY

contents • • • Introduction Types of solids Crystal Structures Elements of Symmetry Bragg’s equation Allotropes of carbon: Diamond, graphite & Fullerene

INTRODUCTION Three phases of matter: ü Gas ü Liquid ü Solid

Gas molecules 4

Liquid molecules 5

Solid molecules 6

What is solid? • • • Definite shape. Definite volume. Highly incompressible. Rigid. Constituent particles held closely by strong intermolecular forces. • Fixed position of constituents.

TYPES OF SOLIDS Two types (based upon atomic arrangement, binding energy, physical & chemical properties): 1. Crystalline 2. Amorphous

Crystalline solids • The building constituents arrange themselves in regular manner throughout the entire three dimensional network. • Existence of crystalline lattice. • A crystalline lattice is a solid figure which has a definite geometrical shape, with flat faces and sharp edges. • Incompressible orderly arranged units. • Definite sharp melting point. • Anisotropy. • Definite geometry. • Give x-ray diffraction bands. • Examples: Na. Cl, Cs. Cl, etc.

AMORPHOUS SOLIDS • Derived from Greek word ‘Omorphe’ meaning shapeless. • No regular but haphazard arrangement of atoms or molecules. • Also considered as non-crystalline solids or supercooled liquids. • No sharp m. p. • Isotropic. • No definite geometrical shape. • Do not give x-ray diffraction bands. • Examples: glass, rubber, plastics.

Types of crystal structures • • Ionic crystals Covalent crystals Molecular crystals Metallic crystals

Ionic crystals • Lattice points are occupied by positive and negative ions. • Hard and brittle solids. • High m. p. due to very strong electrostatic forces of attraction. • Poor conductors of electricity in solid state but good in molten state. • Packing of spheres depends upon: ü presence of charged species present. ü difference in the size of anions and cations. • Two types: ü AB types. ü AB 2 types.

Covalent crystals • • • Lattice points are occupied by neutral atoms. Atoms are held together by covalent bonds Hard solids. High m. p. Poor conductors of electricity. Two common examples: diamond & graphite.

Molecular crystals • Lattice points are occupied by neutral molecules. • The molecules are held together by vander Waal’s forces. • Very soft solids. • Low m. p. • Poor conductors of electricity.

Metallic crystals • Lattice points are occupied by positive metal ions surrounded by a sea of mobile e-. • Soft to very hard. • Metals have high tensile strength. • Good conductors of electricity. • Malleable and ductile. • Bonding electrons in metals remain delocalized over the entire crystal. • High density.

Laws of symmetry • Plane of symmetry • Centre of symmetry • Axis of symmetry.

Elements of symmetry in cubic crystal • • • Rectangular planes of symmetry: 3 Diagonal planes of symmetry: 6 Axes of four-fold symmetry: 3 Axes of three-fold symmetry: 4 Axes of two-fold symmetry: 6 Centre of symmetry: 1 Total symmetry elements: 23

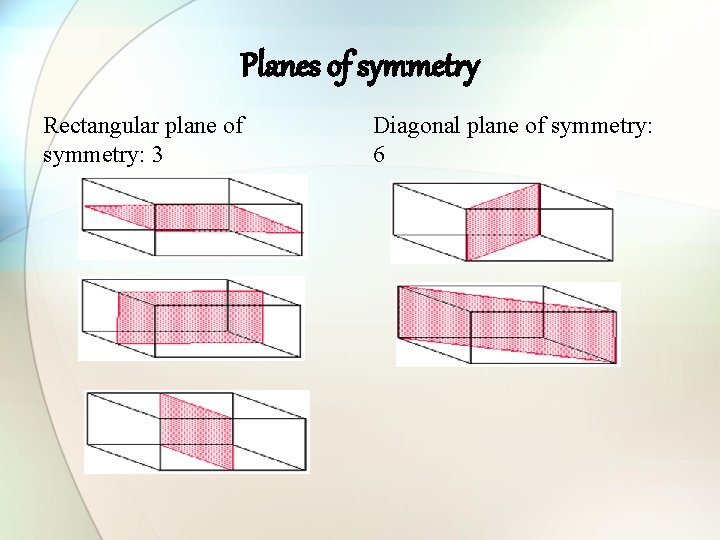
Planes of symmetry Rectangular plane of symmetry: 3 Diagonal plane of symmetry: 6

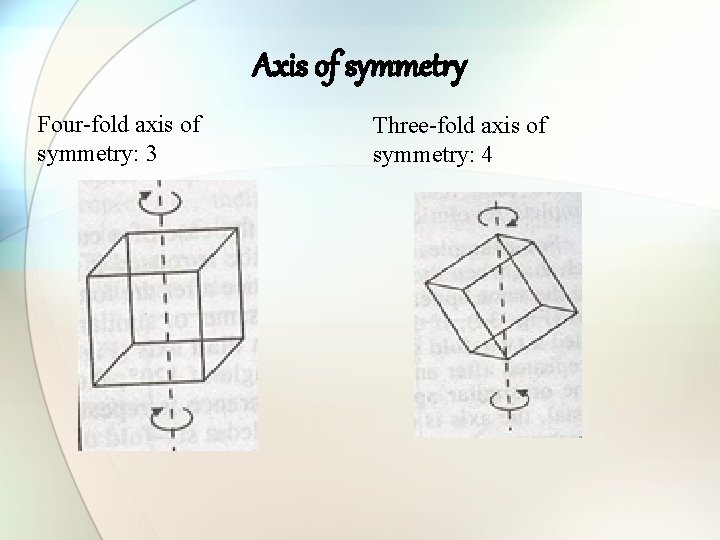
Axis of symmetry Four-fold axis of symmetry: 3 Three-fold axis of symmetry: 4

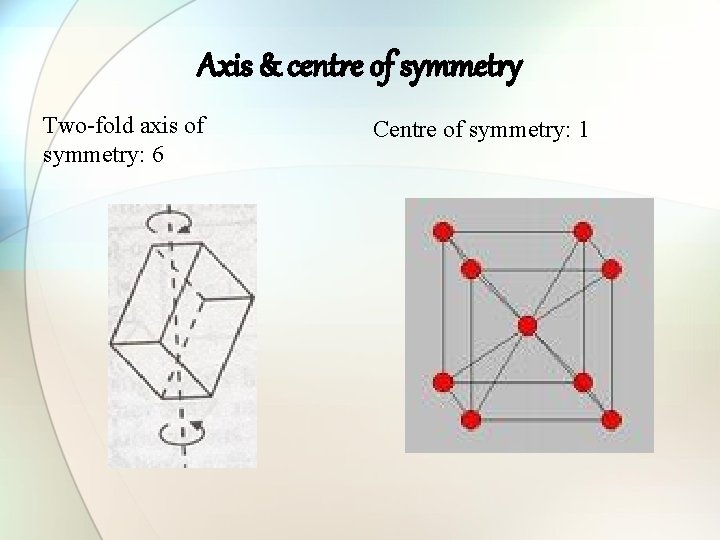
Axis & centre of symmetry Two-fold axis of symmetry: 6 Centre of symmetry: 1

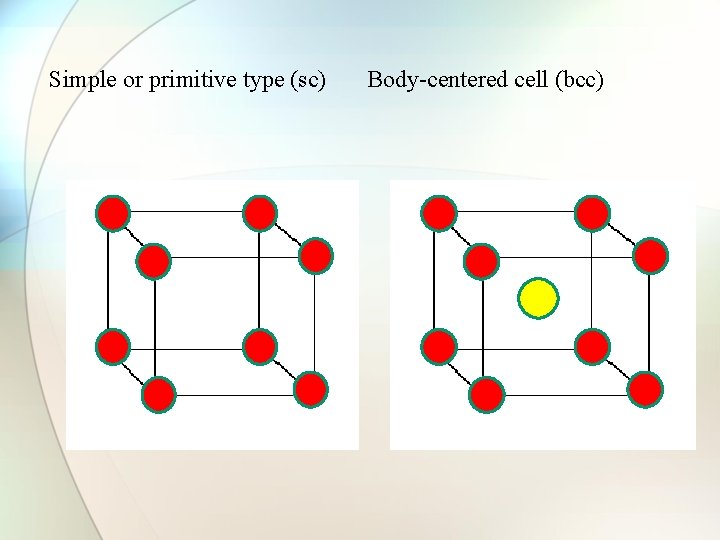
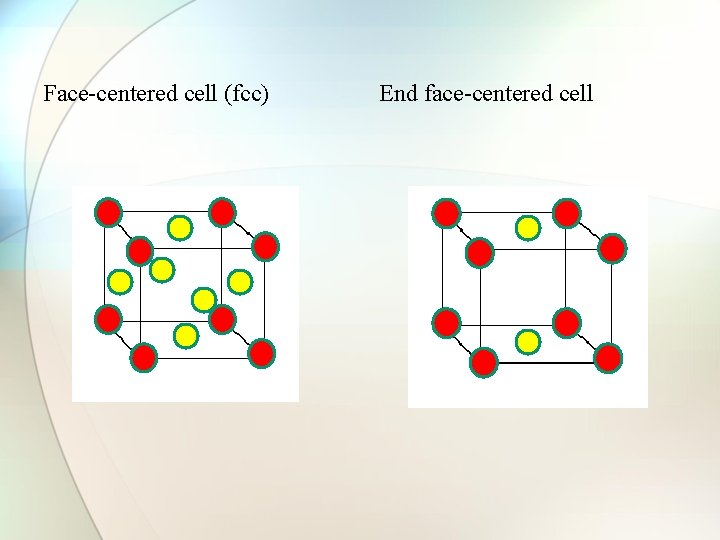
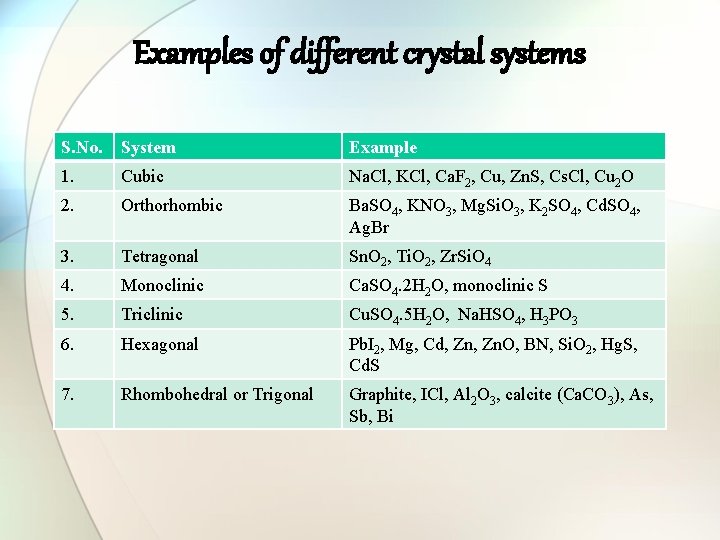
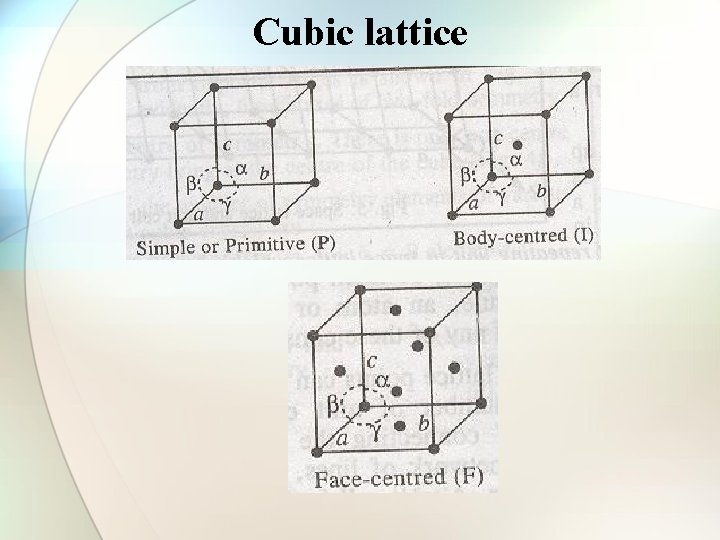
Types of cubic crystals Four types: 1. Simple or primitive type 2. Body-centered 3. Face-centered 4. End face-centered

Simple or primitive type (sc) Body-centered cell (bcc)

Face-centered cell (fcc) End face-centered cell

Number of atoms per unit cell in a cubic lattice • • Simple cubic cell: 1 atom/unit cell of sc Body-centered cell: 2 atoms/unit cell of bcc Face-centered cell: 4 atoms/unit cell of fcc End face-centered cell: 2 atoms/unit cell

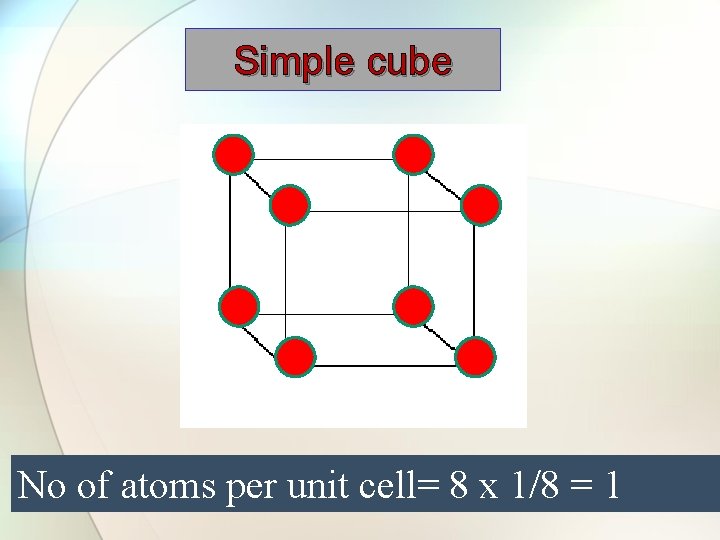
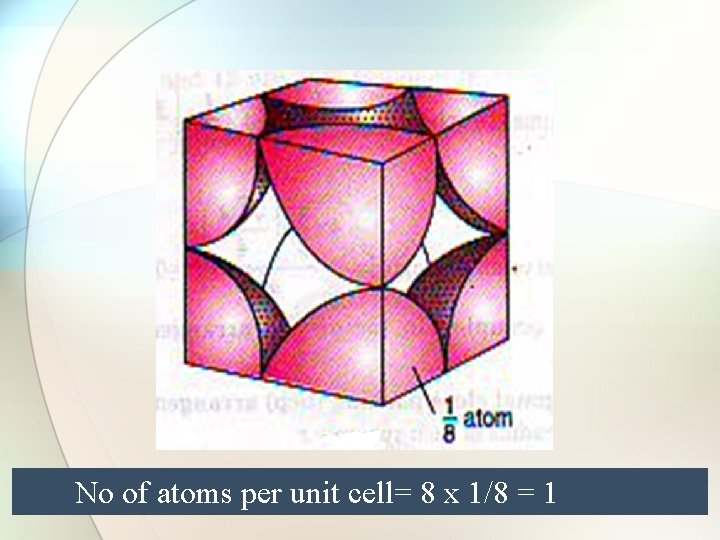
Simple cube No of atoms per unit cell= 8 x 1/8 = 1

No of atoms per unit cell= 8 x 1/8 = 1

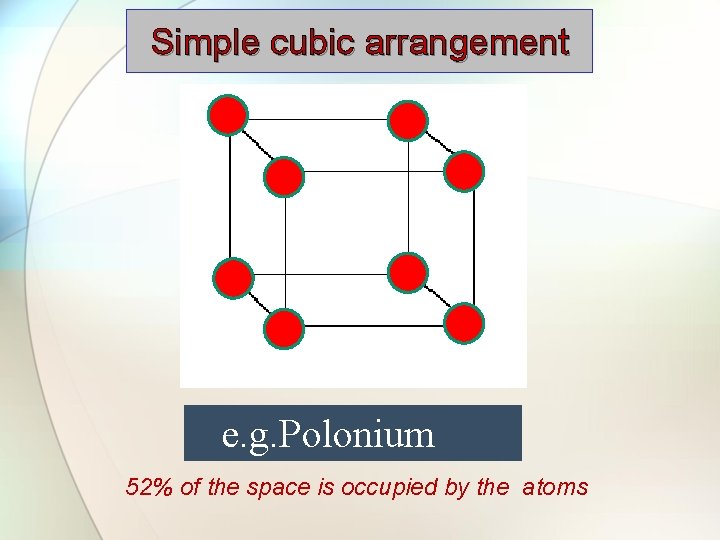
Simple cubic arrangement e. g. Polonium 52% of the space is occupied by the atoms

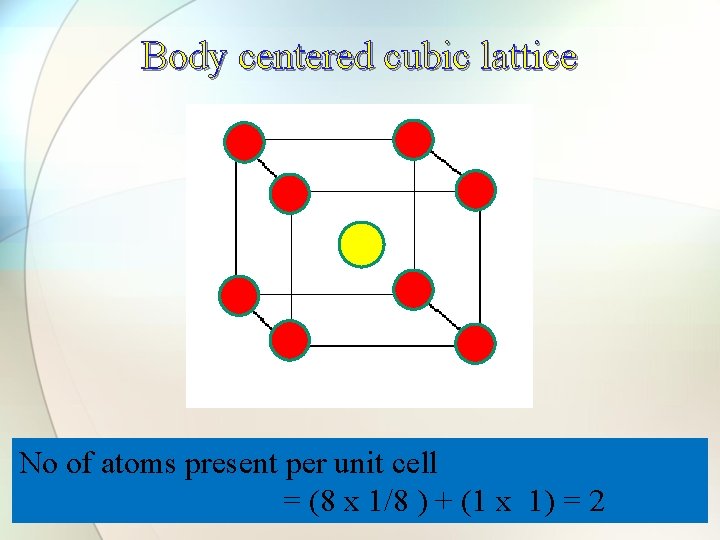
Body centered cubic lattice No of atoms present per unit cell = (8 x 1/8 ) + (1 x 1) = 2

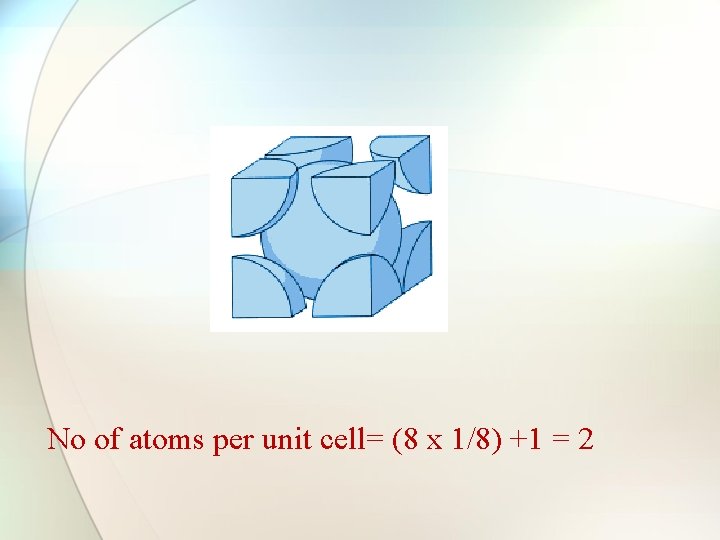
No of atoms per unit cell= (8 x 1/8) +1 = 2

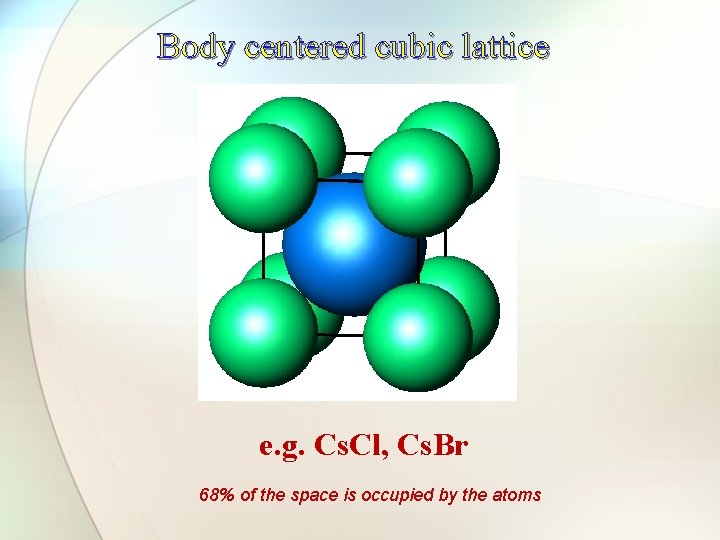
Body centered cubic lattice e. g. Cs. Cl, Cs. Br 68% of the space is occupied by the atoms

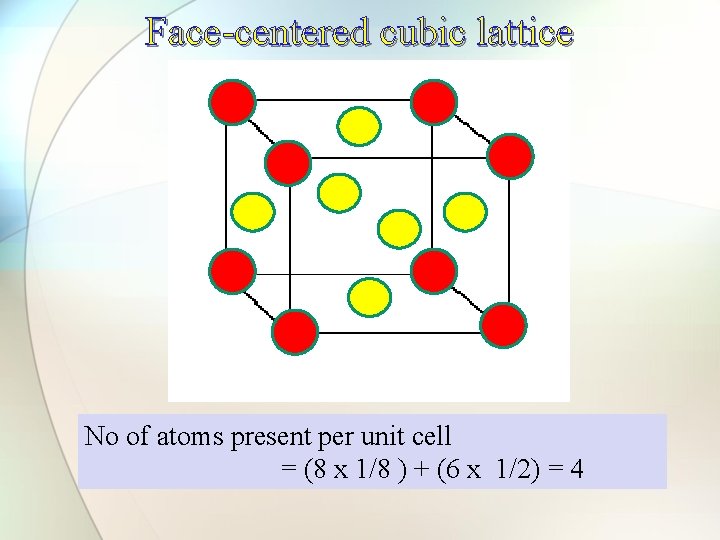
Face-centered cubic lattice No of atoms present per unit cell = (8 x 1/8 ) + (6 x 1/2) = 4

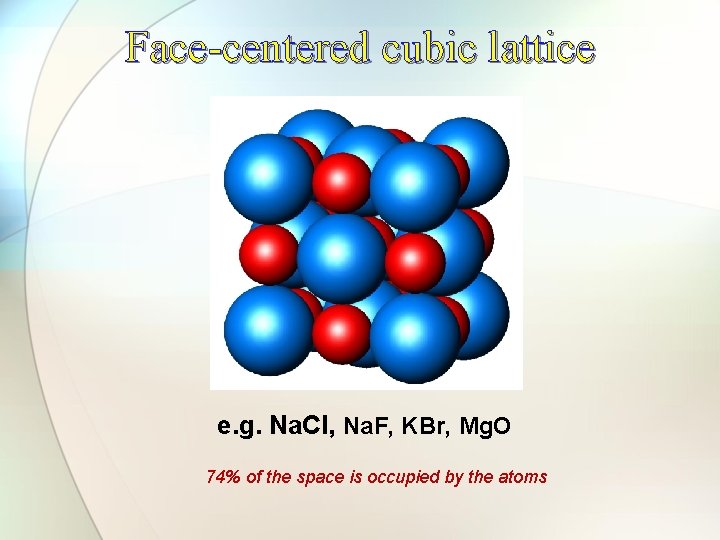
Face-centered cubic lattice e. g. Na. Cl, Na. F, KBr, Mg. O 74% of the space is occupied by the atoms

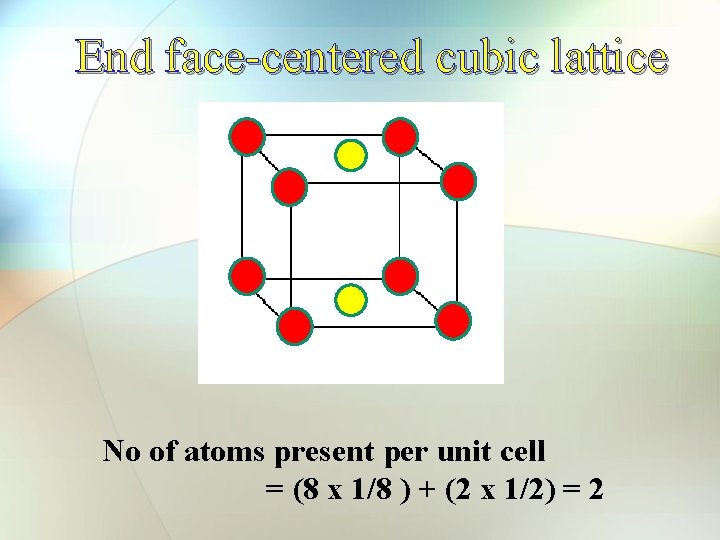
End face-centered cubic lattice No of atoms present per unit cell = (8 x 1/8 ) + (2 x 1/2) = 2

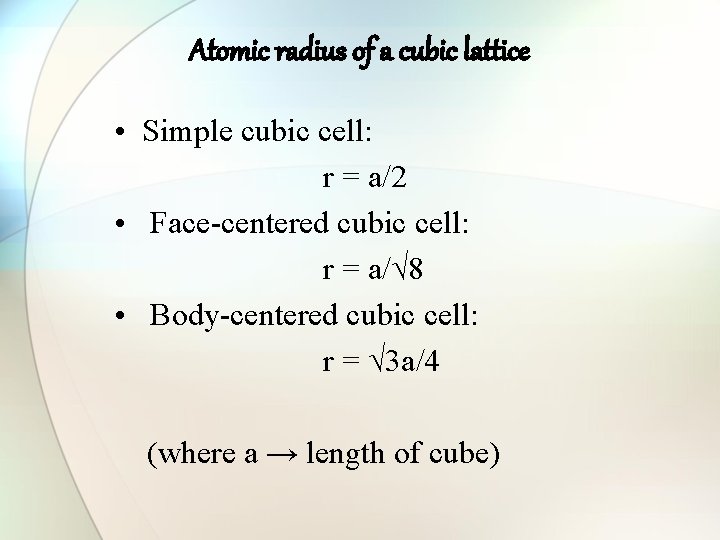
Atomic radius of a cubic lattice • Simple cubic cell: r = a/2 • Face-centered cubic cell: r = a/√ 8 • Body-centered cubic cell: r = √ 3 a/4 (where a → length of cube)

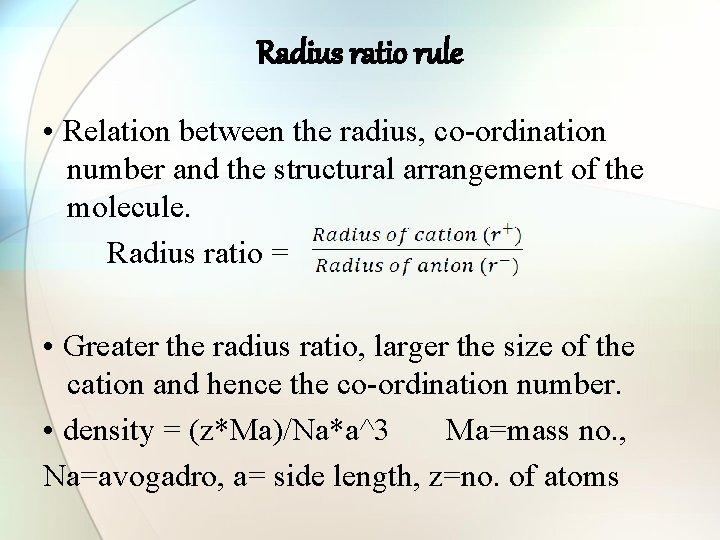
Radius ratio rule • Relation between the radius, co-ordination number and the structural arrangement of the molecule. Radius ratio = • Greater the radius ratio, larger the size of the cation and hence the co-ordination number. • density = (z*Ma)/Na*a^3 Ma=mass no. , Na=avogadro, a= side length, z=no. of atoms

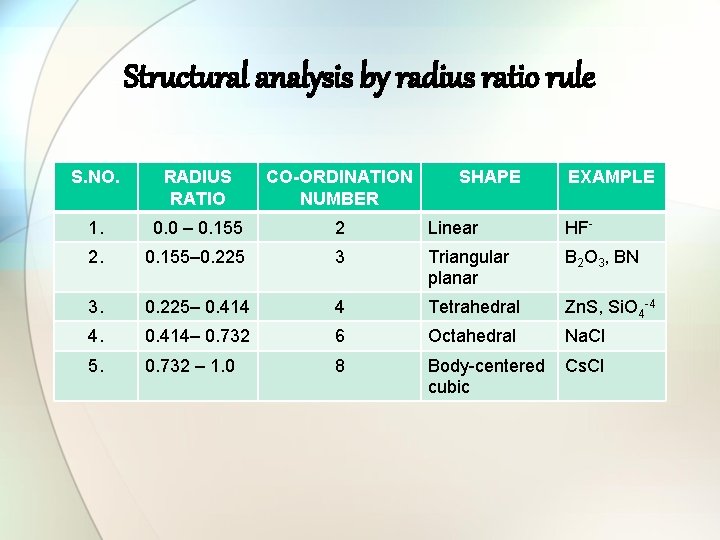
Structural analysis by radius ratio rule S. NO. RADIUS RATIO CO-ORDINATION NUMBER SHAPE EXAMPLE 1. 0. 0 – 0. 155 2 Linear HF- 2. 0. 155– 0. 225 3 Triangular planar B 2 O 3, BN 3. 0. 225– 0. 414 4 Tetrahedral Zn. S, Si. O 4 -4 4. 0. 414– 0. 732 6 Octahedral Na. Cl 5. 0. 732 – 1. 0 8 Body-centered cubic Cs. Cl

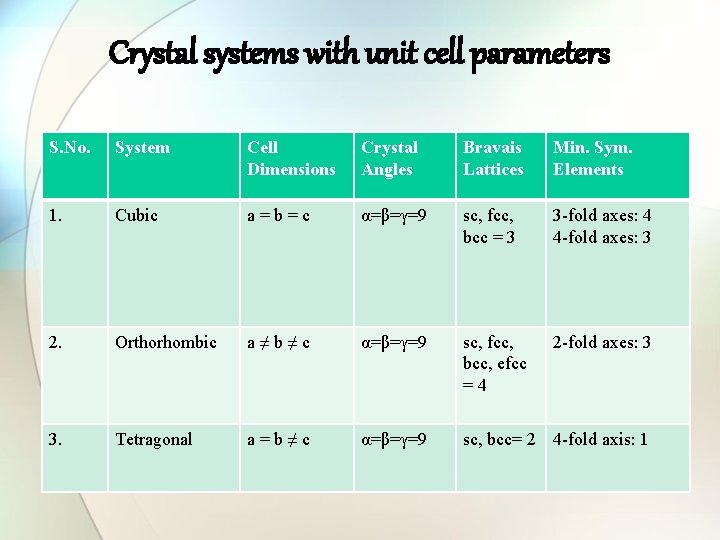
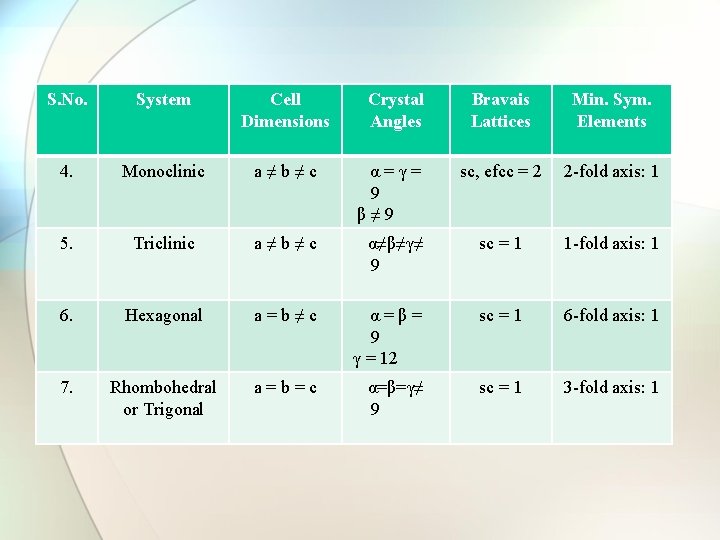
BRAVAIS LATTICES • Unit cell parameters: ü Lengths a, b & c. ü Angles α, β & γ. • Total crystal lattices: 7 • Total Bravais lattices: 14

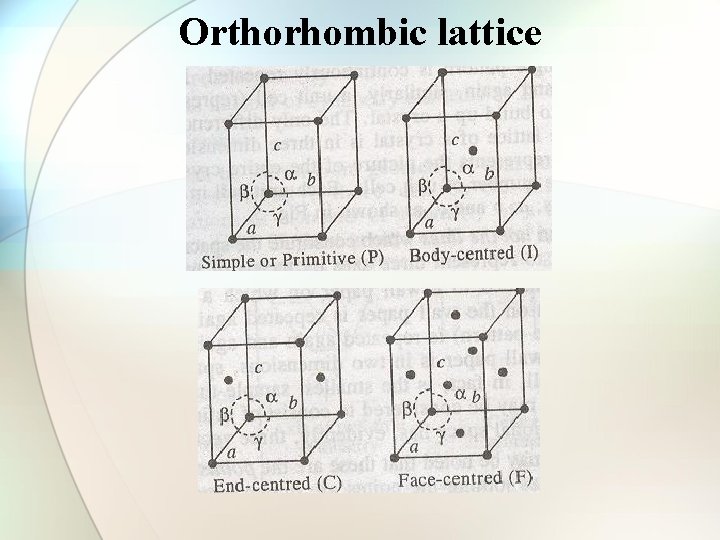
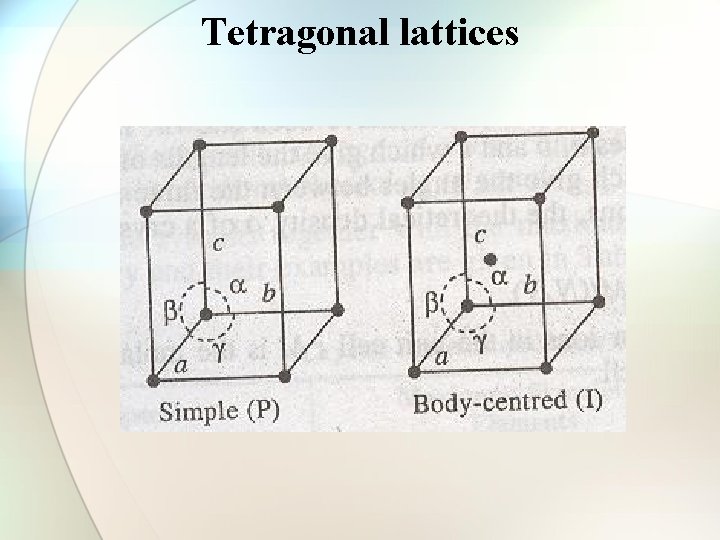
Crystal systems with unit cell parameters S. No. System Cell Dimensions Crystal Angles Bravais Lattices Min. Sym. Elements 1. Cubic a=b=c α=β=γ=9 sc, fcc, bcc = 3 3 -fold axes: 4 4 -fold axes: 3 2. Orthorhombic a≠b≠c α=β=γ=9 sc, fcc, bcc, efcc =4 2 -fold axes: 3 3. Tetragonal a=b≠c α=β=γ=9 sc, bcc= 2 4 -fold axis: 1

S. No. System Cell Dimensions Crystal Angles Bravais Lattices Min. Sym. Elements 4. Monoclinic a≠b≠c α=γ= 9 β≠ 9 sc, efcc = 2 2 -fold axis: 1 5. Triclinic a≠b≠c α≠β≠γ≠ 9 sc = 1 1 -fold axis: 1 6. Hexagonal a=b≠c α=β= 9 γ = 12 sc = 1 6 -fold axis: 1 7. Rhombohedral or Trigonal a=b=c α=β=γ≠ 9 sc = 1 3 -fold axis: 1

Examples of different crystal systems S. No. System Example 1. Cubic Na. Cl, KCl, Ca. F 2, Cu, Zn. S, Cs. Cl, Cu 2 O 2. Orthorhombic Ba. SO 4, KNO 3, Mg. Si. O 3, K 2 SO 4, Cd. SO 4, Ag. Br 3. Tetragonal Sn. O 2, Ti. O 2, Zr. Si. O 4 4. Monoclinic Ca. SO 4. 2 H 2 O, monoclinic S 5. Triclinic Cu. SO 4. 5 H 2 O, Na. HSO 4, H 3 PO 3 6. Hexagonal Pb. I 2, Mg, Cd, Zn. O, BN, Si. O 2, Hg. S, Cd. S 7. Rhombohedral or Trigonal Graphite, ICl, Al 2 O 3, calcite (Ca. CO 3), As, Sb, Bi

Cubic lattice

Orthorhombic lattice

Tetragonal lattices

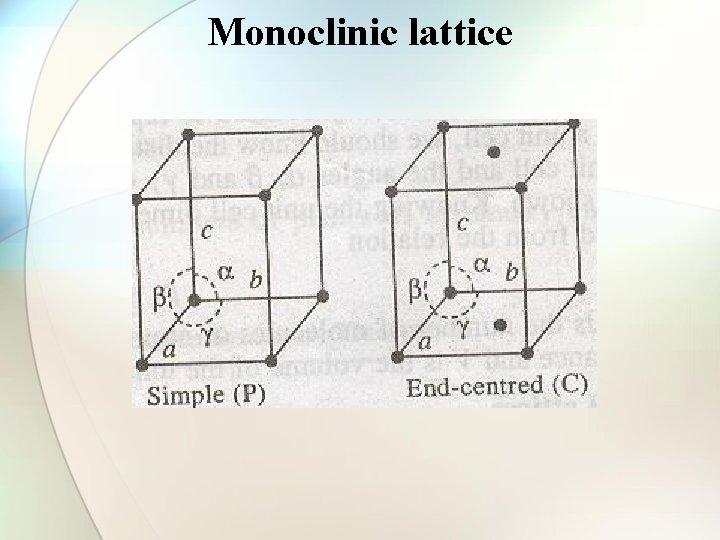
Monoclinic lattice

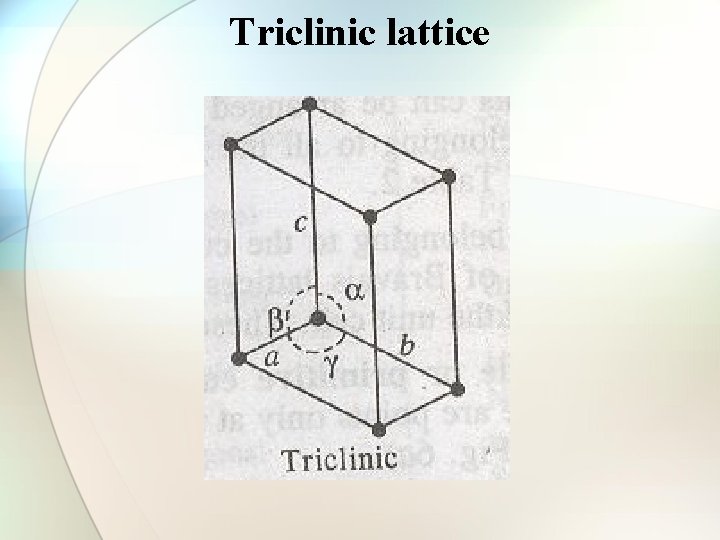
Triclinic lattice

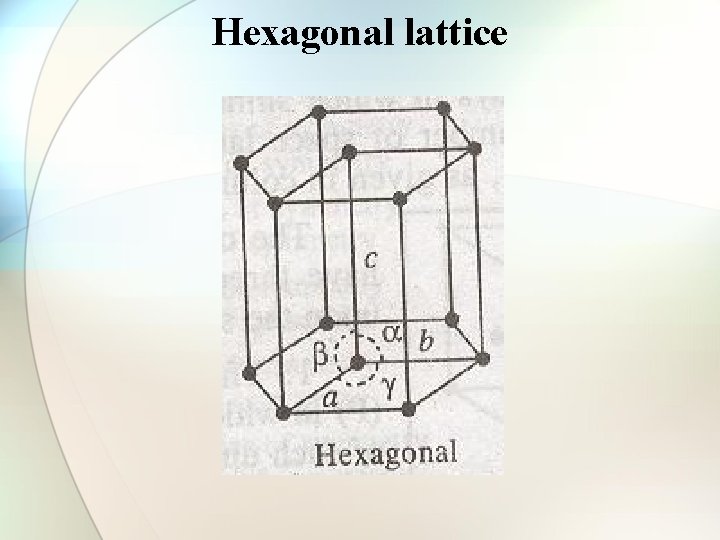
Hexagonal lattice

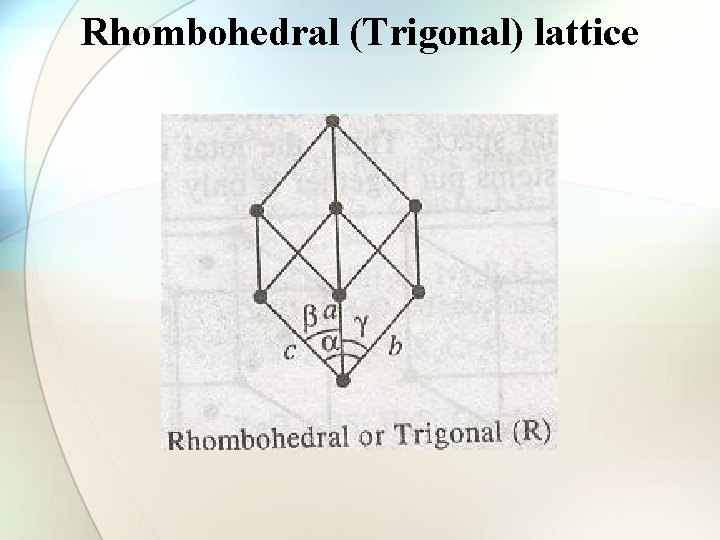
Rhombohedral (Trigonal) lattice

Structures of important ionic compounds 1. AB type: Na. Cl (rock salt) Cs. Cl Zn. S (zinc blende / sphalerite) 2. AB 2 type: Ca. F 2 (fluorite) Ti. O 2 (rutile) Si. O 2 3. A 2 B type: K 2 O (antifluorite)

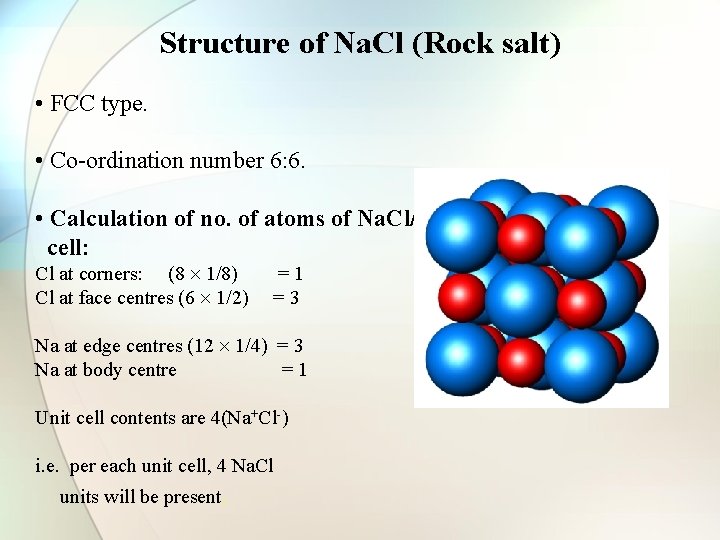
Structure of Na. Cl (Rock salt) • FCC type. • Co-ordination number 6: 6. • Calculation of no. of atoms of Na. Cl/unit cell: Cl at corners: (8 1/8) Cl at face centres (6 1/2) =1 =3 Na at edge centres (12 1/4) = 3 Na at body centre =1 Unit cell contents are 4(Na+Cl-) i. e. per each unit cell, 4 Na. Cl units will be present.

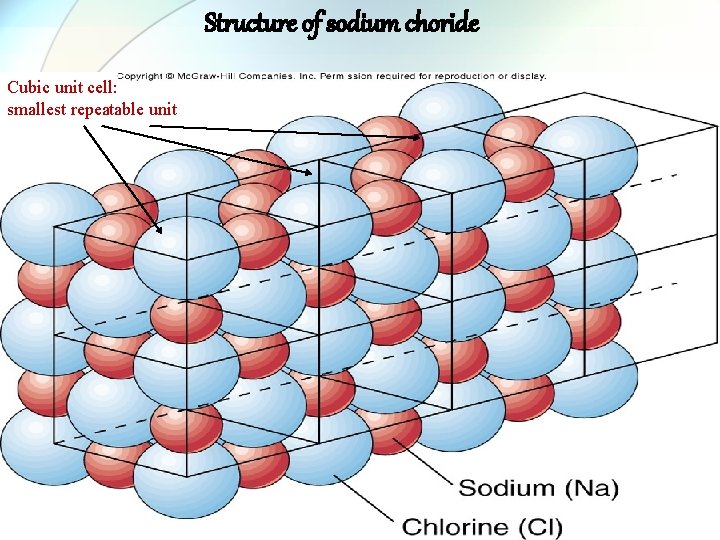
Structure of sodium choride Cubic unit cell: smallest repeatable unit

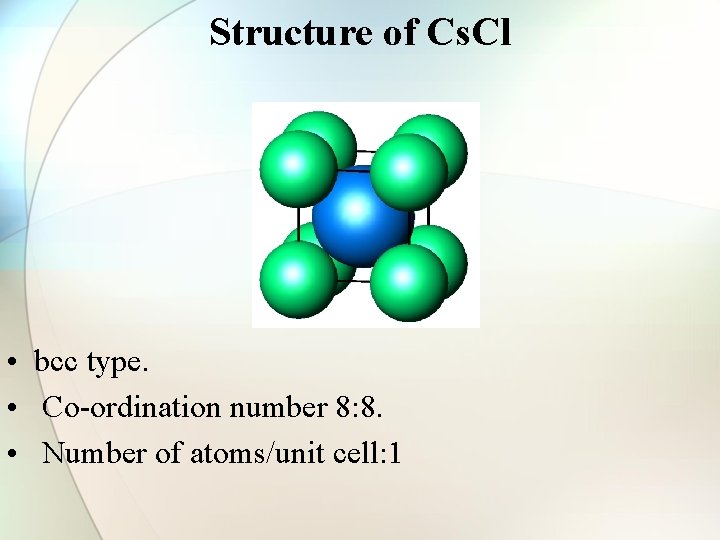
Structure of Cs. Cl • bcc type. • Co-ordination number 8: 8. • Number of atoms/unit cell: 1

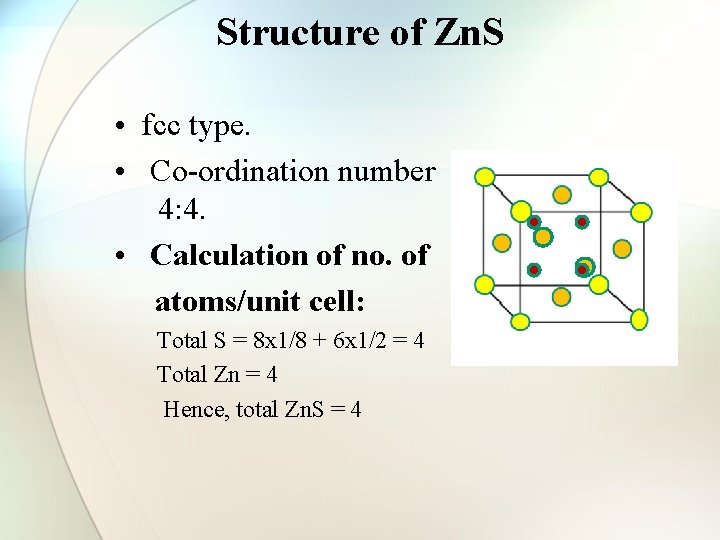
Structure of Zn. S • fcc type. • Co-ordination number 4: 4. • Calculation of no. of atoms/unit cell: Total S = 8 x 1/8 + 6 x 1/2 = 4 Total Zn = 4 Hence, total Zn. S = 4

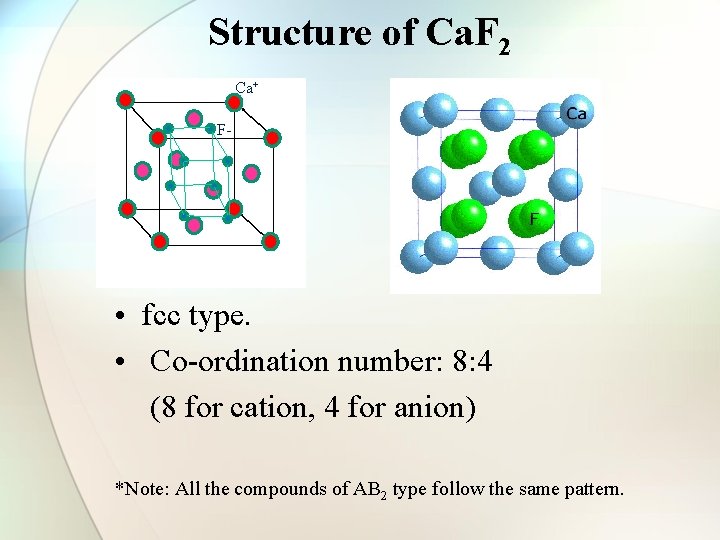
Structure of Ca. F 2 Ca+ F- • fcc type. • Co-ordination number: 8: 4 (8 for cation, 4 for anion) *Note: All the compounds of AB 2 type follow the same pattern.

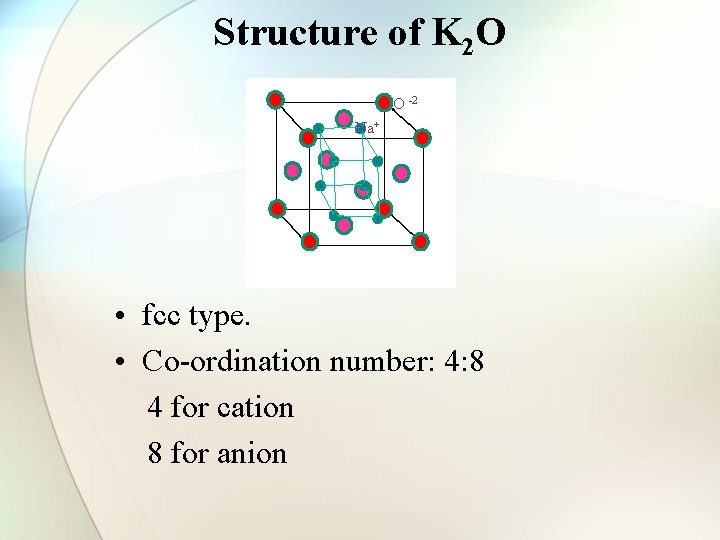
Structure of K 2 O O -2 Na+ • fcc type. • Co-ordination number: 4: 8 4 for cation 8 for anion

Structure of important covalent compounds 1. Diamond 2. Graphite

Diamond

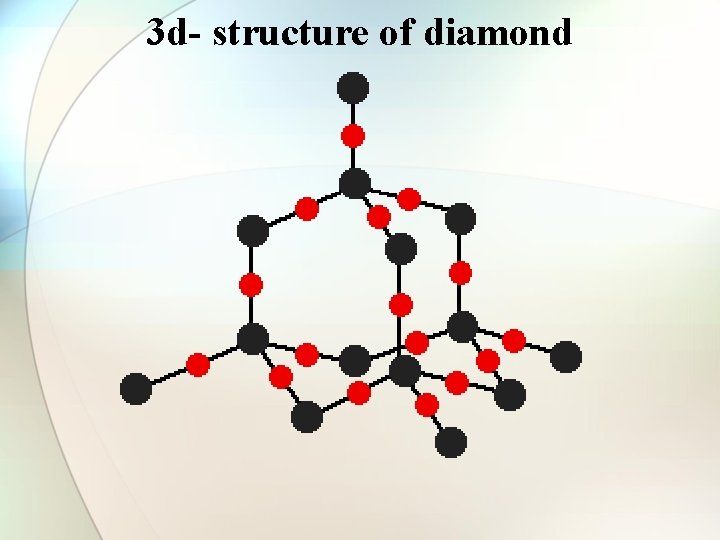
Structure of diamond • • • fcc type. Tetrahedral C-C bond length = 1. 34 A Refractive index = 2. 4 High dispersive power of light Non-conductor of electricity 3 d network Hardest substance ever known. Used as abrasive.

3 d- structure of diamond

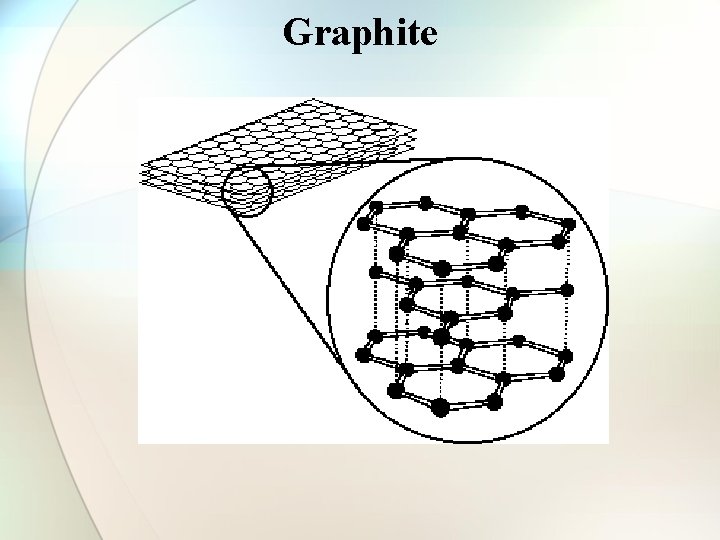
Graphite

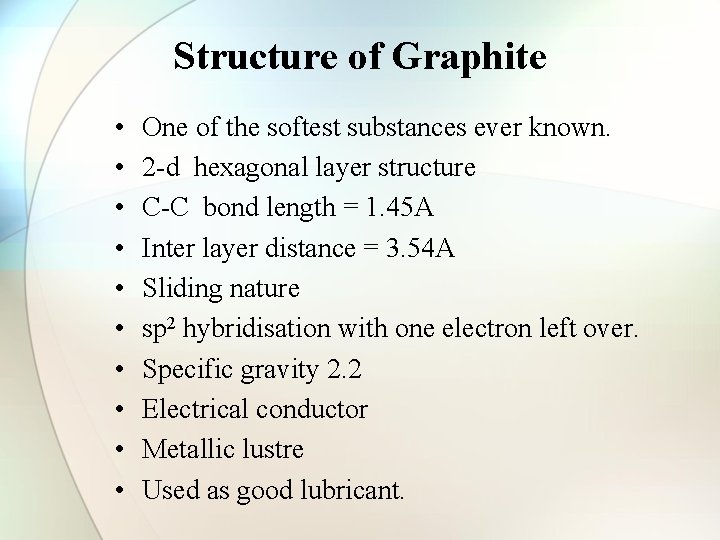
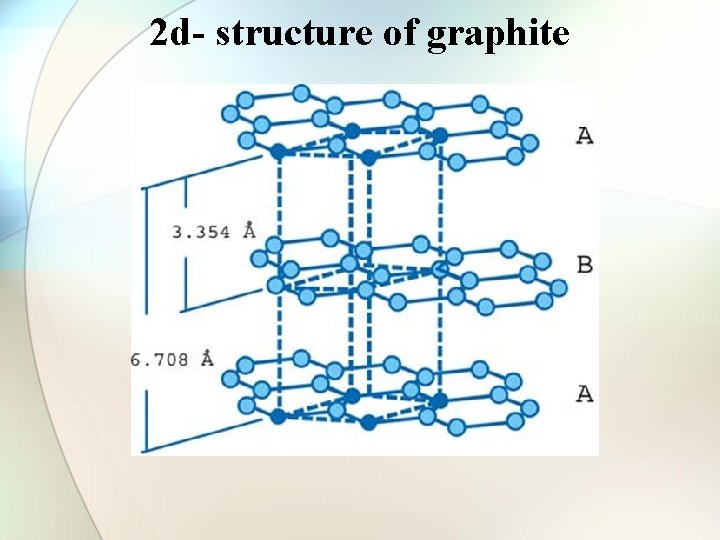
Structure of Graphite • • • One of the softest substances ever known. 2 -d hexagonal layer structure C-C bond length = 1. 45 A Inter layer distance = 3. 54 A Sliding nature sp 2 hybridisation with one electron left over. Specific gravity 2. 2 Electrical conductor Metallic lustre Used as good lubricant.

2 d- structure of graphite

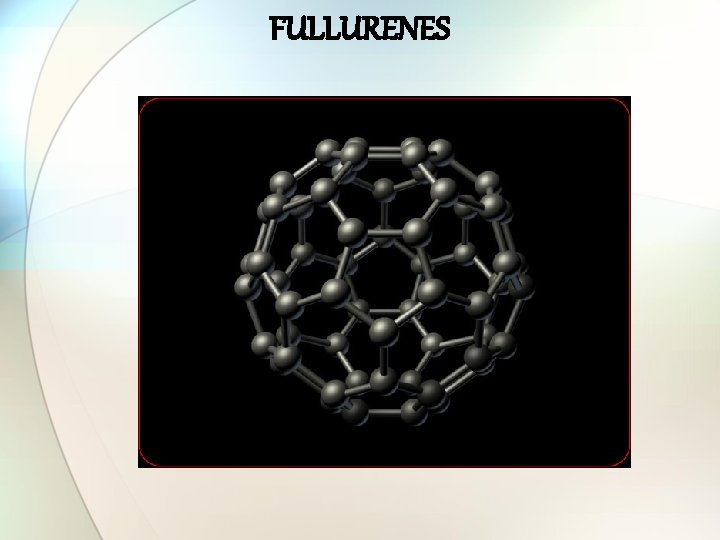
FULLURENES

Important points about Fullurenes • Discovered in 1985 as C 60. • Consists of spherical, ellipsoid or cylindrical arrangement of dozens of C-atoms. • 3 types: ü Spherical: Also called ‘bucky balls’. Molecule of the year 1991 by Science magazine. ü Cylindrical: C nanotubes or buckytubes. ü Planar.

Structure of fullurenes • 60 C-atoms arranged in pentagons and hexagons. • 7Å in diameter. • Soccer-ball shaped molecule with 20 six-membered & 12 five-membered rings. • Each pentagon is surrounded by five hexagons. • No two pentagons are adjecent. • Each carbon is sp 2 -hybridized. • Used: ü as photoresistant. ü in the preparation of super-conductors. ü in optical devices. ü in batteries as charge carriers.

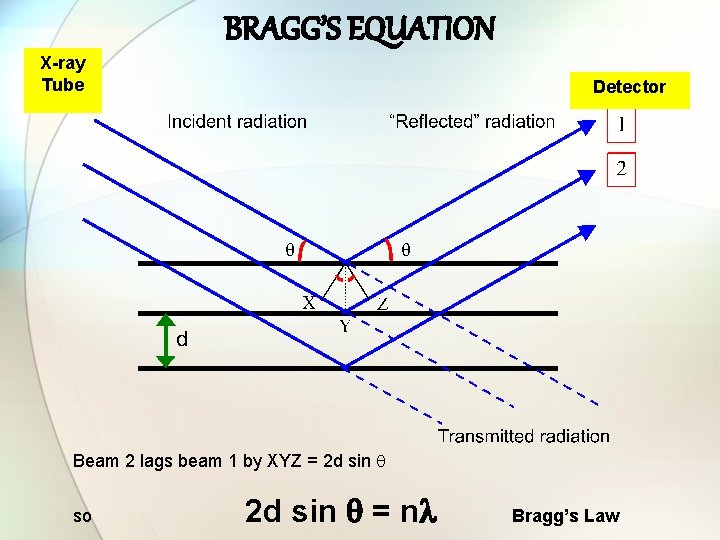
BRAGG’S EQUATION X-ray Tube Detector Beam 2 lags beam 1 by XYZ = 2 d sin so 2 d sin = n Bragg’s Law