Covalent Covalent compounds Covalent compounds are two nonmetals









- Slides: 14

Covalent

Covalent compounds • Covalent compounds are two nonmetals bonded together • Naming covalent compounds is easy! • Name the first element • Name the second element ending in –ide • Use prefixes to tell the number of atoms

Prefixes 1. 1. Mono (only on 2 nd element) 2. Di 3. Tri 4. Tetra 5. Penta 6. Hexa 7. Hepta 8. Octa 9. Nona 10. Deca

Examples • SO 2 – sulfur dioxide • CH 4 – carbon tetrahydride • N 2 O 4 – dinitrogen tetroxide

Practice – write the names 1. CO 2 2. CCl 4 3. P 2 O 5 4. H 4 N 5. HCl

Summary • Ionic compounds have metals with a nonmetal • Covalent compounds have nonmetals only • Ionic compounds can have polyatomic ions • Ionic compounds can have transition metals that need a Roman numeral in the name • Covalent compounds can have prefixes • Both compounds end in –ide (unless polyatomic ion)

Do you understand? 1. 2. 3. 4. How can you tell if a formula is ionic or covalent? How can you tell if a formula will require a Roman numeral? How can you tell if a formula includes a polyatomic ion? How can you tell if a formula will need prefixes?

Covalent compounds • Molecular compound because covalently bonded atoms are what makes up a molecule

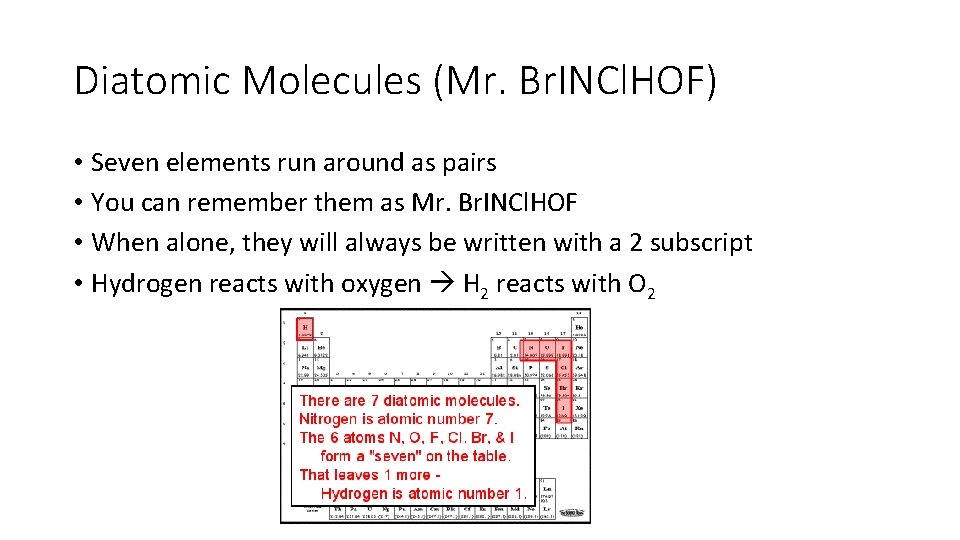
Diatomic Molecules (Mr. Br. INCl. HOF) • Seven elements run around as pairs • You can remember them as Mr. Br. INCl. HOF • When alone, they will always be written with a 2 subscript • Hydrogen reacts with oxygen H 2 reacts with O 2

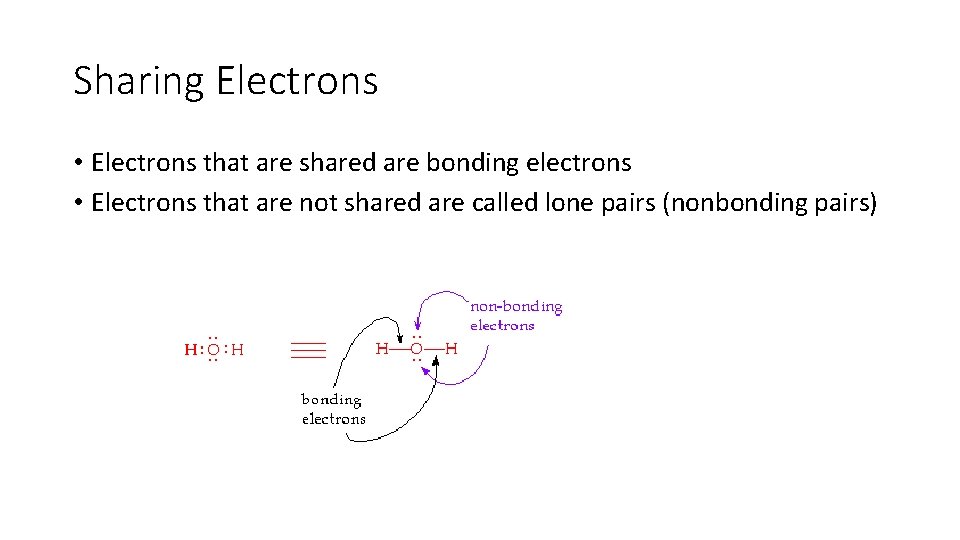
Sharing Electrons • Electrons that are shared are bonding electrons • Electrons that are not shared are called lone pairs (nonbonding pairs)

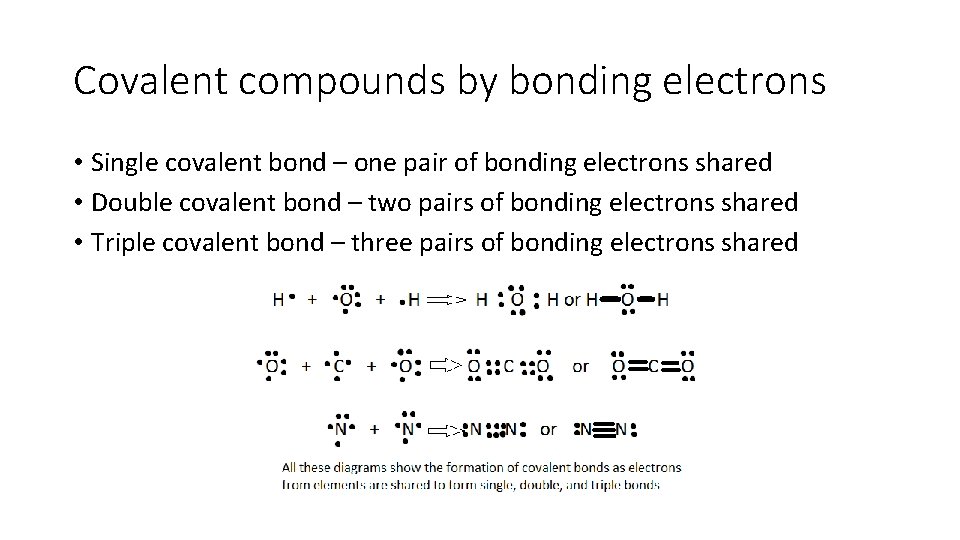
Covalent compounds by bonding electrons • Single covalent bond – one pair of bonding electrons shared • Double covalent bond – two pairs of bonding electrons shared • Triple covalent bond – three pairs of bonding electrons shared

Bond properties based on bond types • Bond length: the length of the bond between two atoms; decreases as more electrons are shared • Shorter…triple < double < single…Longer • Bond energy: energy required to break the shared electron bond; increases as more electrons are shared • Easier to break…single < double < triple… more energy to break

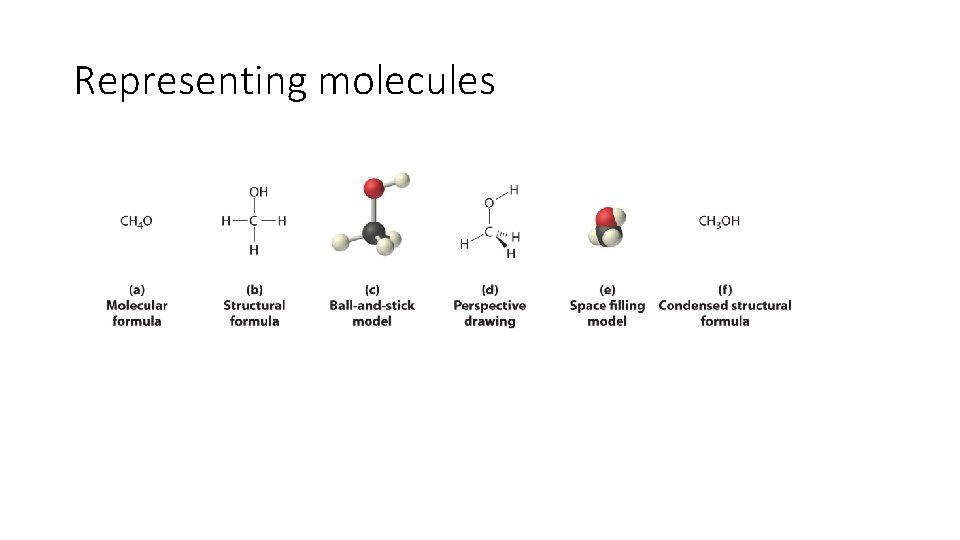
Representing molecules

Do you understand? 1. What’s another name for a covalent compound? 2. Draw the Lewis dot structure for H and O. 3. Now draw a structural formula for water, using lines to represent the shared electrons (bond). 4. Why would it take more energy to break a triple bond than an single bond? 5. Each molecule has a particular shape. What do you think determines how the atoms align in space?