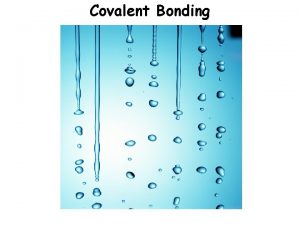
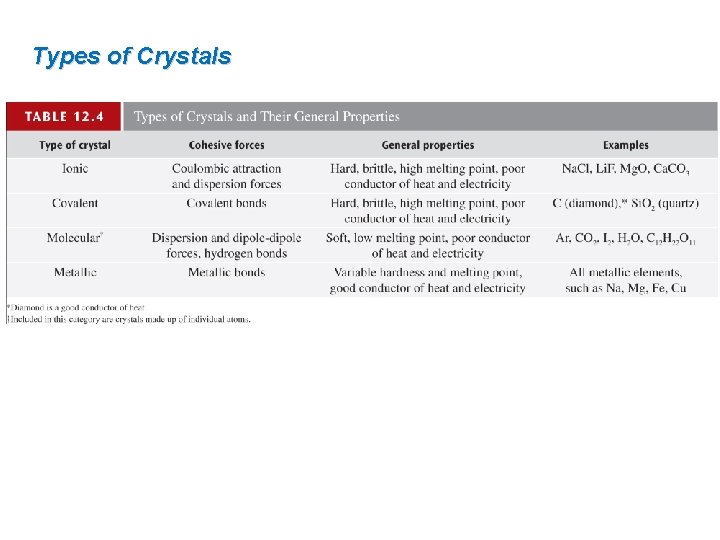
12 4 Types of solids In covalent solids





- Slides: 23

12. 4 Types of solids In covalent solids, atoms are held together in an extensive threedimensional network entirely by covalent bonds.

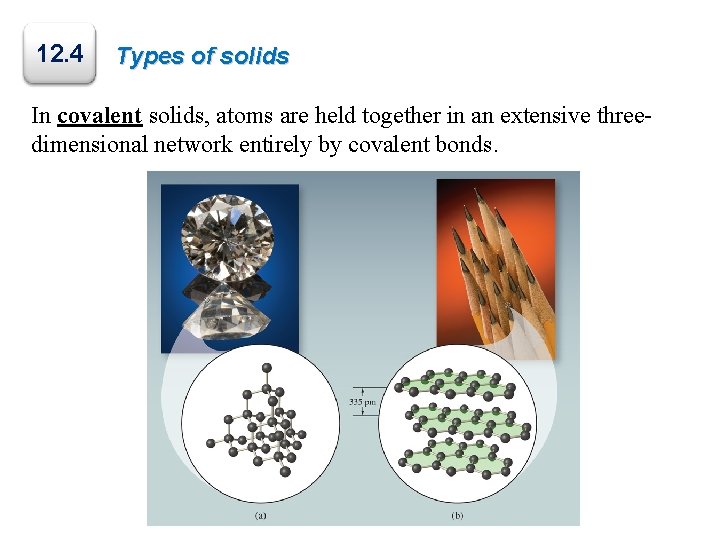
Types of Crystals In molecular solids, the lattice points are occupied by molecules; the attractive forces between them are van der Waals forces and/or hydrogen bonding.

Types of Crystals In metallic solids, every lattice point is occupied by an atom of the same metal. Electrons are delocalized over the entire crystal. Delocalized electrons make metals good conductors. Large cohesive force resulting from delocalization makes metals strong.

Types of Crystals

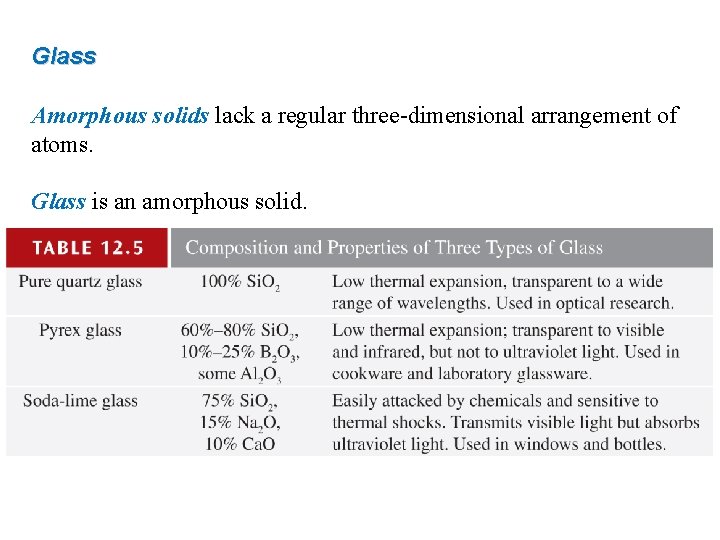
Glass Amorphous solids lack a regular three-dimensional arrangement of atoms. Glass is an amorphous solid.

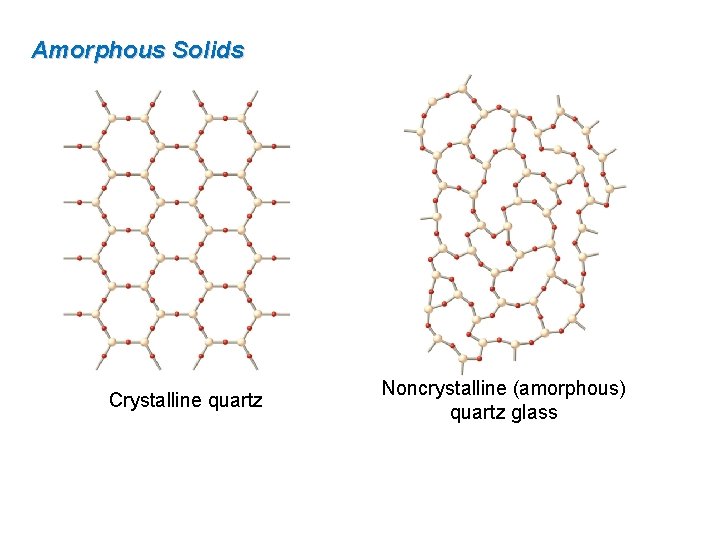
Amorphous Solids Crystalline quartz Noncrystalline (amorphous) quartz glass

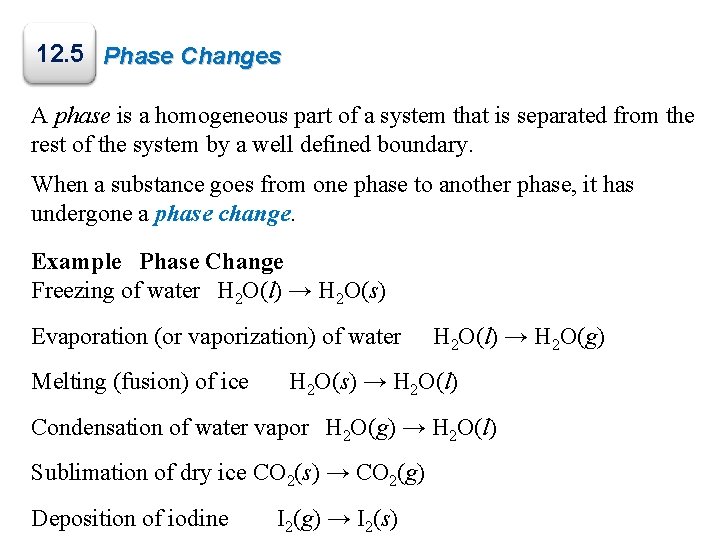
12. 5 Phase Changes A phase is a homogeneous part of a system that is separated from the rest of the system by a well defined boundary. When a substance goes from one phase to another phase, it has undergone a phase change. Example Phase Change Freezing of water H 2 O(l) → H 2 O(s) Evaporation (or vaporization) of water Melting (fusion) of ice H 2 O(l) → H 2 O(g) H 2 O(s) → H 2 O(l) Condensation of water vapor H 2 O(g) → H 2 O(l) Sublimation of dry ice CO 2(s) → CO 2(g) Deposition of iodine I 2(g) → I 2(s)

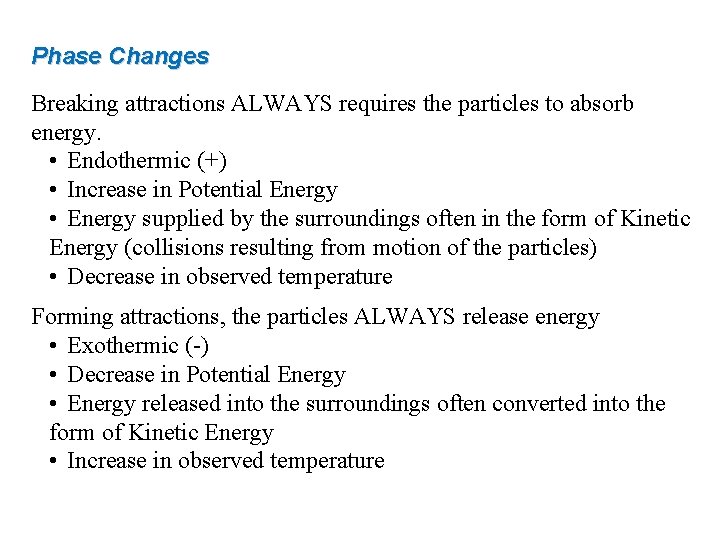
Phase Changes Breaking attractions ALWAYS requires the particles to absorb energy. • Endothermic (+) • Increase in Potential Energy • Energy supplied by the surroundings often in the form of Kinetic Energy (collisions resulting from motion of the particles) • Decrease in observed temperature Forming attractions, the particles ALWAYS release energy • Exothermic (-) • Decrease in Potential Energy • Energy released into the surroundings often converted into the form of Kinetic Energy • Increase in observed temperature

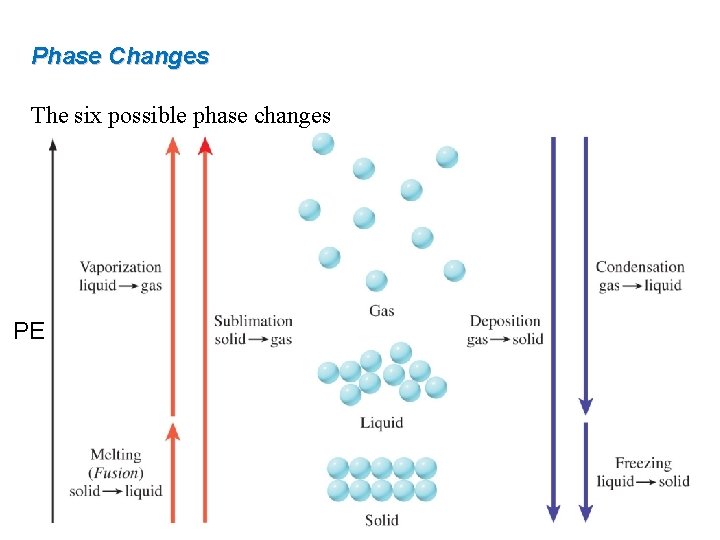
Phase Changes The six possible phase changes PE

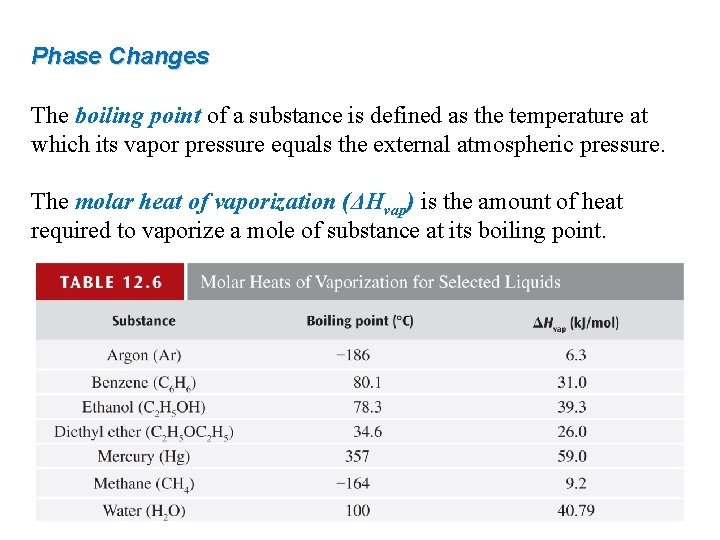
Phase Changes The boiling point of a substance is defined as the temperature at which its vapor pressure equals the external atmospheric pressure. The molar heat of vaporization (ΔHvap) is the amount of heat required to vaporize a mole of substance at its boiling point.

Phase Changes The transformation of a liquid to a solid is called freezing. The reverse process is called melting, or fusion. The melting point (freezing point) of a solid (or liquid) is the temperature at which the solid and liquid phases coexist in equilibrium. ice ⇌ water H 2 O(s) ⇌ H 2 O(l) In dynamic equilibrium, the forward and reverse process are occurring at the same rate.

Phase Changes The molar heat of fusion (ΔHfus) is the energy required to melt 1 mol of a solid.

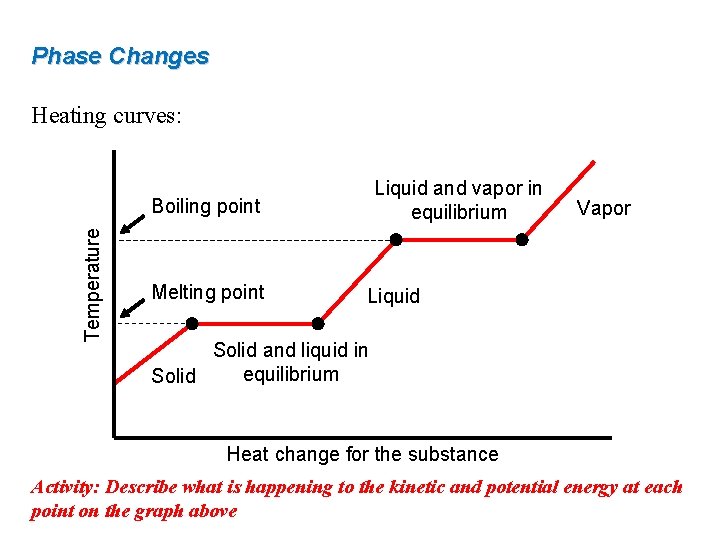
Phase Changes Heating curves: Liquid and vapor in equilibrium Temperature Boiling point Melting point Vapor Liquid Solid and liquid in equilibrium Solid Heat change for the substance Activity: Describe what is happening to the kinetic and potential energy at each point on the graph above

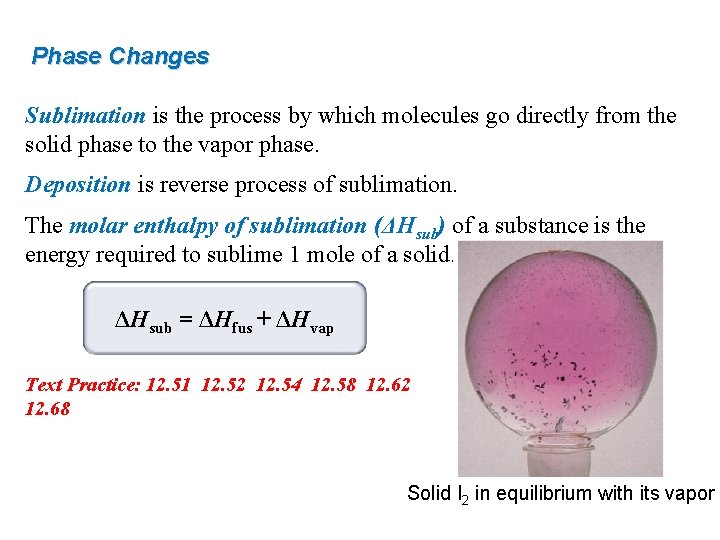
Phase Changes Sublimation is the process by which molecules go directly from the solid phase to the vapor phase. Deposition is reverse process of sublimation. The molar enthalpy of sublimation (ΔHsub) of a substance is the energy required to sublime 1 mole of a solid. ΔHsub = ΔHfus + ΔHvap Text Practice: 12. 51 12. 52 12. 54 12. 58 12. 62 12. 68 Solid I 2 in equilibrium with its vapor

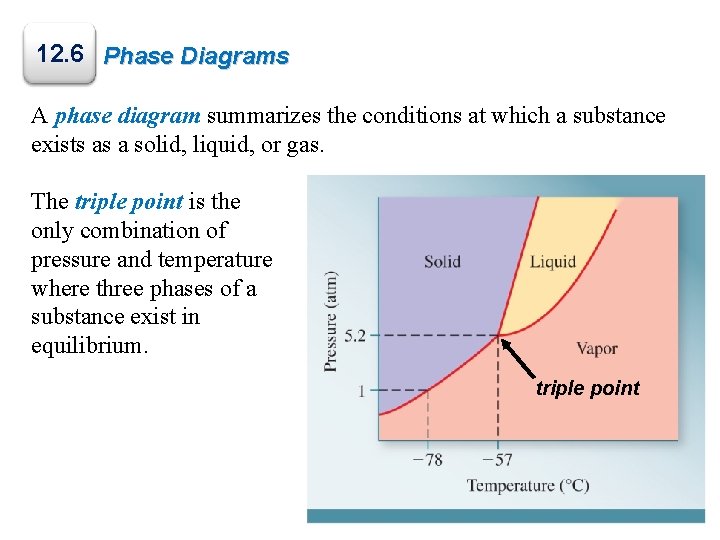
12. 6 Phase Diagrams A phase diagram summarizes the conditions at which a substance exists as a solid, liquid, or gas. The triple point is the only combination of pressure and temperature where three phases of a substance exist in equilibrium. triple point

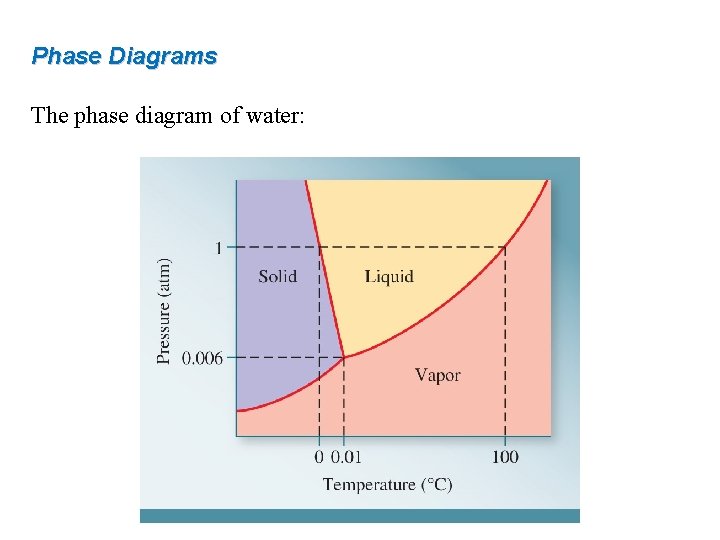
Phase Diagrams The phase diagram of water:

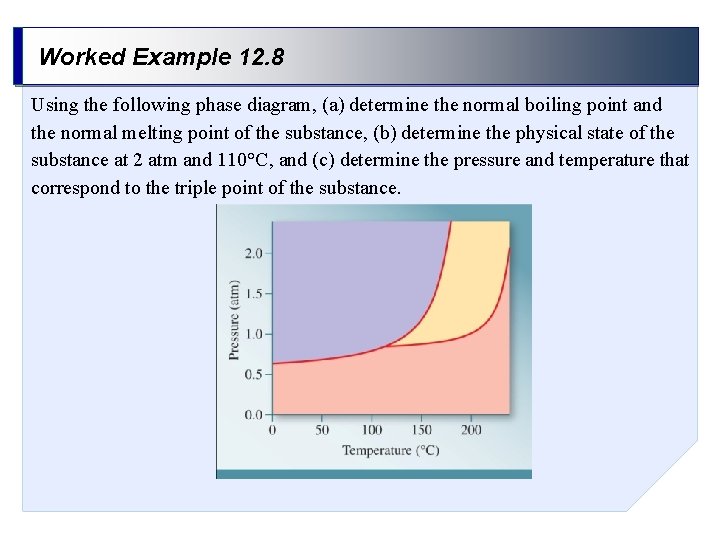
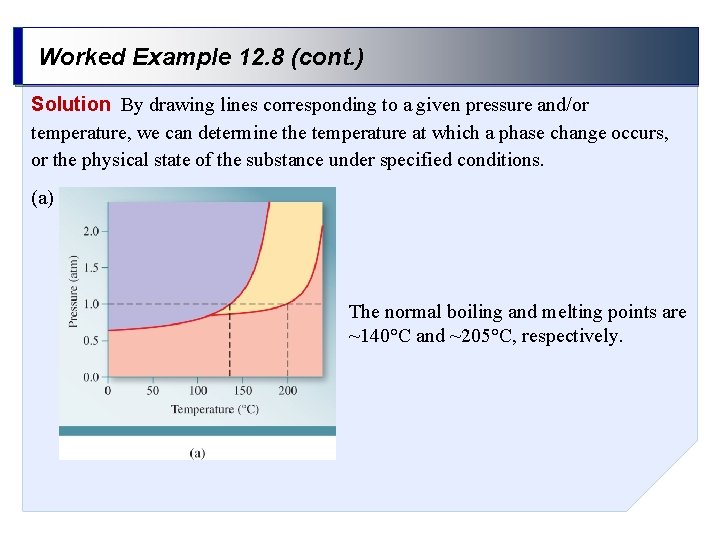
Worked Example 12. 8 Using the following phase diagram, (a) determine the normal boiling point and the normal melting point of the substance, (b) determine the physical state of the substance at 2 atm and 110°C, and (c) determine the pressure and temperature that correspond to the triple point of the substance.

Worked Example 12. 8 (cont. ) Solution By drawing lines corresponding to a given pressure and/or temperature, we can determine the temperature at which a phase change occurs, or the physical state of the substance under specified conditions. (a) The normal boiling and melting points are ~140°C and ~205°C, respectively.

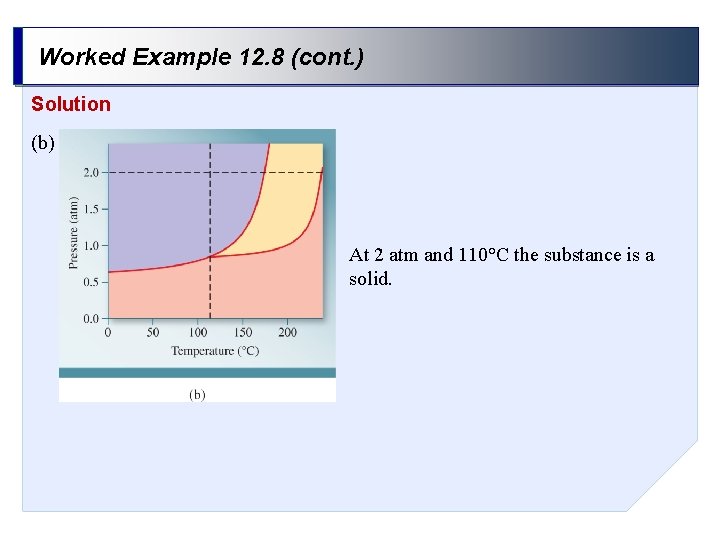
Worked Example 12. 8 (cont. ) Solution (b) At 2 atm and 110°C the substance is a solid.

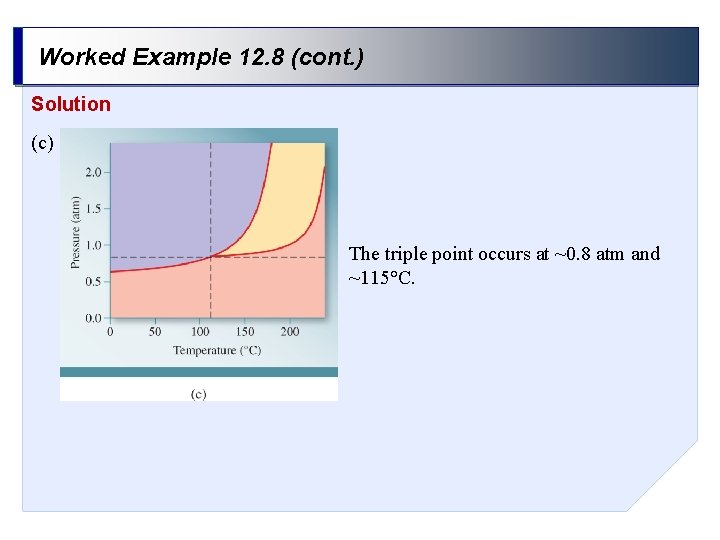
Worked Example 12. 8 (cont. ) Solution (c) The triple point occurs at ~0. 8 atm and ~115°C.

Study Guide for Sections 12. 4 -12. 6 DAY 19, Terms to know: Sections 12. 4 -12. 6 phase, phase change, boiling point, molar heat of a process, melting point, heat of fusion, heating curve, sublimation, deposition, phase diagram DAY 19, Specific outcomes and skills that may be tested on exam 3: Sections 12. 4 -12. 6 • Be able to describe and explain the differences between covalent, molecular, ionic, and metallic solids and the differences between crystals and amorphous solids • Given a molecular formula or Lewis structure, be able to predict whether the molecules will form a covalent, molecular, ionic, or metallic solid and a crystalline or amorphous solid • Be able to give examples of each of the 6 phase changes discussed • Be able to predict and explain temperature and heat changes for any of the phase changes or areas between phase changes on a heating curve • Given a phase diagram, be able to label the areas of solid, liquid, and gas, determine the state of matter at a given temperature and pressure and be able to find the melting point, boiling point, and triple point • Be able to draw a phase diagram and label each of the three sections of the diagram

Extra Practice Problems for Sections 12. 4 -12. 6 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 12. 41 12. 43 12. 67 12. 69 12. 77 12. 79 12. 81 12. 85 12. 103 12. 111 12. 115

Prep for Day 20 Must Watch videos: https: //www. youtube. com/watch? v=SXf 9 r. Dn. VFao (molarity, Tyler De. Witt) https: //www. youtube. com/watch? v=s. Wfn 8 hb. XRp 8 (molarity, Tyler De. Witt) http: //www. youtube. com/watch? v=v 6 dn. Ep 58 m. Vk (dilution, thechemistrysolution) https: //www. youtube. com/watch? v=96 o. Nr. Vn. Tk 50 (molality, Tyler De. Witt) https: //www. youtube. com/watch? v=LPj 73 Gj. BDkg (molality II, Tyler De. Witt) https: //www. youtube. com/watch? v=UL 1 jm. Ja. Uka. Q (balancing, crash course chemistry) https: //www. youtube. com/watch? v=S 6 UQX 7 Zdk. Tg (mole ratios, Tyler De. Witt) https: //www. youtube. com/watch? v=_B 735 tur. Do. M (balancing, Bozeman) Other helpful videos: https: //www. youtube. com/watch? v=ZXn. RMVh. Rptk (concentration units, Isaacs) http: //www. youtube. com/watch? v=yb 4 FW 6 E 1 HKE (molarity, thechemistrysolution) http: //www. learnerstv. com/video/Free-video-Lecture-29336 -Chemistry. htm (concentrations, Bozeman) https: //www. youtube. com/watch? v=SXf 9 r. Dn. VFao (molality problems, Tyler De. Witt) http: //ps. uci. edu/content/chem-1 p-preparation-general-chemistry (UC-Irvine, lectures 13) Read Sections 9. 5 (pages 344 -352), 13. 3, 8. 1, 8. 3, 11. 8
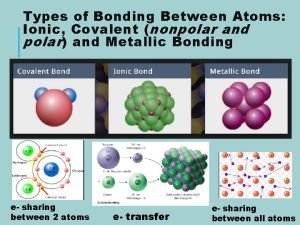
 Types of bonds between atoms
Types of bonds between atoms The nature of covalent bonding
The nature of covalent bonding Covalent molecular and covalent network
Covalent molecular and covalent network Hydrogen or covalent bond stronger
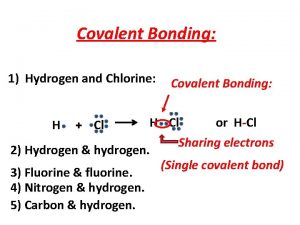
Hydrogen or covalent bond stronger Covalent molecular and covalent network
Covalent molecular and covalent network Silicon carbide covalent network
Silicon carbide covalent network Sic covalent bond
Sic covalent bond Chlorine covalent bond
Chlorine covalent bond Covalent vs coordinate covalent
Covalent vs coordinate covalent C-o covalent bond
C-o covalent bond Nature of covalent bond
Nature of covalent bond Covalent compound formula
Covalent compound formula Two types of covalent bonds
Two types of covalent bonds What are two types of solids
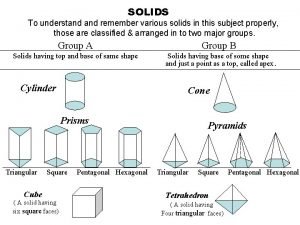
What are two types of solids Types of solids
Types of solids Sectioning of solids
Sectioning of solids Viscoelasticity
Viscoelasticity Four types of solids
Four types of solids A pentagonal pyramid base 25mm side and axis 50mm long
A pentagonal pyramid base 25mm side and axis 50mm long Sectional plane are represented by
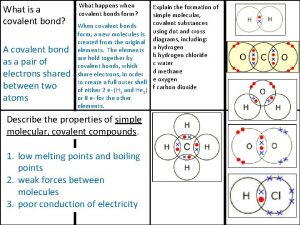
Sectional plane are represented by Physical state of covalent compounds
Physical state of covalent compounds Covalent bonds form when nonmetals
Covalent bonds form when nonmetals Melting and boiling point of oxygen
Melting and boiling point of oxygen Carbon four bonds
Carbon four bonds