Reactions in Aqueous Solution Chapter 4 Copyright The










- Slides: 51

Reactions in Aqueous Solution Chapter 4 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

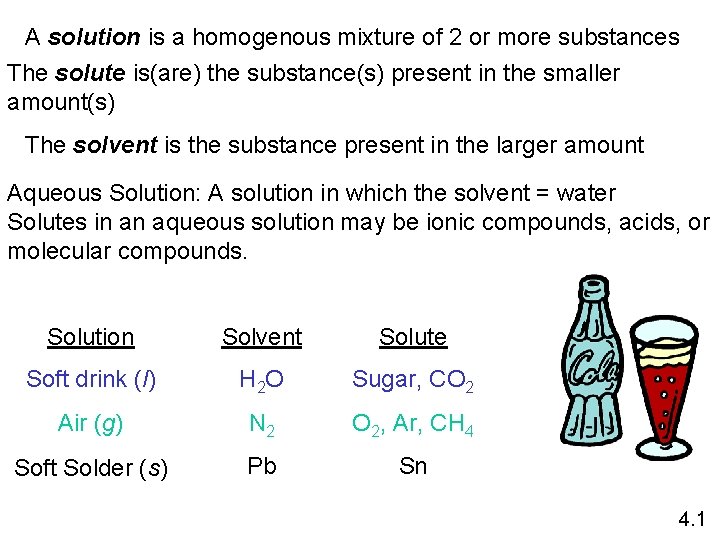
A solution is a homogenous mixture of 2 or more substances The solute is(are) the substance(s) present in the smaller amount(s) The solvent is the substance present in the larger amount Aqueous Solution: A solution in which the solvent = water Solutes in an aqueous solution may be ionic compounds, acids, or molecular compounds. Solution Solvent Solute Soft drink (l) H 2 O Sugar, CO 2 Air (g) N 2 O 2, Ar, CH 4 Soft Solder (s) Pb Sn 4. 1

Behavior of Solutes in Aqueous Solution & Their Classification • Water causes some solutes to dissociate or ionize. • Dissociation or Ionization is the separation of a compound into separated cation and anion. • The solutes that undergo dissociation or ionization include water soluble ionic compounds and acids. • Molecular compounds dissolve intact in aqueous solutions. They do not dissociate or ionize. • When a solute dissociates (ionizes), ions are generated in solution and the solution is therefore able to conduct electricity. • If a lot of ions are generated, the solution conducts electricity strongly. • If only a small amount of ions are generated, the ability to conduct is weak. • If no ions are generated, the solution cannot conduct electricity.

An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. That is all solutes that ionize or dissociate are electrolytes. A strong electrolyte ionizes nearly completely generating a lot of ions. A weak electrolyte only ionizes partially and hence fewer ions are generated. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. nonelectrolyte weak electrolyte 4. 1 strong electrolyte

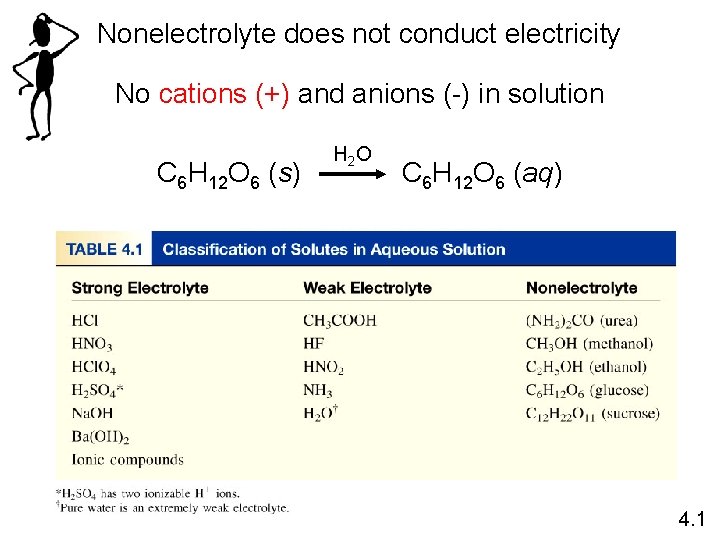
Strong Electrolytes: all water soluble ionic compounds (see solubility rules) & the 7 strong acids (memorize list) 7 Strong acids: HCl, HBr, HI, HNO 3, H 2 SO 4, HCl. O 3 Weak Electrolytes: All weak acids (not on list of 7: HC 2 H 3 O 2, HF, HCN, H 3 PO 4, H 2 CO 3), NH 3 Nonelectrolytes: All molecular compounds that are not weak acids or weak bases. Examples: alcohol, sugar

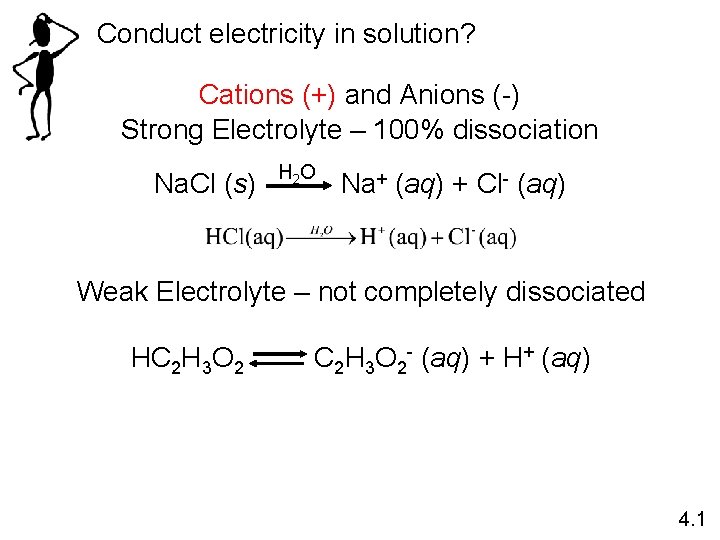
Conduct electricity in solution? Cations (+) and Anions (-) Strong Electrolyte – 100% dissociation Na. Cl (s) H 2 O Na+ (aq) + Cl- (aq) Weak Electrolyte – not completely dissociated HC 2 H 3 O 2 - (aq) + H+ (aq) 4. 1

Nonelectrolyte does not conduct electricity No cations (+) and anions (-) in solution C 6 H 12 O 6 (s) H 2 O C 6 H 12 O 6 (aq) 4. 1

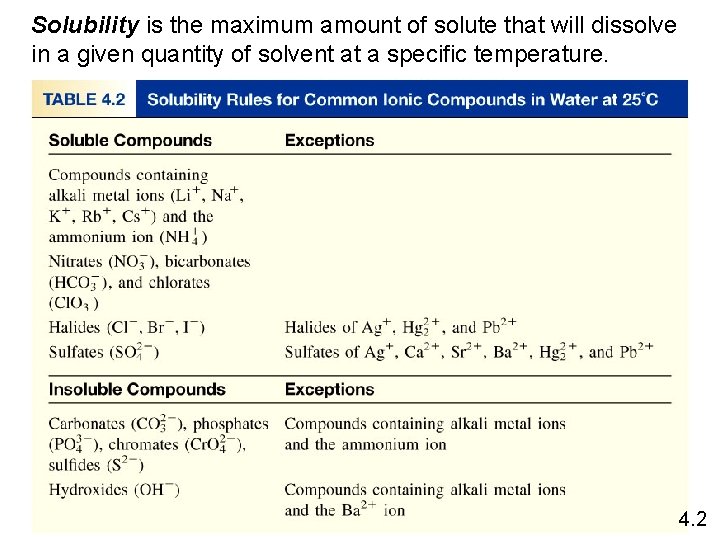
Solubility is the maximum amount of solute that will dissolve in a given quantity of solvent at a specific temperature. 4. 2

Metathesis Reactions = Double Displacement Reactions Metathesis Reaction: A reaction in which two reactants exchange ions (cations or anions). These reactions therefore involve ionic compounds, acids as reactants. A metathesis reaction will result in a net change in the solution if one of the following happens when ions are exchanged: 1. At least 1 new pairing of ions results in the formation of a gas which escapes from the solution. (bubbling may be observed). Gases commonly occurring as products: CO 2, NH 3, H 2 S, SO 2 2. At least 1 new pairing of ions results in the formation of a water insoluble compound or precipitate. Such a metathesis reaction is called a precipitation reaction. (settling of a solid, cloudiness or turbidity will be observed) 3. At least 1 new pairing of ions results in the formation of a weak electrolyte or a nonelectrolyte. A visual change may not be observed for these reactions. The reaction between an acid & a base falls under this category

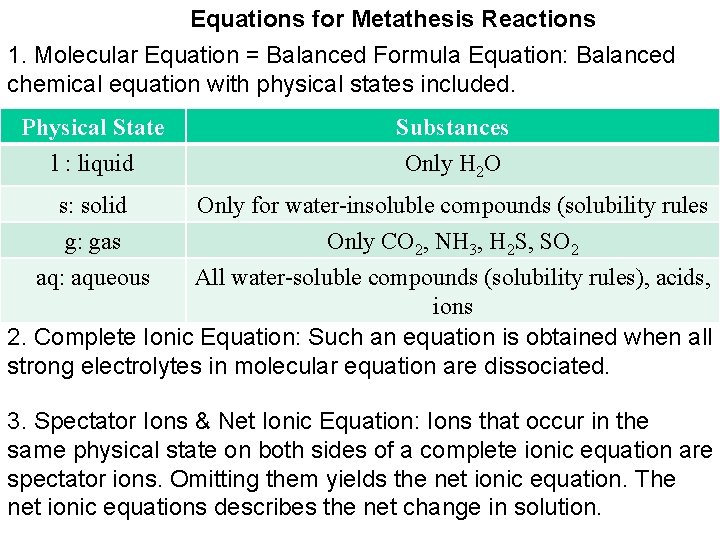
Equations for Metathesis Reactions 1. Molecular Equation = Balanced Formula Equation: Balanced chemical equation with physical states included. Physical State Substances l : liquid Only H 2 O s: solid g: gas aq: aqueous Only for water-insoluble compounds (solubility rules Only CO 2, NH 3, H 2 S, SO 2 All water-soluble compounds (solubility rules), acids, ions 2. Complete Ionic Equation: Such an equation is obtained when all strong electrolytes in molecular equation are dissociated. 3. Spectator Ions & Net Ionic Equation: Ions that occur in the same physical state on both sides of a complete ionic equation are spectator ions. Omitting them yields the net ionic equation. The net ionic equations describes the net change in solution.

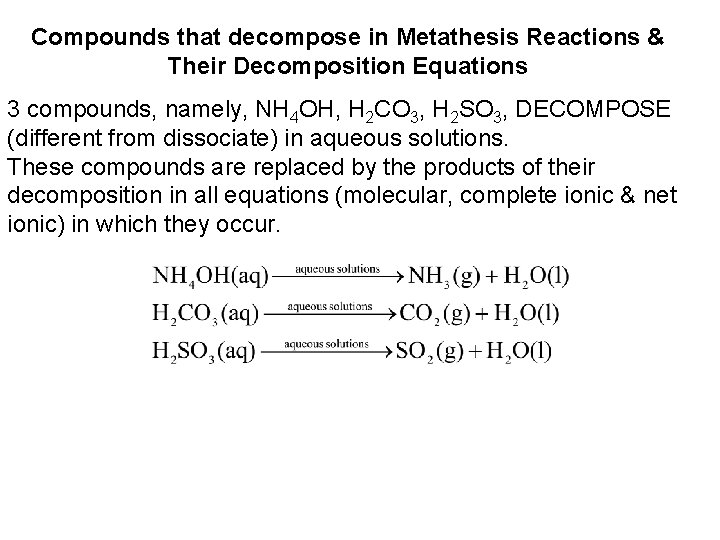
Compounds that decompose in Metathesis Reactions & Their Decomposition Equations 3 compounds, namely, NH 4 OH, H 2 CO 3, H 2 SO 3, DECOMPOSE (different from dissociate) in aqueous solutions. These compounds are replaced by the products of their decomposition in all equations (molecular, complete ionic & net ionic) in which they occur.

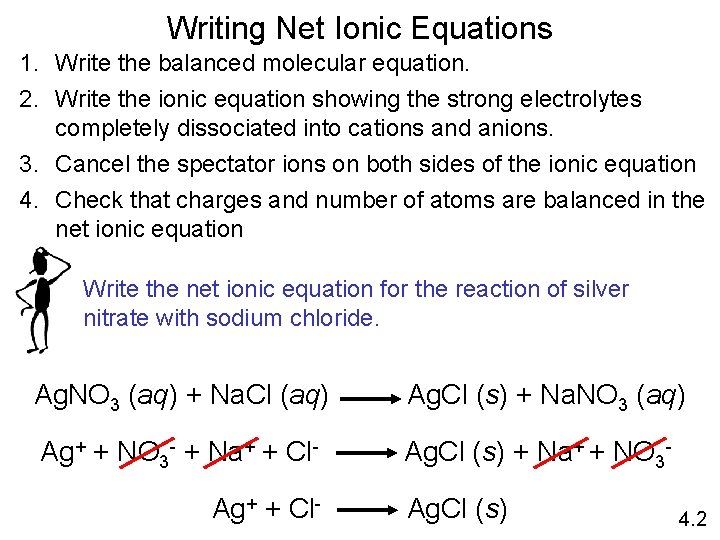
Writing Net Ionic Equations 1. Write the balanced molecular equation. 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation 4. Check that charges and number of atoms are balanced in the net ionic equation Write the net ionic equation for the reaction of silver nitrate with sodium chloride. Ag. NO 3 (aq) + Na. Cl (aq) Ag. Cl (s) + Na. NO 3 (aq) Ag+ + NO 3 - + Na+ + Cl- Ag. Cl (s) + Na+ + NO 3 - Ag+ + Cl- Ag. Cl (s) 4. 2

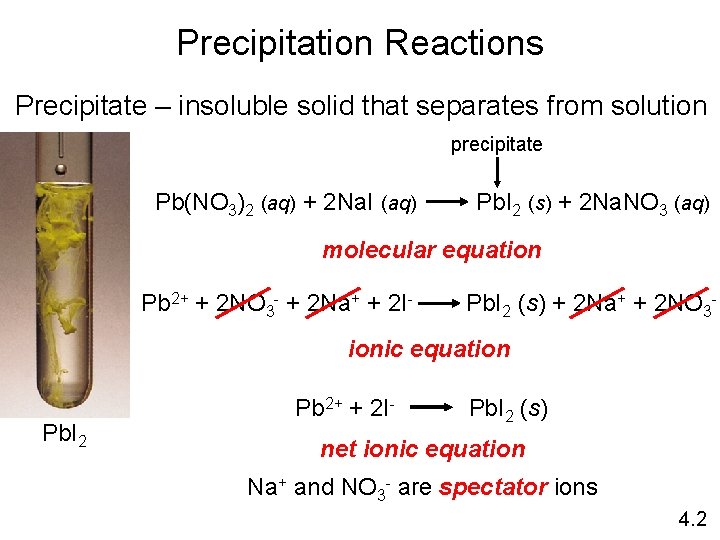
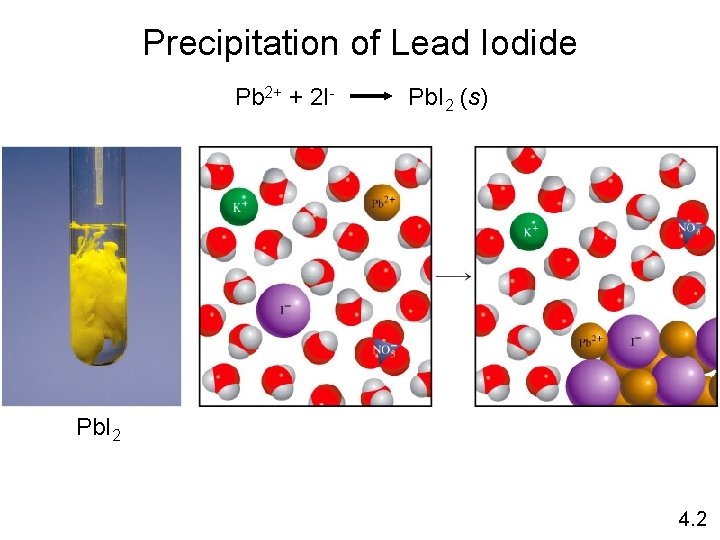
Precipitation Reactions Precipitate – insoluble solid that separates from solution precipitate Pb(NO 3)2 (aq) + 2 Na. I (aq) Pb. I 2 (s) + 2 Na. NO 3 (aq) molecular equation Pb 2+ + 2 NO 3 - + 2 Na+ + 2 I- Pb. I 2 (s) + 2 Na+ + 2 NO 3 - ionic equation Pb. I 2 Pb 2+ + 2 I- Pb. I 2 (s) net ionic equation Na+ and NO 3 - are spectator ions 4. 2

Precipitation of Lead Iodide Pb 2+ + 2 I- Pb. I 2 (s) Pb. I 2 4. 2

Acids Have a sour taste. Vinegar owes its taste to acetic acid. Citrus fruits contain citric acid. React with certain metals to produce hydrogen gas. 2 HCl (aq) + Mg (s) Mg. Cl 2 (aq) + H 2 (g) React with carbonates and bicarbonates to produce carbon dioxide gas 2 HCl (aq) + Ca. CO 3 (s) Ca. Cl 2 (aq) + CO 2 (g) + H 2 O (l) Aqueous acid solutions conduct electricity. 4. 3

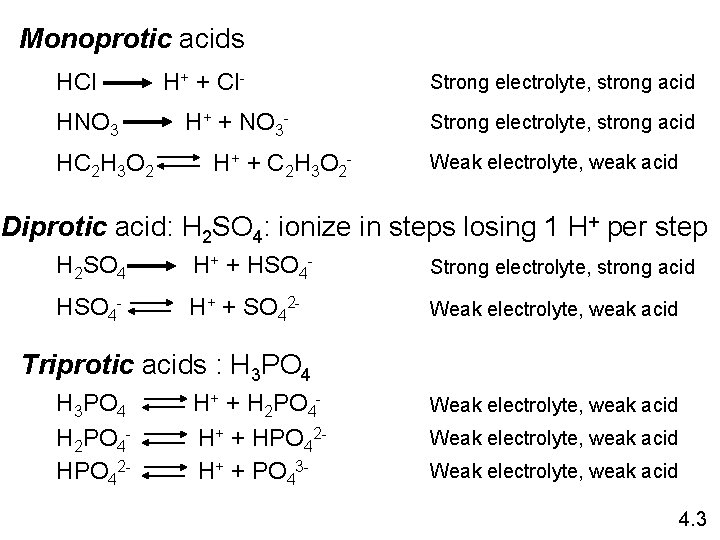
Monoprotic acids HCl HNO 3 HC 2 H 3 O 2 H+ + Cl. H+ + NO 3 H + + C 2 H 3 O 2 - Strong electrolyte, strong acid Weak electrolyte, weak acid Diprotic acid: H 2 SO 4: ionize in steps losing 1 H+ per step H 2 SO 4 H+ + HSO 4 - Strong electrolyte, strong acid HSO 4 - H+ + SO 42 - Weak electrolyte, weak acid Triprotic acids : H 3 PO 4 H 2 PO 4 HPO 42 - H+ + H 2 PO 4 H+ + HPO 42 H+ + PO 43 - Weak electrolyte, weak acid 4. 3

Bases (Arrhenius Definition): These are compounds that increase the amount of OH- in aqueous solution. Examples: all water soluble metal hydroxides; NH 3 Strong bases: These generate a lot of OH- in aqueous solution. Examples: all water soluble metal hydroxides; Na. OH(aq) Na+(aq) + OH-(aq) (dissociation) Ba(OH)2(aq) Ba+2(aq) + 2 OH-(aq) (dissociation) Weak Bases: These generate a small amount of OH- in aqueous solution. Example: NH 3(aq) + H 2 O NH 4+(aq) + OH-(aq) (hydrolysis)

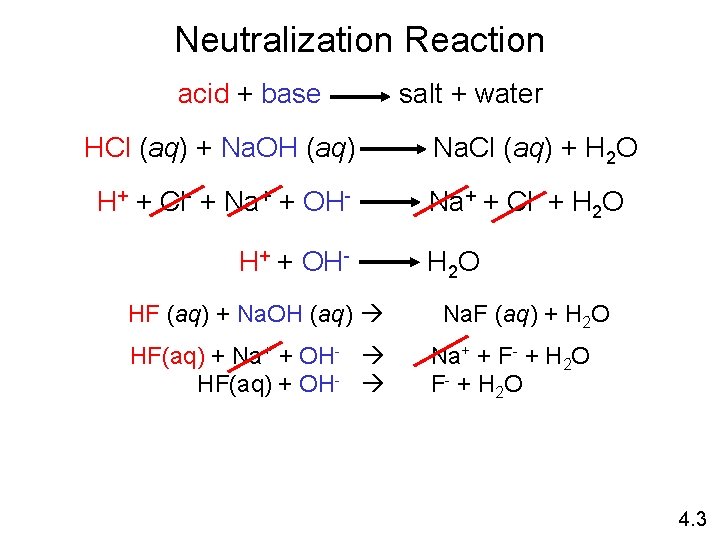
Neutralization Reaction acid + base HCl (aq) + Na. OH (aq) H+ + Cl- + Na+ + OHHF (aq) + Na. OH (aq) HF(aq) + Na+ + OH- HF(aq) + OH- salt + water Na. Cl (aq) + H 2 O Na+ + Cl- + H 2 O Na. F (aq) + H 2 O Na+ + F- + H 2 O F - + H 2 O 4. 3

Metal hydroxide + acid water + salt; salt: cation = metal cation; anion = anion of acid Balancing the above equation: number of H+ from acid = number of OH- from base = number of H 2 O formed 3 Ca(OH)2 + 2 H 3 PO 4 6 H 2 O + Ca 3(PO 4)2; salt = Ca 3(PO 4)2; Ca(OH)2 + H 2 SO 4 2 H 2 O + Ca. SO 4; salt = Ca. SO 4; 3 Na. OH + H 3 PO 4 3 H 2 O + Na 3 PO 4; salt = Na 3 PO 4; Ammonia + acid ammonium salt; cation = NH 4+; anion = anion of acid; To balance: number of H+ from acid= number of NH 3 = number of NH 4+ 3 NH 3 + H 3 PO 4 (NH 4)3 PO 4; ammonium salt = (NH 4)3 PO 4; 2 NH 3 + H 2 SO 4 (NH 4)2 SO 4; ammonium salt = (NH 4)2 SO 4 NH 3 + HNO 3 NH 4 NO 3; ammonium salt = NH 4 NO 3

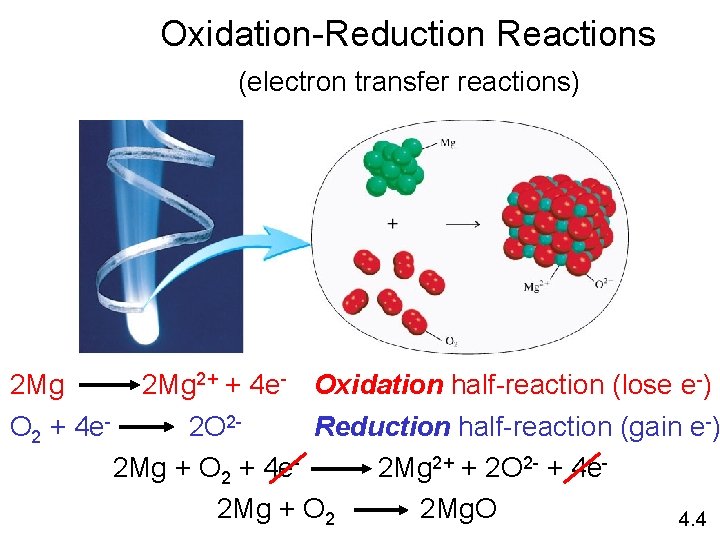
Oxidation-Reduction Reactions (electron transfer reactions) 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) O 2 + 4 e 2 O 2 Reduction half-reaction (gain e-) 2 Mg + O 2 + 4 e 2 Mg 2+ + 2 O 2 - + 4 e 2 Mg + O 2 2 Mg. O 4. 4

• Redox reactions or oxidation-reduction reactions involve electron transfer from one reactant to another. • Oxidation is the loss of electrons: OIL • Reduction is the gain of electrons: RIG • The two are dependant and you cannot have one without other • The reactant that loses electrons is said to undergo oxidation. • The reactant that gains electrons is said to undergo reduction • The reactant that undergoes oxidation causes the other reactant to undergo reduction and is hence called the REDUCING AGENT. • The reactant that undergoes reduction takes away electrons from the reactant causing its oxidation. It is therefore the OXIDIZING AGENT. • Reducing agent is oxidized, the oxidizing agent is reduced. • The electrons flow from the reducing agent to the oxidizing agent • When a metal & nonmetal react, metal =reducing agent, nonmetal = oxidizing agent.

4. 4

• The transfer of electrons in a redox reaction is followed by looking at changes in OXIDATION NUMBER. • Oxidation numbers are imaginary numbers obtained from a set of rules (MEMORIZE) • Every atom in a molecule or polyatomic ion is assigned an oxidation number • OXIDATION NUMBERS SHOULD NOT BE CONFUSED WITH CHARGES FOR POLYATOMIC SPECIES • The oxidation number of monoatomic ions (NOT POLYATOMIC IONS) = the charge of ion • The oxidation number of elements (NOT COMPOUNDS) = 0 • When a reactant gives up electrons, the oxidation number of at least one of its constituent elements becomes less negative or more positive (larger). • When a reactant gains electrons, the oxidation number of at least one of its constituent elements becomes more negative or less positive (smaller).

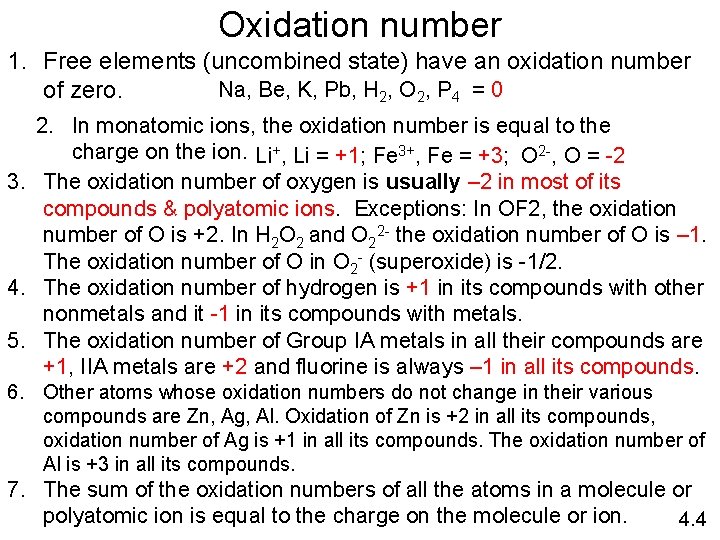
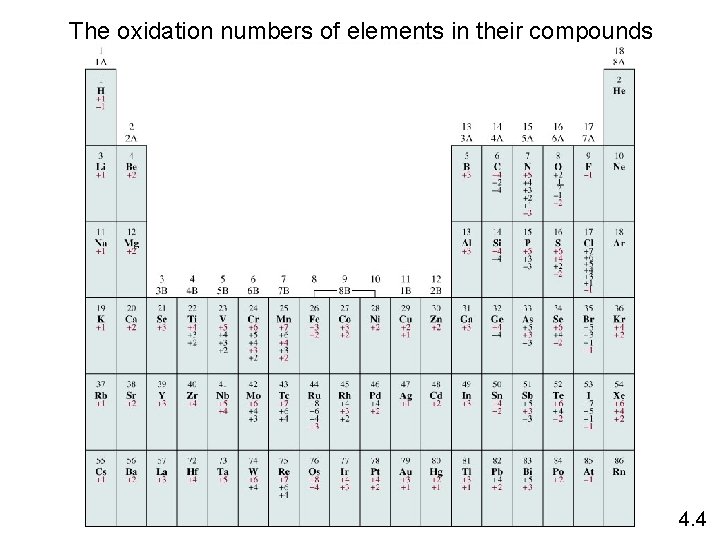
Oxidation number 1. Free elements (uncombined state) have an oxidation number Na, Be, K, Pb, H 2, O 2, P 4 = 0 of zero. 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2 in most of its compounds & polyatomic ions. Exceptions: In OF 2, the oxidation number of O is +2. In H 2 O 2 and O 22 - the oxidation number of O is – 1. The oxidation number of O in O 2 - (superoxide) is -1/2. 4. The oxidation number of hydrogen is +1 in its compounds with other nonmetals and it -1 in its compounds with metals. 5. The oxidation number of Group IA metals in all their compounds are +1, IIA metals are +2 and fluorine is always – 1 in all its compounds. 6. Other atoms whose oxidation numbers do not change in their various compounds are Zn, Ag, Al. Oxidation of Zn is +2 in all its compounds, oxidation number of Ag is +1 in all its compounds. The oxidation number of Al is +3 in all its compounds. 7. The sum of the oxidation numbers of all the atoms in a molecule or polyatomic ion is equal to the charge on the molecule or ion. 4. 4

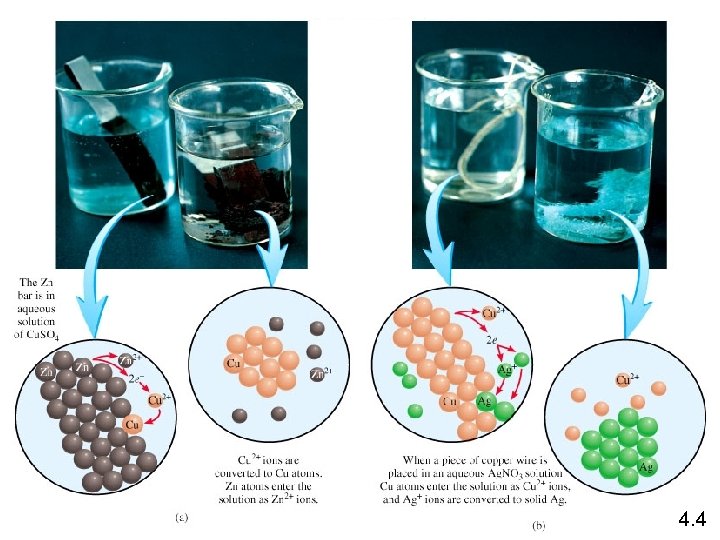
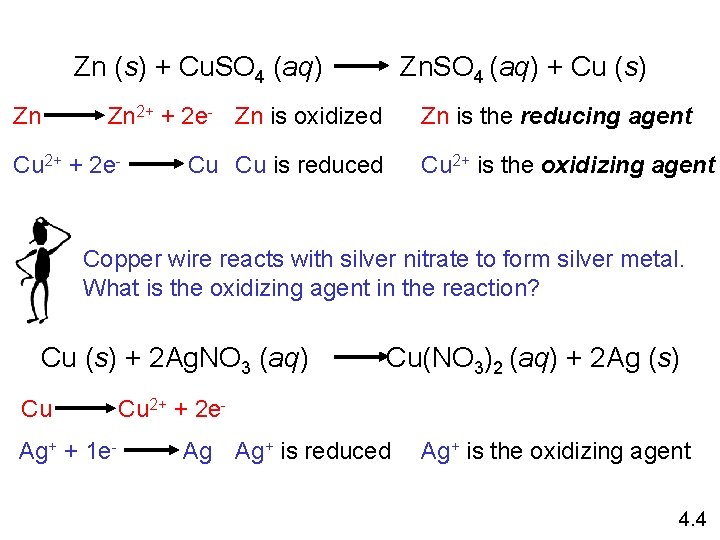
Zn (s) + Cu. SO 4 (aq) Zn Zn. SO 4 (aq) + Cu (s) Zn is the reducing agent Zn 2+ + 2 e- Zn is oxidized Cu 2+ + 2 e- Cu Cu is reduced Cu 2+ is the oxidizing agent Copper wire reacts with silver nitrate to form silver metal. What is the oxidizing agent in the reaction? Cu (s) + 2 Ag. NO 3 (aq) Cu Ag+ + 1 e- Cu(NO 3)2 (aq) + 2 Ag (s) Cu 2+ + 2 e. Ag Ag+ is reduced Ag+ is the oxidizing agent 4. 4

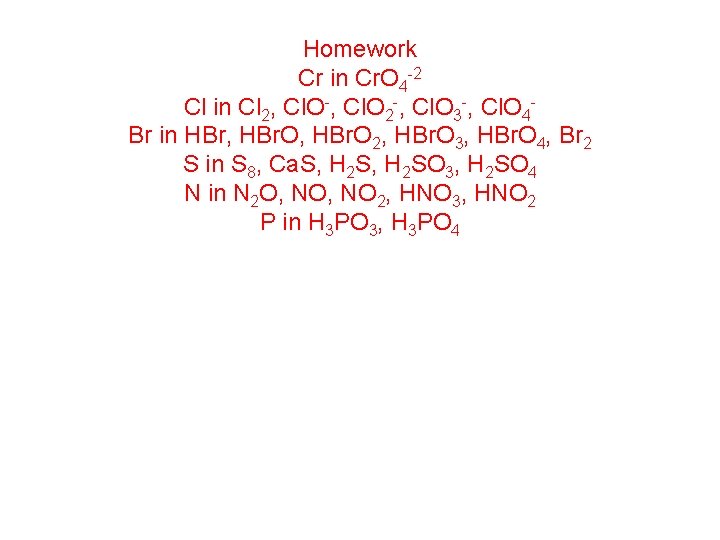
Homework Cr in Cr. O 4 -2 Cl in Cl 2, Cl. O-, Cl. O 2 -, Cl. O 3 -, Cl. O 4 Br in HBr, HBr. O 2, HBr. O 3, HBr. O 4, Br 2 S in S 8, Ca. S, H 2 SO 3, H 2 SO 4 N in N 2 O, NO 2, HNO 3, HNO 2 P in H 3 PO 3, H 3 PO 4

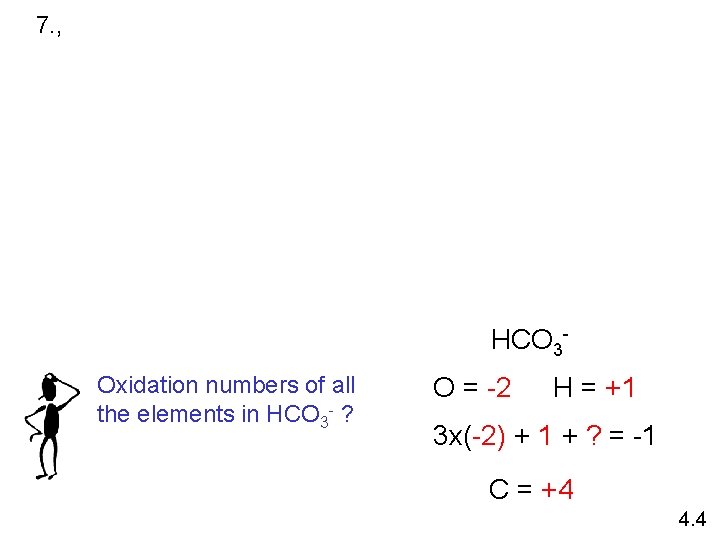
7. , HCO 3 Oxidation numbers of all the elements in HCO 3 - ? O = -2 H = +1 3 x(-2) + 1 + ? = -1 C = +4 4. 4

The oxidation numbers of elements in their compounds 4. 4

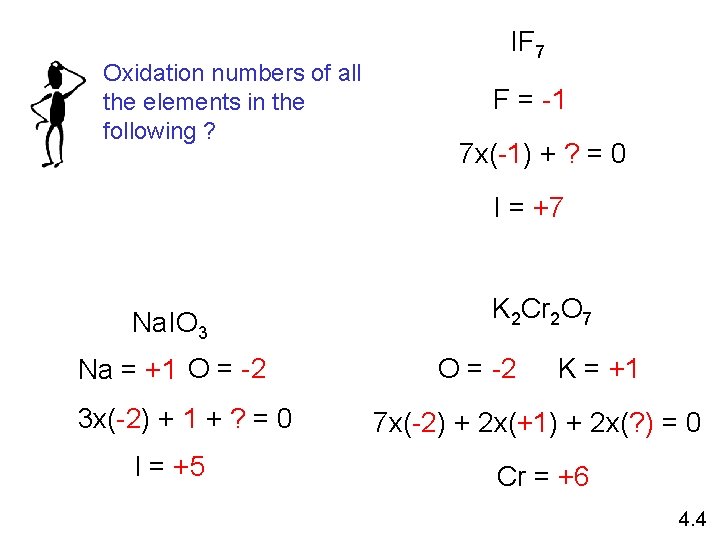
Oxidation numbers of all the elements in the following ? IF 7 F = -1 7 x(-1) + ? = 0 I = +7 Na. IO 3 Na = +1 O = -2 3 x(-2) + 1 + ? = 0 I = +5 K 2 Cr 2 O 7 O = -2 K = +1 7 x(-2) + 2 x(+1) + 2 x(? ) = 0 Cr = +6 4. 4

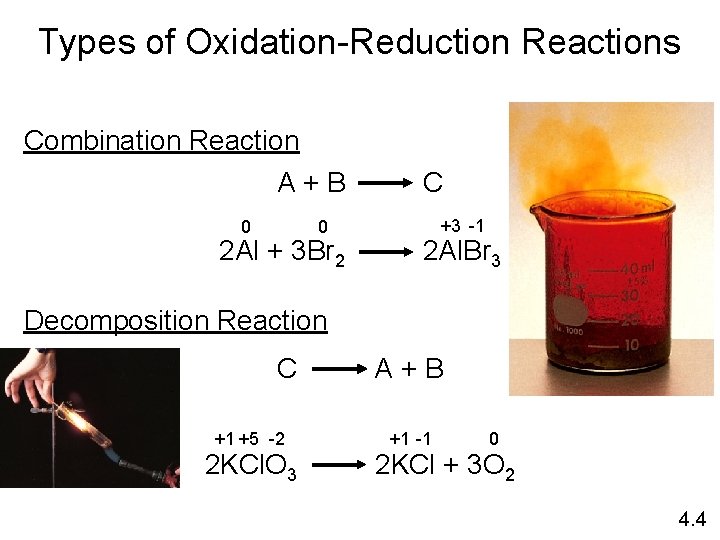
Types of Oxidation-Reduction Reactions Combination Reaction A+B 0 0 2 Al + 3 Br 2 C +3 -1 2 Al. Br 3 Decomposition Reaction C +1 +5 -2 2 KCl. O 3 A+B +1 -1 0 2 KCl + 3 O 2 4. 4

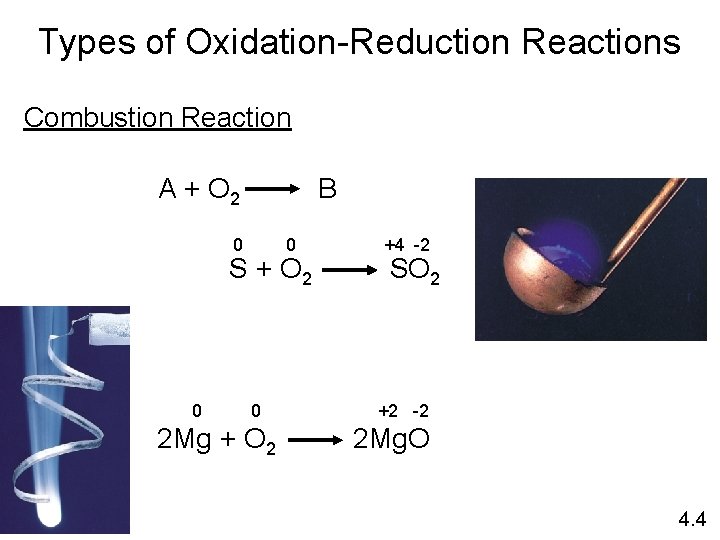
Types of Oxidation-Reduction Reactions Combustion Reaction A + O 2 B 0 0 S + O 2 0 0 2 Mg + O 2 +4 -2 SO 2 +2 -2 2 Mg. O 4. 4

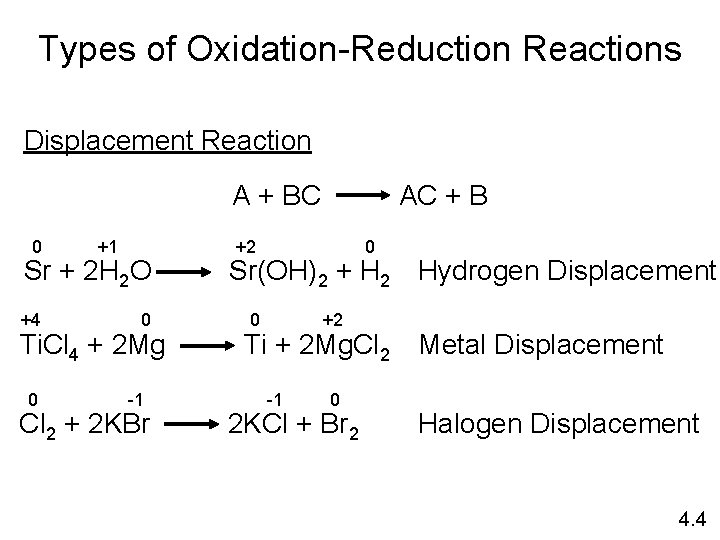
Types of Oxidation-Reduction Reactions Displacement Reaction A + BC 0 +1 Sr + 2 H 2 O +4 0 Ti. Cl 4 + 2 Mg 0 -1 Cl 2 + 2 KBr AC + B +2 0 Sr(OH)2 + H 2 Hydrogen Displacement 0 +2 Ti + 2 Mg. Cl 2 Metal Displacement -1 0 2 KCl + Br 2 Halogen Displacement 4. 4

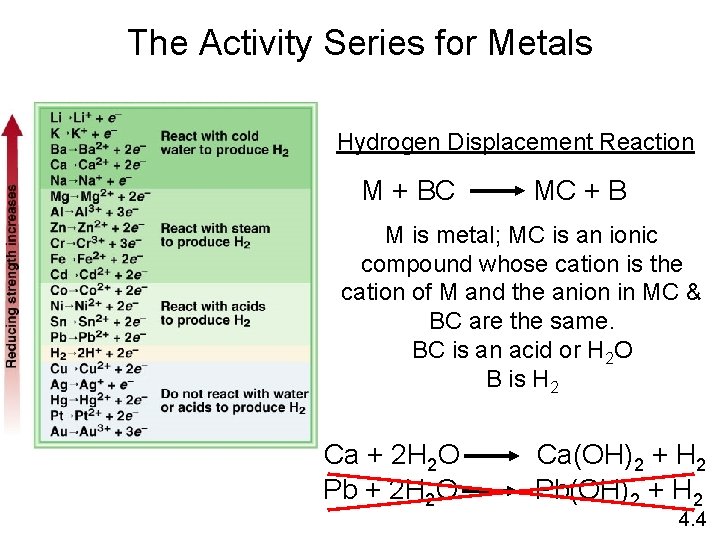
The Activity Series for Metals Hydrogen Displacement Reaction M + BC MC + B M is metal; MC is an ionic compound whose cation is the cation of M and the anion in MC & BC are the same. BC is an acid or H 2 O B is H 2 Ca + 2 H 2 O Pb + 2 H 2 O Ca(OH)2 + H 2 Pb(OH)2 + H 2 4. 4

• All metal above H 2 in the activity series will react with acids such as HCl to produce H 2(g) • Metal(s) + HCl(aq) metal chloride + H 2(g); for all metals more active than H 2(g). • The oxidizing agent = H+ of the acid • The reducing agent = metal • Similarly, any metal will react with salts of less active metals. The cation of the less active metal will be reduced to the element. The more active metal will be oxidized to its corresponding cation. • Mmore active + Mless active. C Mmore active. C + Mless active • Mmore active: more active metal; Mless active: less active metal Mless active. C, Mmore active. C: ionic compounds with the same anion C but the cations differ. • More active metal(s) + cation of less active metal(aq) cation of less more active metal (aq) + less active metal(s) • Metals will not react with salts of more active metals. • Less active metal + cation of more active metal no reaction.

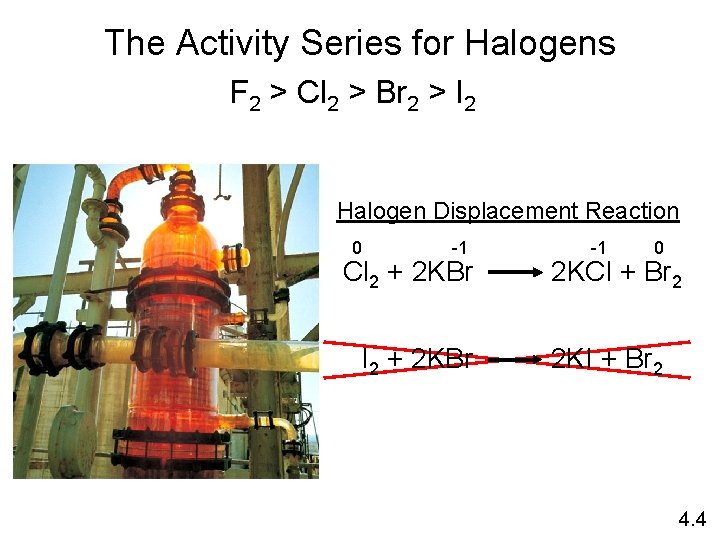
The Activity Series for Halogens F 2 > Cl 2 > Br 2 > I 2 Halogen Displacement Reaction 0 -1 Cl 2 + 2 KBr I 2 + 2 KBr -1 0 2 KCl + Br 2 2 KI + Br 2 4. 4

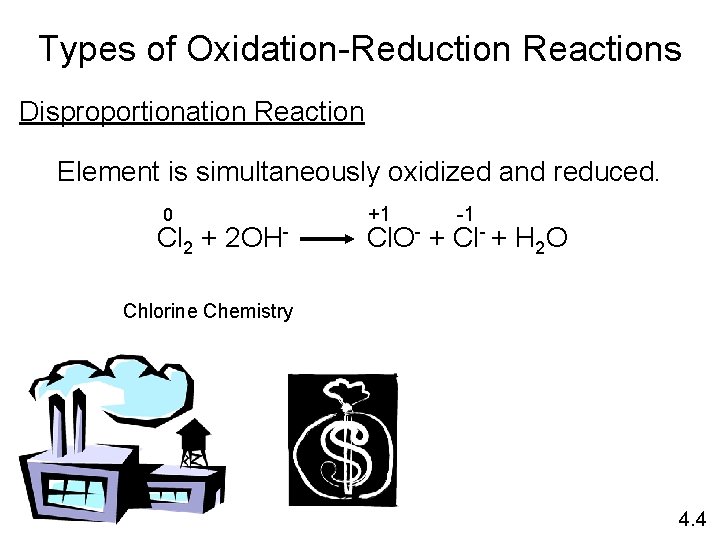
Types of Oxidation-Reduction Reactions Disproportionation Reaction Element is simultaneously oxidized and reduced. 0 Cl 2 + 2 OH- +1 -1 Cl. O- + Cl- + H 2 O Chlorine Chemistry 4. 4

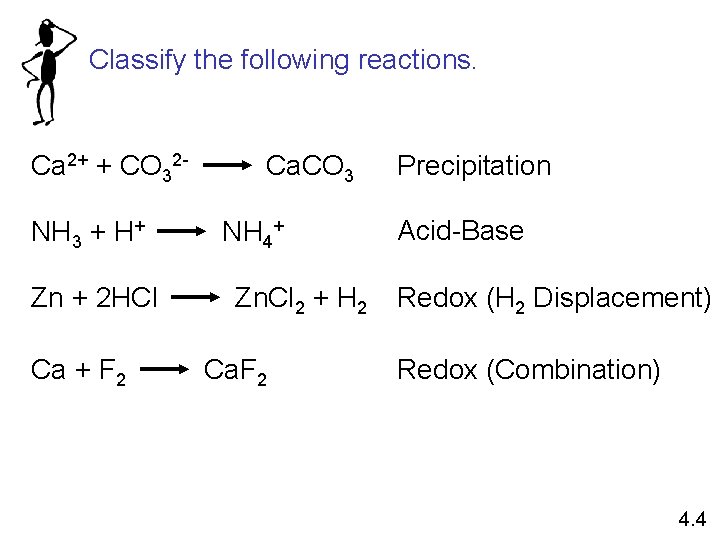
Classify the following reactions. Ca 2+ + CO 32 NH 3 + H+ Zn + 2 HCl Ca + F 2 Ca. CO 3 NH 4+ Zn. Cl 2 + H 2 Ca. F 2 Precipitation Acid-Base Redox (H 2 Displacement) Redox (Combination) 4. 4

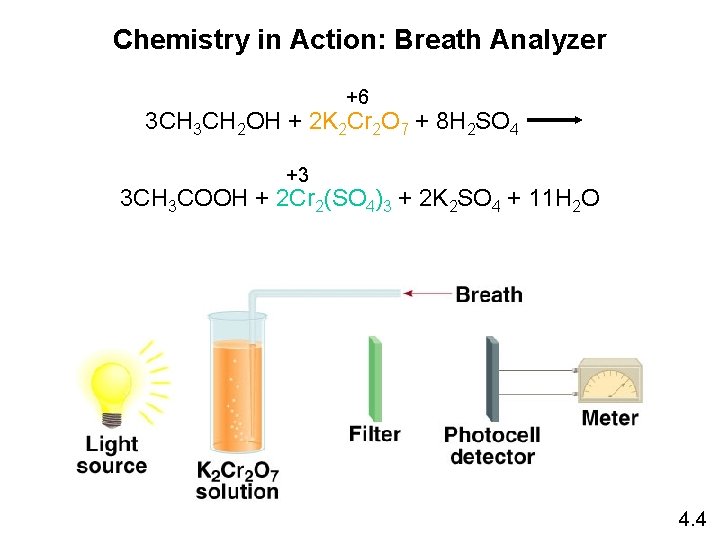
Chemistry in Action: Breath Analyzer +6 3 CH 2 OH + 2 K 2 Cr 2 O 7 + 8 H 2 SO 4 +3 3 CH 3 COOH + 2 Cr 2(SO 4)3 + 2 K 2 SO 4 + 11 H 2 O 4. 4

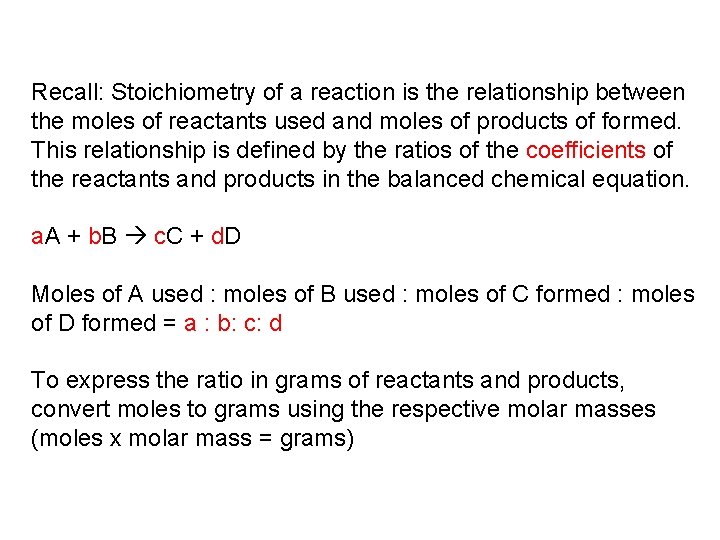
Recall: Stoichiometry of a reaction is the relationship between the moles of reactants used and moles of products of formed. This relationship is defined by the ratios of the coefficients of the reactants and products in the balanced chemical equation. a. A + b. B c. C + d. D Moles of A used : moles of B used : moles of C formed : moles of D formed = a : b: c: d To express the ratio in grams of reactants and products, convert moles to grams using the respective molar masses (moles x molar mass = grams)

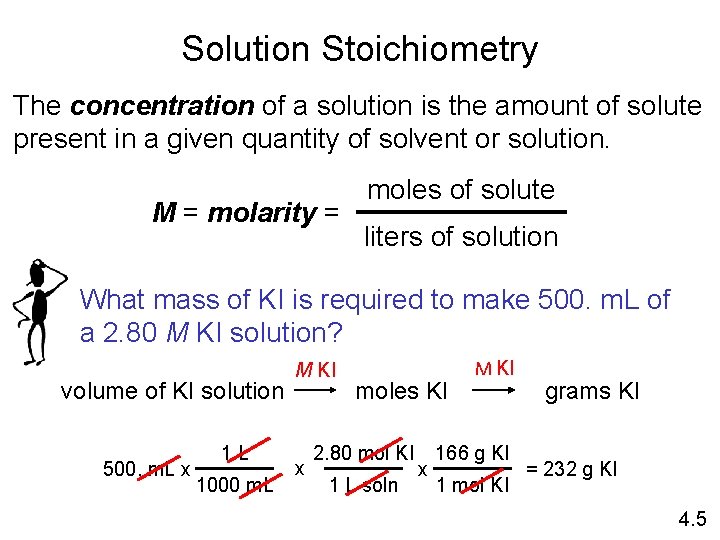
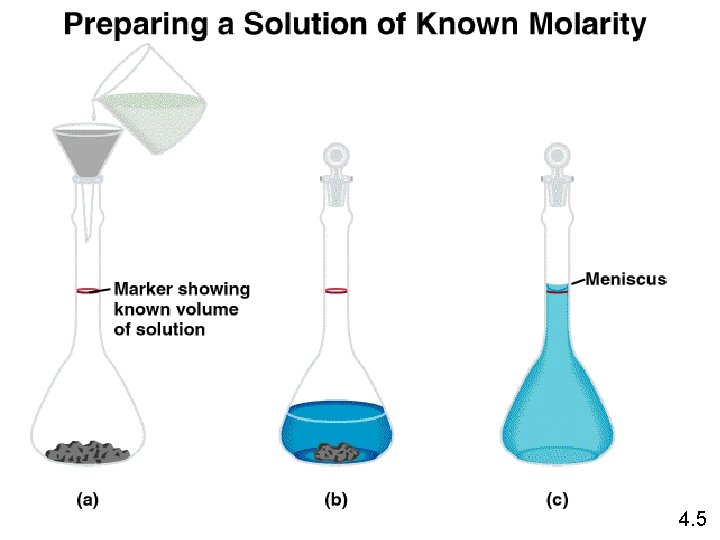
Solution Stoichiometry The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. M = molarity = moles of solute liters of solution What mass of KI is required to make 500. m. L of a 2. 80 M KI solution? volume of KI solution 500. m. L x 1 L 1000 m. L M KI x moles KI 2. 80 mol KI 1 L soln x M KI 166 g KI 1 mol KI grams KI = 232 g KI 4. 5

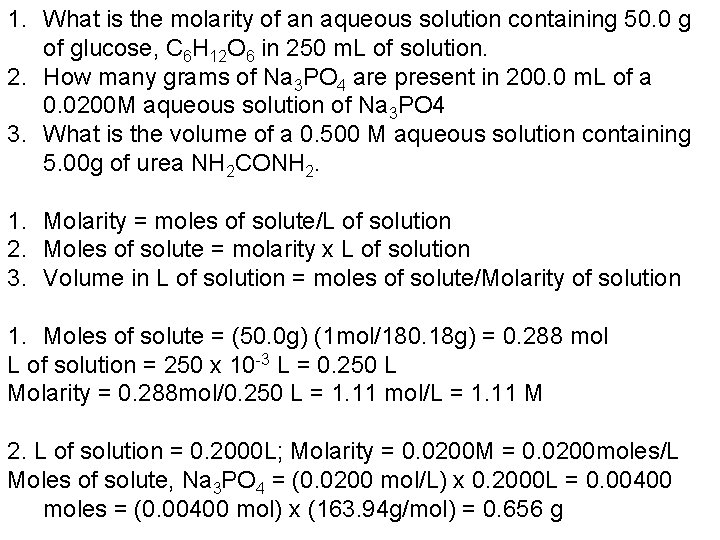
1. What is the molarity of an aqueous solution containing 50. 0 g of glucose, C 6 H 12 O 6 in 250 m. L of solution. 2. How many grams of Na 3 PO 4 are present in 200. 0 m. L of a 0. 0200 M aqueous solution of Na 3 PO 4 3. What is the volume of a 0. 500 M aqueous solution containing 5. 00 g of urea NH 2 CONH 2. 1. Molarity = moles of solute/L of solution 2. Moles of solute = molarity x L of solution 3. Volume in L of solution = moles of solute/Molarity of solution 1. Moles of solute = (50. 0 g) (1 mol/180. 18 g) = 0. 288 mol L of solution = 250 x 10 -3 L = 0. 250 L Molarity = 0. 288 mol/0. 250 L = 1. 11 mol/L = 1. 11 M 2. L of solution = 0. 2000 L; Molarity = 0. 0200 M = 0. 0200 moles/L Moles of solute, Na 3 PO 4 = (0. 0200 mol/L) x 0. 2000 L = 0. 00400 moles = (0. 00400 mol) x (163. 94 g/mol) = 0. 656 g

Solute: 5. 00 g of NH 2 CONH 2 = (5. 00 g) x (1 mol/60. 06 g) = 0. 0833 mol Molarity of solution = 0. 500 M = 0. 500 moles/L Volume of solution = mol/molarity = 0. 0833 mol/(0. 500 mol/L) = 0. 167 L = 167 m. L

4. 5

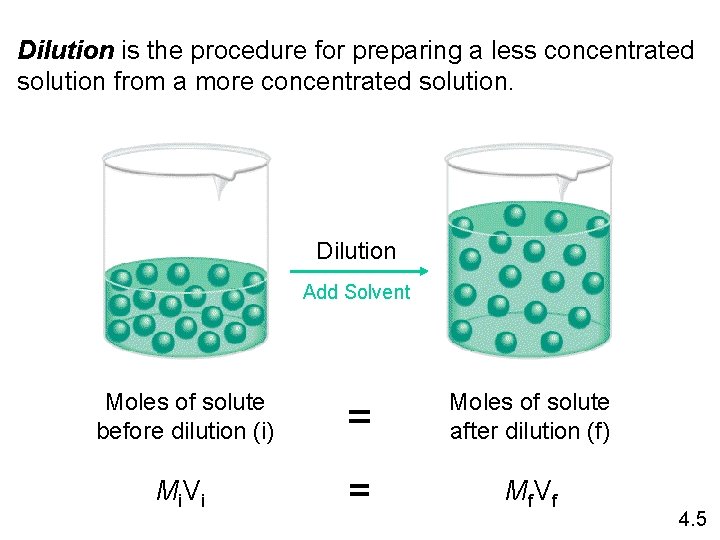
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) = Moles of solute after dilution (f) Mi V i = Mf V f 4. 5

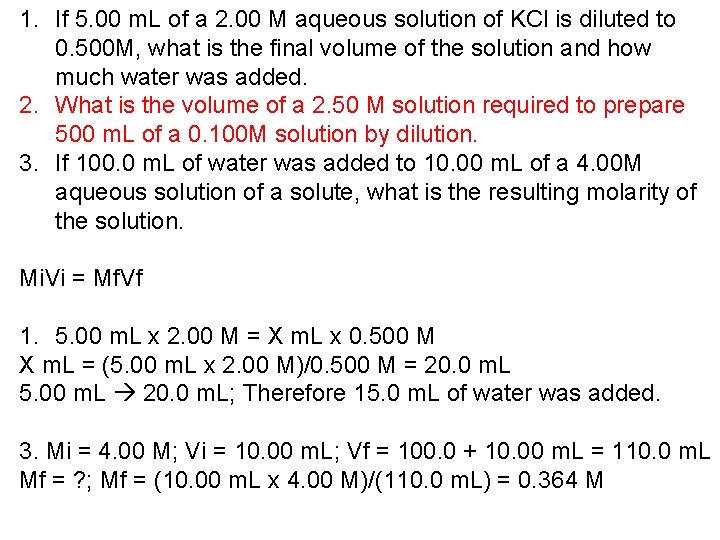
1. If 5. 00 m. L of a 2. 00 M aqueous solution of KCl is diluted to 0. 500 M, what is the final volume of the solution and how much water was added. 2. What is the volume of a 2. 50 M solution required to prepare 500 m. L of a 0. 100 M solution by dilution. 3. If 100. 0 m. L of water was added to 10. 00 m. L of a 4. 00 M aqueous solution of a solute, what is the resulting molarity of the solution. Mi. Vi = Mf. Vf 1. 5. 00 m. L x 2. 00 M = X m. L x 0. 500 M X m. L = (5. 00 m. L x 2. 00 M)/0. 500 M = 20. 0 m. L 5. 00 m. L 20. 0 m. L; Therefore 15. 0 m. L of water was added. 3. Mi = 4. 00 M; Vi = 10. 00 m. L; Vf = 100. 0 + 10. 00 m. L = 110. 0 m. L Mf = ? ; Mf = (10. 00 m. L x 4. 00 M)/(110. 0 m. L) = 0. 364 M

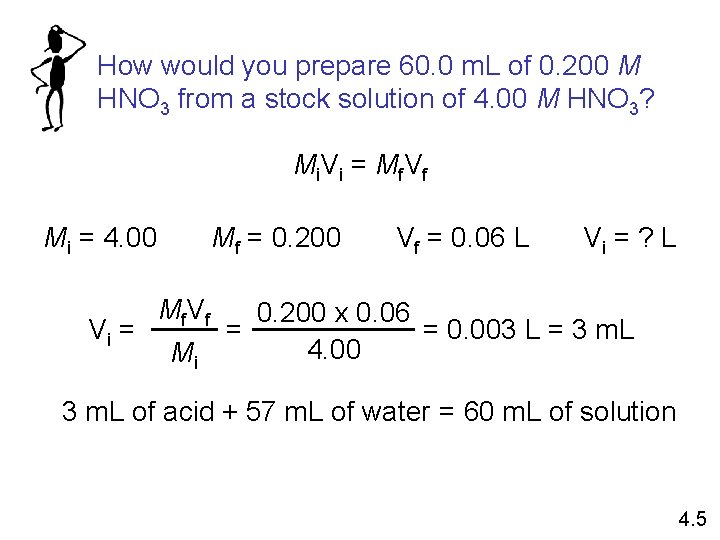
How would you prepare 60. 0 m. L of 0. 200 M HNO 3 from a stock solution of 4. 00 M HNO 3? Mi V i = Mf V f Mi = 4. 00 Vi = Mf = 0. 200 Mf V f Mi Vf = 0. 06 L Vi = ? L 0. 200 x 0. 06 = 0. 003 L = 3 m. L = 4. 00 3 m. L of acid + 57 m. L of water = 60 m. L of solution 4. 5

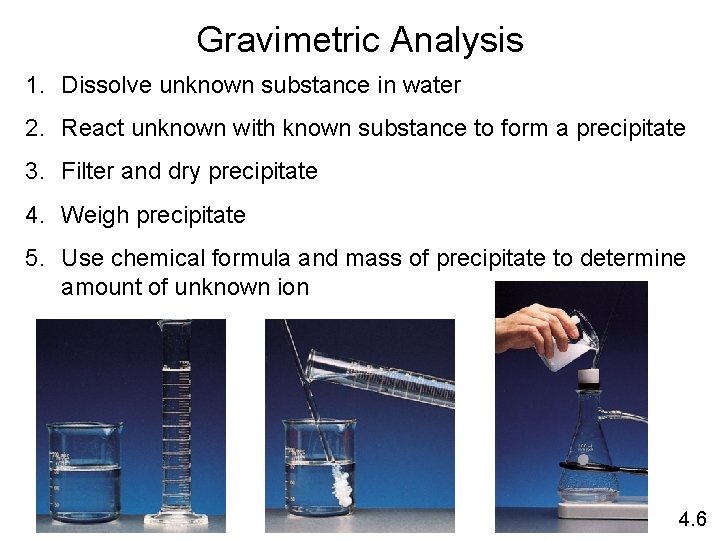
Gravimetric Analysis 1. Dissolve unknown substance in water 2. React unknown with known substance to form a precipitate 3. Filter and dry precipitate 4. Weigh precipitate 5. Use chemical formula and mass of precipitate to determine amount of unknown ion 4. 6

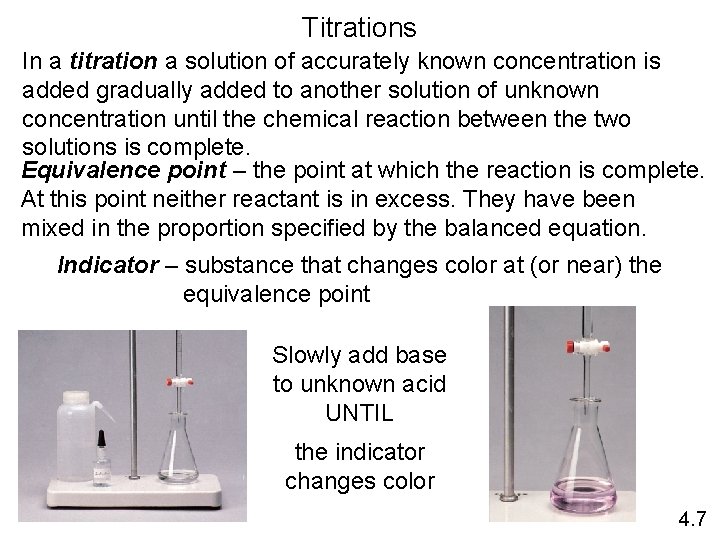
Titrations In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete. At this point neither reactant is in excess. They have been mixed in the proportion specified by the balanced equation. Indicator – substance that changes color at (or near) the equivalence point Slowly add base to unknown acid UNTIL the indicator changes color 4. 7

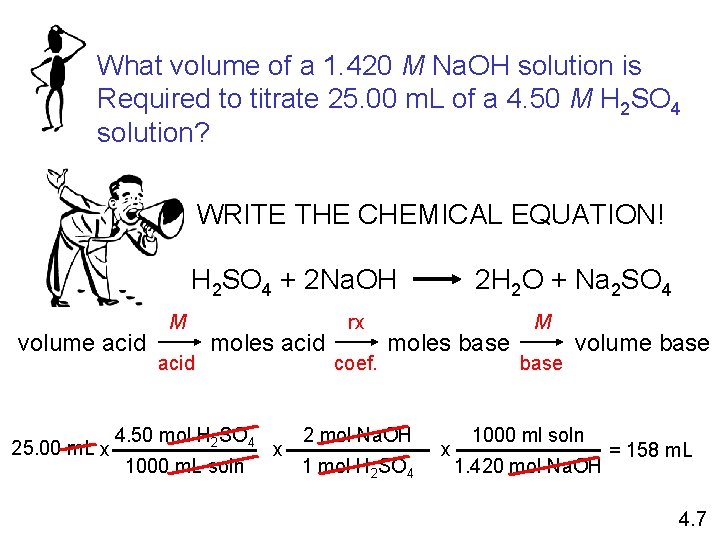
What volume of a 1. 420 M Na. OH solution is Required to titrate 25. 00 m. L of a 4. 50 M H 2 SO 4 solution? WRITE THE CHEMICAL EQUATION! H 2 SO 4 + 2 Na. OH volume acid 25. 00 m. L x M acid moles acid 4. 50 mol H 2 SO 4 1000 m. L soln x rx coef. 2 H 2 O + Na 2 SO 4 moles base 2 mol Na. OH 1 mol H 2 SO 4 x M base volume base 1000 ml soln 1. 420 mol Na. OH = 158 m. L 4. 7

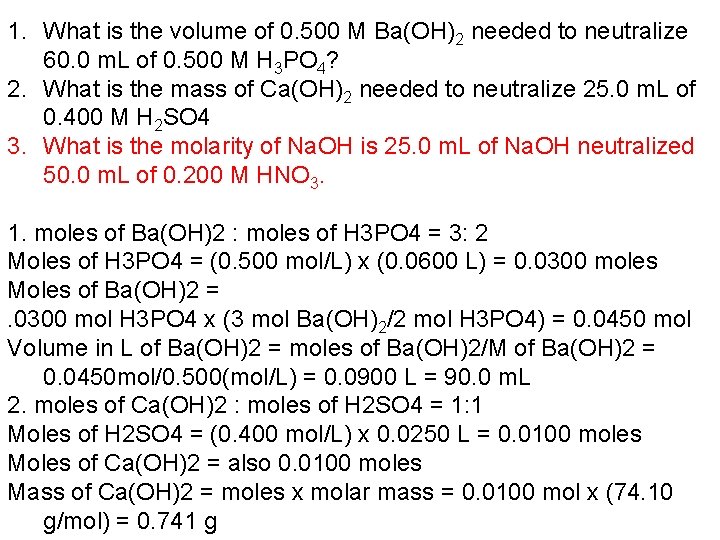
1. What is the volume of 0. 500 M Ba(OH)2 needed to neutralize 60. 0 m. L of 0. 500 M H 3 PO 4? 2. What is the mass of Ca(OH)2 needed to neutralize 25. 0 m. L of 0. 400 M H 2 SO 4 3. What is the molarity of Na. OH is 25. 0 m. L of Na. OH neutralized 50. 0 m. L of 0. 200 M HNO 3. 1. moles of Ba(OH)2 : moles of H 3 PO 4 = 3: 2 Moles of H 3 PO 4 = (0. 500 mol/L) x (0. 0600 L) = 0. 0300 moles Moles of Ba(OH)2 =. 0300 mol H 3 PO 4 x (3 mol Ba(OH)2/2 mol H 3 PO 4) = 0. 0450 mol Volume in L of Ba(OH)2 = moles of Ba(OH)2/M of Ba(OH)2 = 0. 0450 mol/0. 500(mol/L) = 0. 0900 L = 90. 0 m. L 2. moles of Ca(OH)2 : moles of H 2 SO 4 = 1: 1 Moles of H 2 SO 4 = (0. 400 mol/L) x 0. 0250 L = 0. 0100 moles Moles of Ca(OH)2 = also 0. 0100 moles Mass of Ca(OH)2 = moles x molar mass = 0. 0100 mol x (74. 10 g/mol) = 0. 741 g

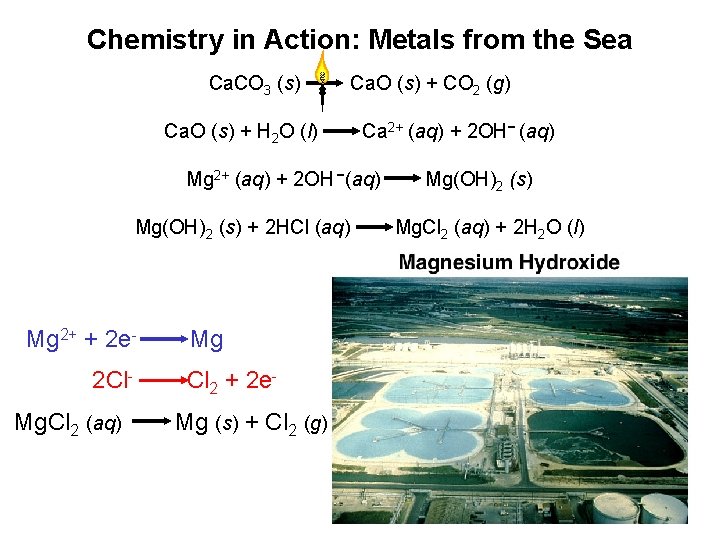
Chemistry in Action: Metals from the Sea Ca. CO 3 (s) Ca. O (s) + CO 2 (g) Ca. O (s) + H 2 O (l) Ca 2+ (aq) + 2 OH- (aq) Mg 2+ (aq) + 2 OH -(aq) Mg(OH)2 (s) + 2 HCl (aq) Mg 2+ + 2 e 2 Cl. Mg. Cl 2 (aq) Mg Cl 2 + 2 e. Mg (s) + Cl 2 (g) Mg(OH)2 (s) Mg. Cl 2 (aq) + 2 H 2 O (l)