Covalent Compounds Section 4 1 Covalent Compounds Covalent




- Slides: 10

Covalent Compounds Section 4. 1

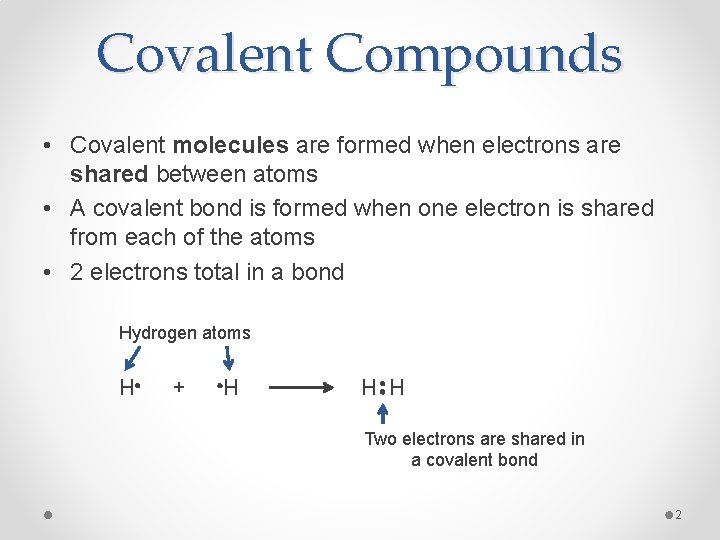
Covalent Compounds • Covalent molecules are formed when electrons are shared between atoms • A covalent bond is formed when one electron is shared from each of the atoms • 2 electrons total in a bond Hydrogen atoms H + H H H Two electrons are shared in a covalent bond 2

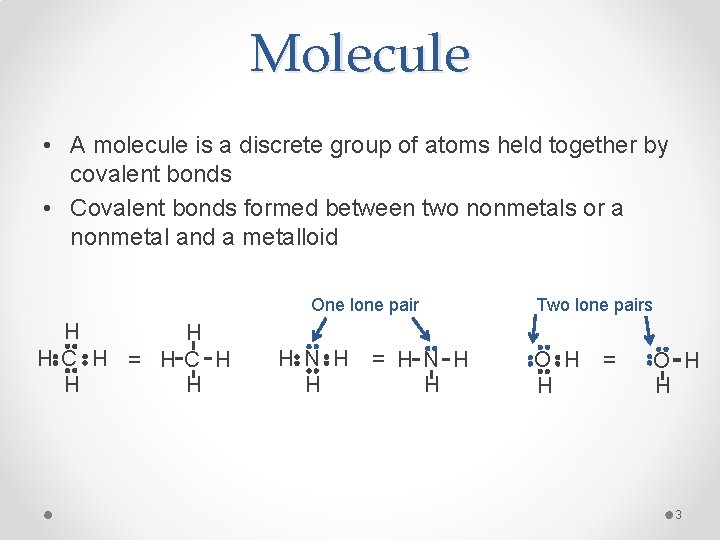
Molecule • A molecule is a discrete group of atoms held together by covalent bonds • Covalent bonds formed between two nonmetals or a nonmetal and a metalloid One lone pair H H HC H = HC H H N H H = H N H H Two lone pairs O H H = O H H 3

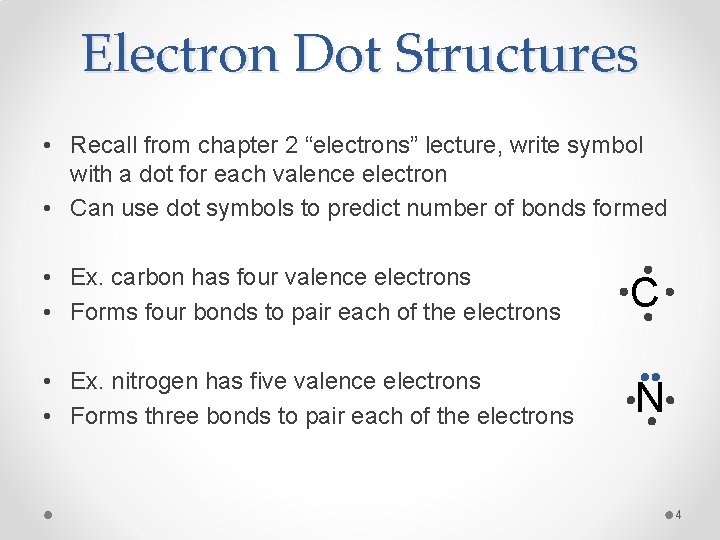
Electron Dot Structures • Recall from chapter 2 “electrons” lecture, write symbol with a dot for each valence electron • Can use dot symbols to predict number of bonds formed • Ex. carbon has four valence electrons • Forms four bonds to pair each of the electrons C • Ex. nitrogen has five valence electrons • Forms three bonds to pair each of the electrons N 4

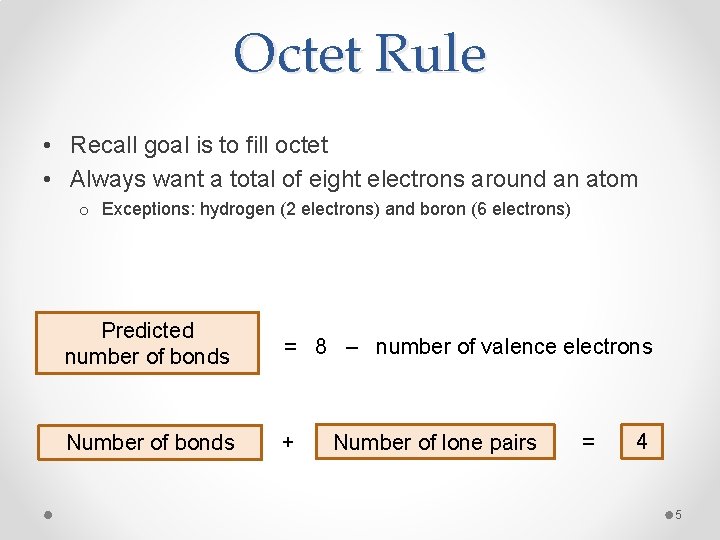
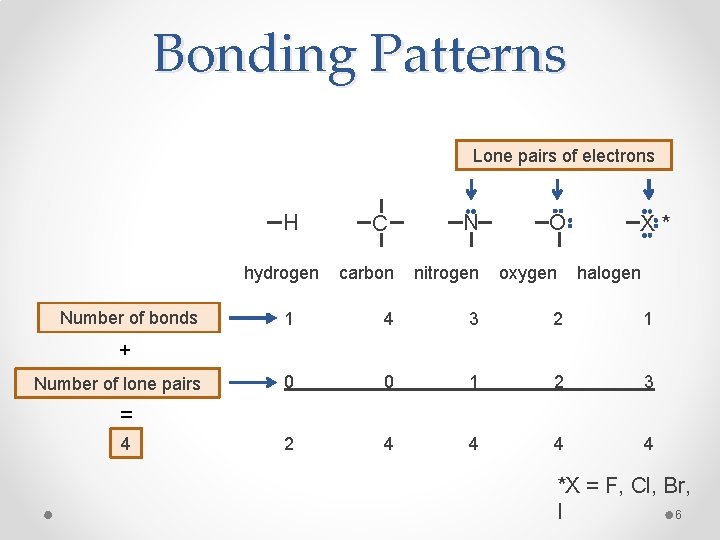
Octet Rule • Recall goal is to fill octet • Always want a total of eight electrons around an atom o Exceptions: hydrogen (2 electrons) and boron (6 electrons) Predicted number of bonds = 8 – number of valence electrons Number of bonds + Number of lone pairs = 4 5

Bonding Patterns Lone pairs of electrons H hydrogen Number of bonds C N O oxygen X* carbon nitrogen halogen 1 4 3 2 1 0 0 1 2 3 2 4 4 + Number of lone pairs = 4 *X = F, Cl, Br, I 6

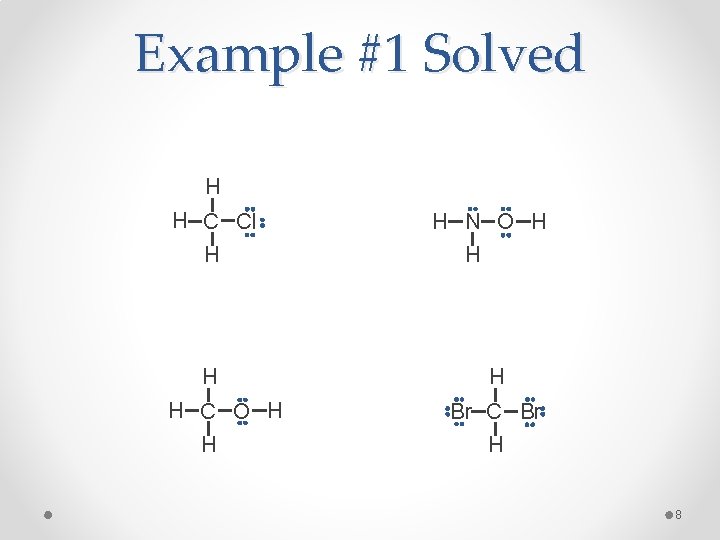
Example #1 Fill in the lone pairs on each atom to give every main group element except hydrogen an octet. H H C Cl H H H C O H H H N O H H H Br C Br H 7

Example #1 Solved H H C Cl H H H C O H H H N O H H H Br C Br H 8

Example #2 Identify which of the following compounds are covalent: a. CBr 4 e. NO 2 b. SF 6 f. Pt. Cl 4 c. KNO 3 g. H 2 S d. Ag 2 O h. PCl 3 9

Example #3 A nonmetal like oxygen forms both ionic and covalent bonds, depending on the identity of the element to which it bonds. What type of bonding is observed in Ca. O and CO 2? Explain why two different types of bonding are observed. 10