Covalent Bonding What is a covalent bond Covalent

Covalent Bonding

What is a covalent bond? • Covalent bonds are made by the sharing of electrons by two atoms – Single Bond- sharing 1 pair (2) electrons – Double Bond- sharing 2 pair (4) electrons – Triple Bond- sharing 3 pairs (6) electrons

Lewis Structure • A Lewis Structure (sometimes called electron dot diagram) allows chemists to model what a molecule might look like • Dots are used to represent electrons • Lines are used to represent bonds (shared electrons)

Steps in Drawing a Lewis Structure • Draw the Lewis Structure for Carbon Tetrafluoride.

1) Determine how many valence electrons each atom has and add them up • C-4 x 1 = 4 • F-7 x 4 = 28 • Total e- = 32

2) Draw a skeletal structure (least electronegative element is placed in center F F C F F
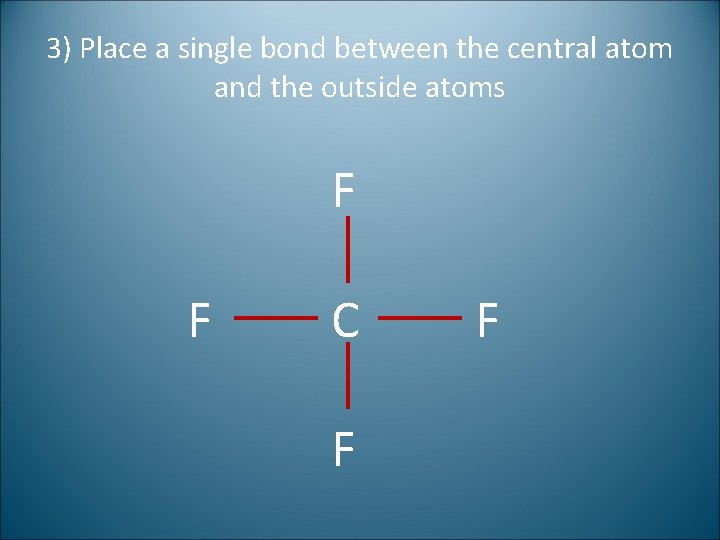
3) Place a single bond between the central atom and the outside atoms F F C F F

4) Subtract 2 electrons for each single bond created C-4 x 1 = 4 F-7 x 4 = 28 Total e- = 32 2 x 4 e- = 24 F F C F F
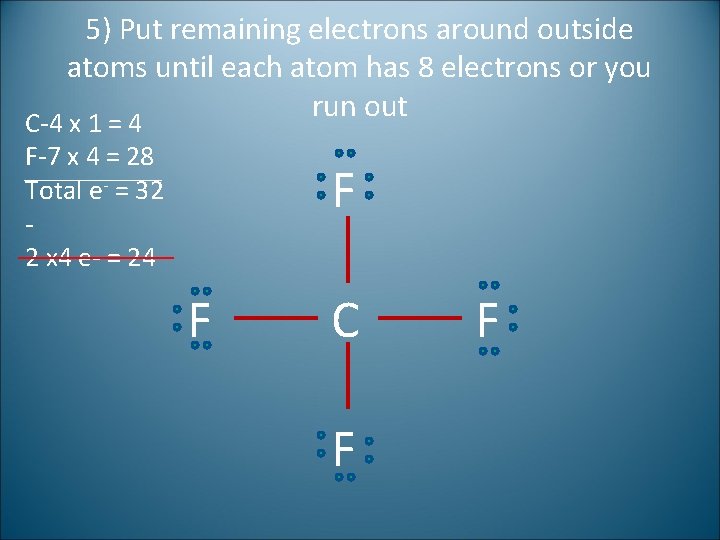
5) Put remaining electrons around outside atoms until each atom has 8 electrons or you run out C-4 x 1 = 4 F-7 x 4 = 28 Total e- = 32 2 x 4 e- = 24 F F C F F

6) Once all atoms have 8 electrons, if there any leftovers, place on central atom C-4 x 1 = 4 F-7 x 4 = 28 Total e- = 32 2 x 4 e- = 24 F F C F F

7) Check to make sure all atoms fullfill the octet rule (have 8 electrons) except for H, which can only have 2 C-4 x 1 = 4 F-7 x 4 = 28 Total e- = 32 2 x 4 e- = 24 F F C 8 F 8 8 8 F 8
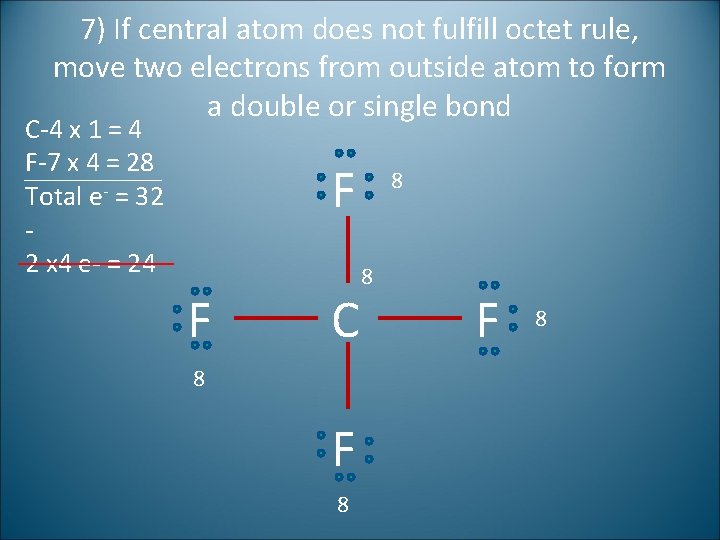
7) If central atom does not fulfill octet rule, move two electrons from outside atom to form a double or single bond C-4 x 1 = 4 F-7 x 4 = 28 Total e- = 32 2 x 4 e- = 24 F F C 8 F 8 8 8 F 8

Draw the Lewis dot structure for CO 2

Draw the Lewis Structure for Ammonia (NH 3)

Draw the electron dot diagram for NO 3 - • Note: Add 1 electron for each negative charge on an ion, subtract 1 for each positive. • Note: Lewis structures for ions must have brackets around them.
- Slides: 15