Reactions in Aqueous Solution Chapter 4 A solution

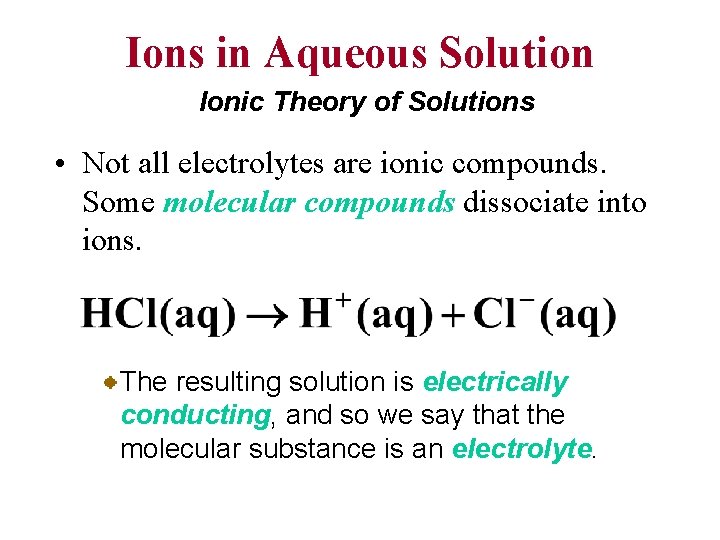
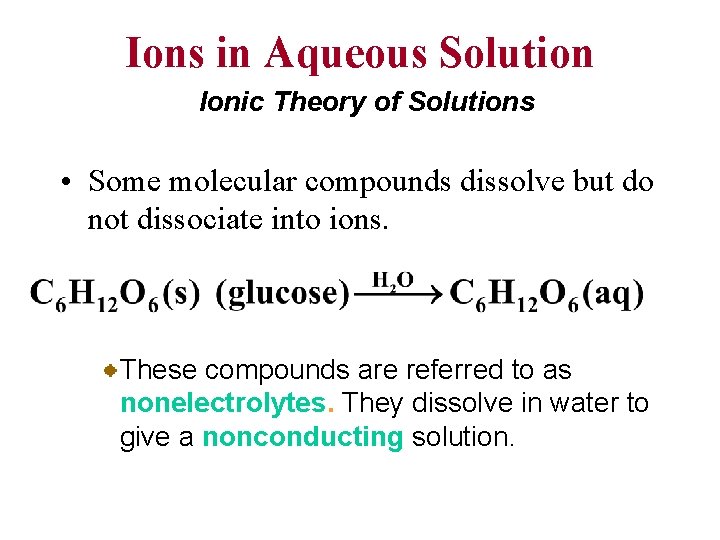


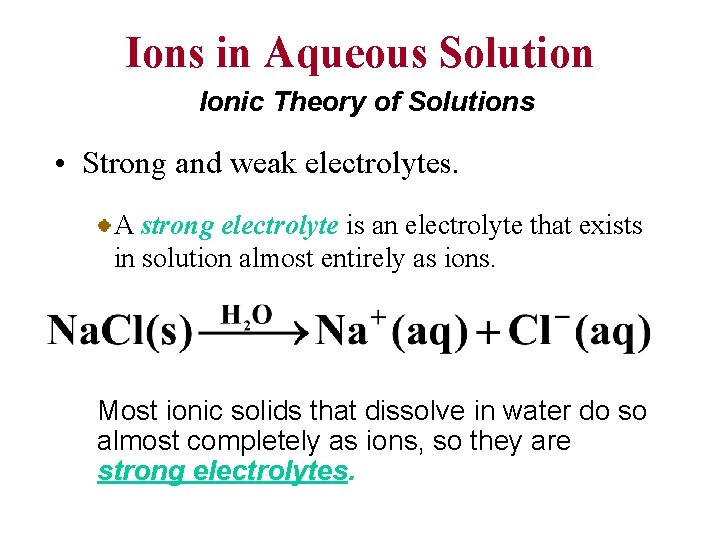
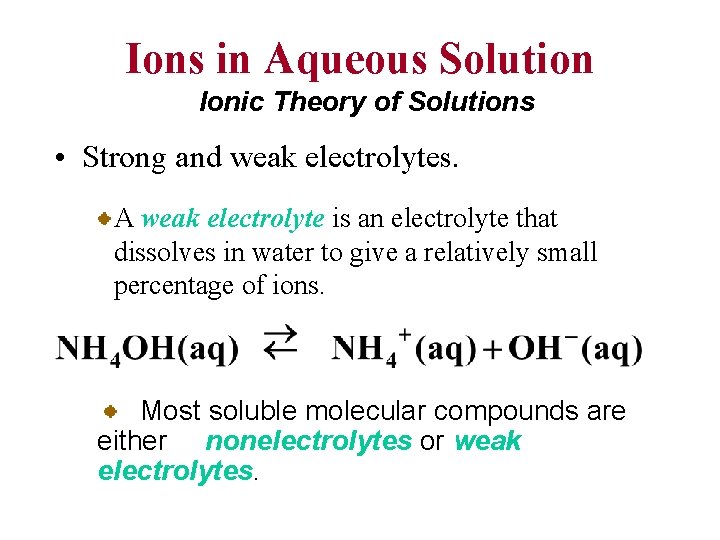



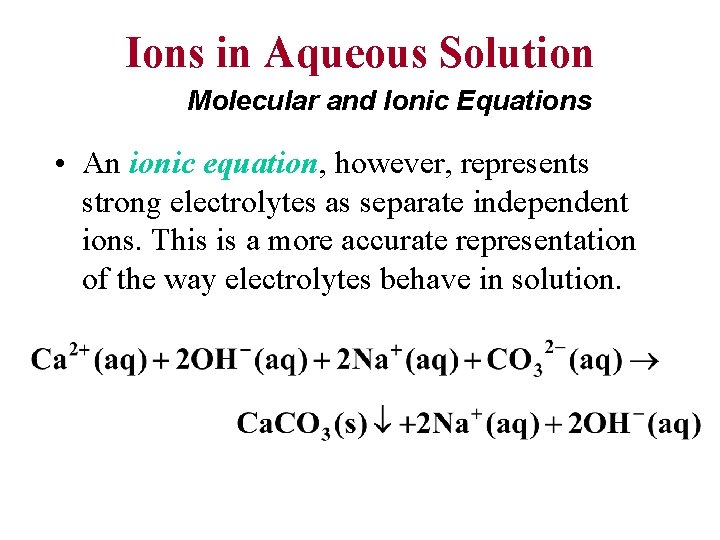
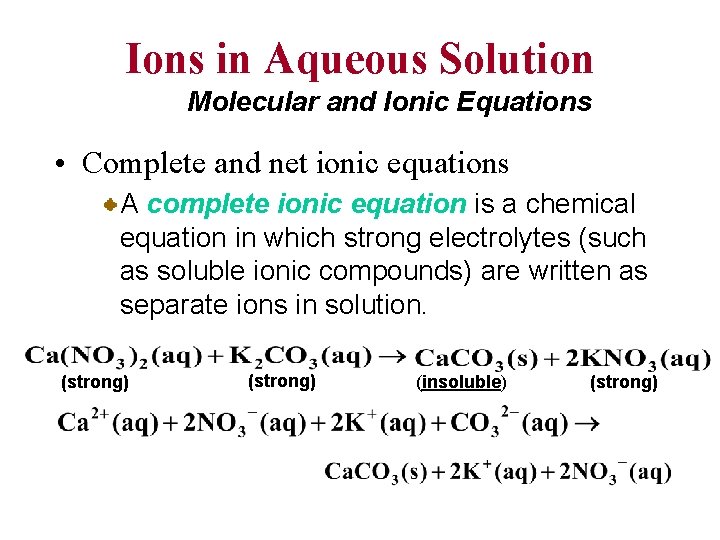
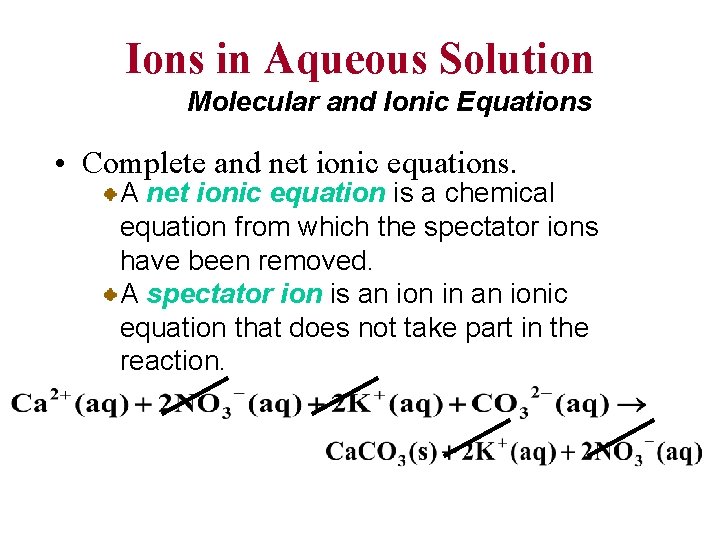

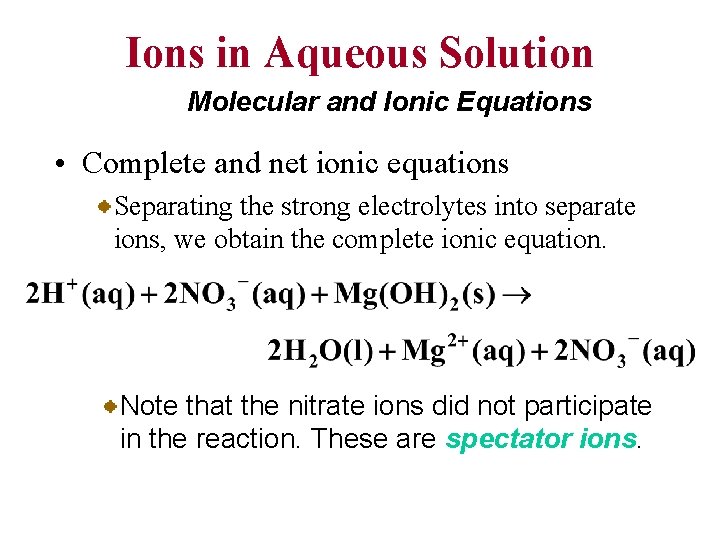
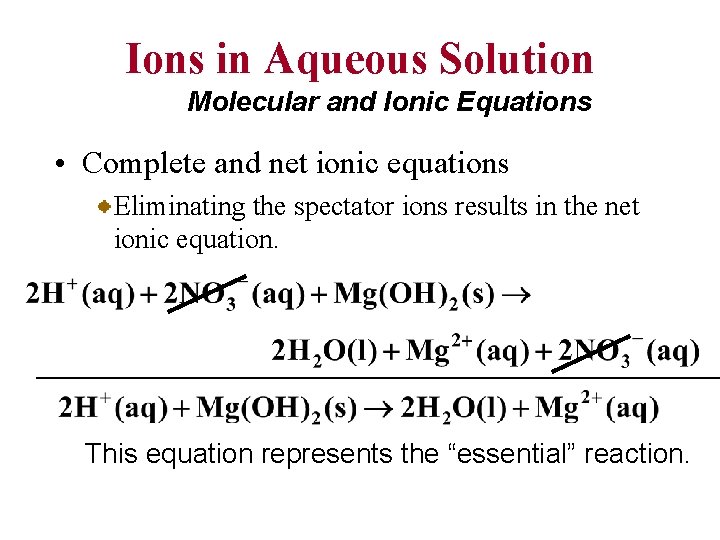

















- Slides: 145

Reactions in Aqueous Solution Chapter 4

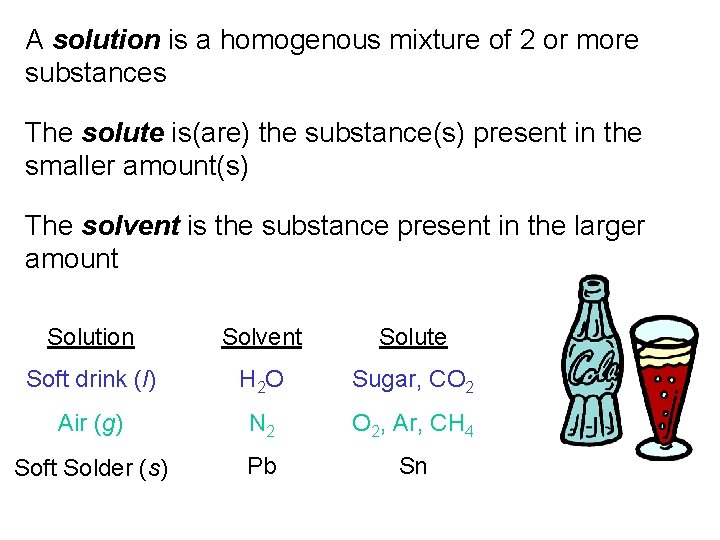
A solution is a homogenous mixture of 2 or more substances The solute is(are) the substance(s) present in the smaller amount(s) The solvent is the substance present in the larger amount Solution Solvent Solute Soft drink (l) H 2 O Sugar, CO 2 Air (g) N 2 O 2, Ar, CH 4 Soft Solder (s) Pb Sn

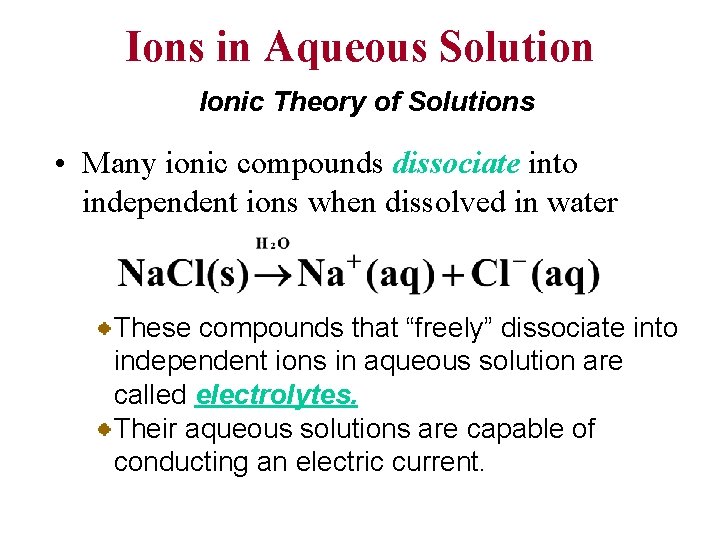
Ions in Aqueous Solution Ionic Theory of Solutions • Many ionic compounds dissociate into independent ions when dissolved in water These compounds that “freely” dissociate into independent ions in aqueous solution are called electrolytes. Their aqueous solutions are capable of conducting an electric current.
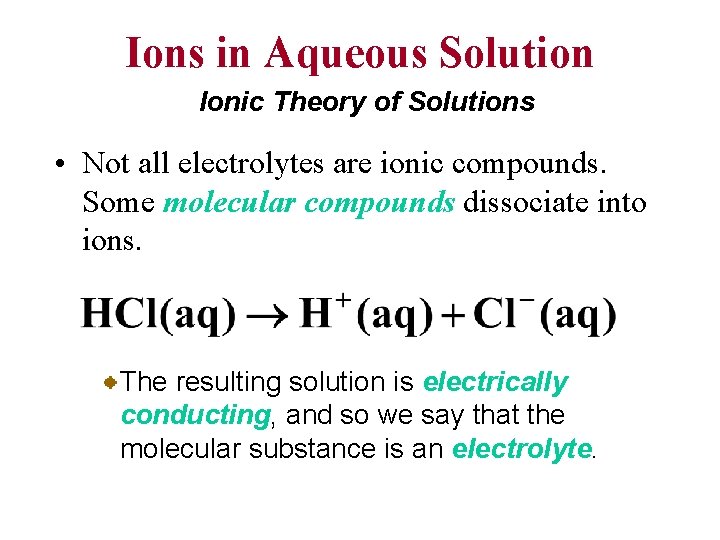
Ions in Aqueous Solution Ionic Theory of Solutions • Not all electrolytes are ionic compounds. Some molecular compounds dissociate into ions. The resulting solution is electrically conducting, and so we say that the molecular substance is an electrolyte.
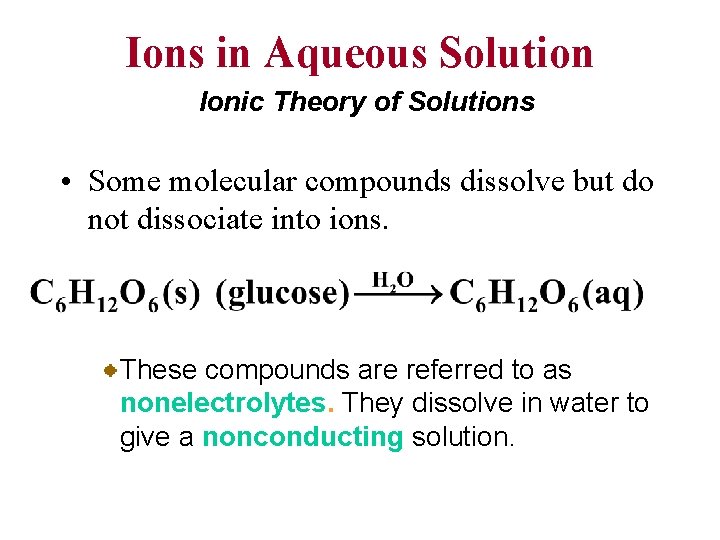
Ions in Aqueous Solution Ionic Theory of Solutions • Some molecular compounds dissolve but do not dissociate into ions. These compounds are referred to as nonelectrolytes. They dissolve in water to give a nonconducting solution.

Ions in Aqueous Solution Ionic Theory of Solutions • Electrolytes are substances that dissolve in water to give an electrically conducting solution. Thus, in general, ionic solids that dissolve in water are electrolytes. Some molecular compounds, such as acids, also dissociate in aqueous solution and are considered electrolytes. .

An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. nonelectrolyte weak electrolyte strong electrolyte

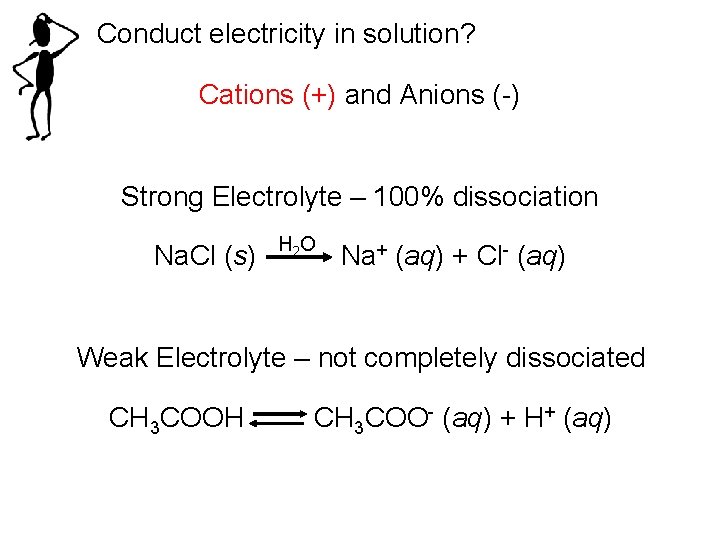
Conduct electricity in solution? Cations (+) and Anions (-) Strong Electrolyte – 100% dissociation Na. Cl (s) H 2 O Na+ (aq) + Cl- (aq) Weak Electrolyte – not completely dissociated CH 3 COOH CH 3 COO- (aq) + H+ (aq)

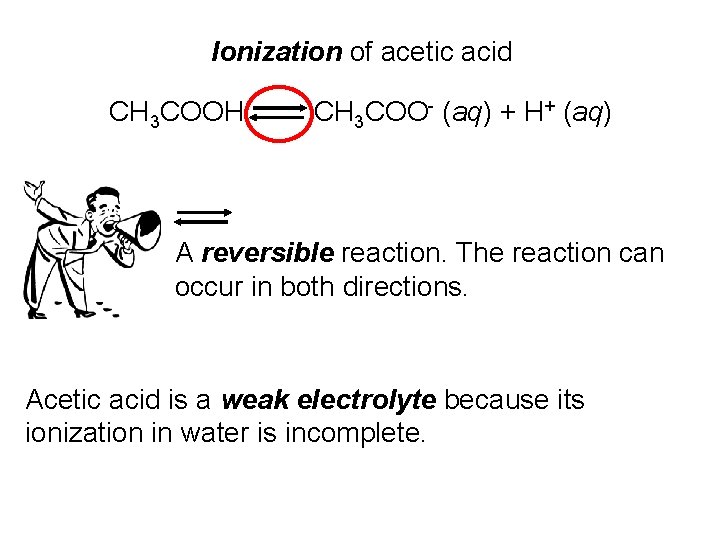
Ionization of acetic acid CH 3 COOH CH 3 COO- (aq) + H+ (aq) A reversible reaction. The reaction can occur in both directions. Acetic acid is a weak electrolyte because its ionization in water is incomplete.
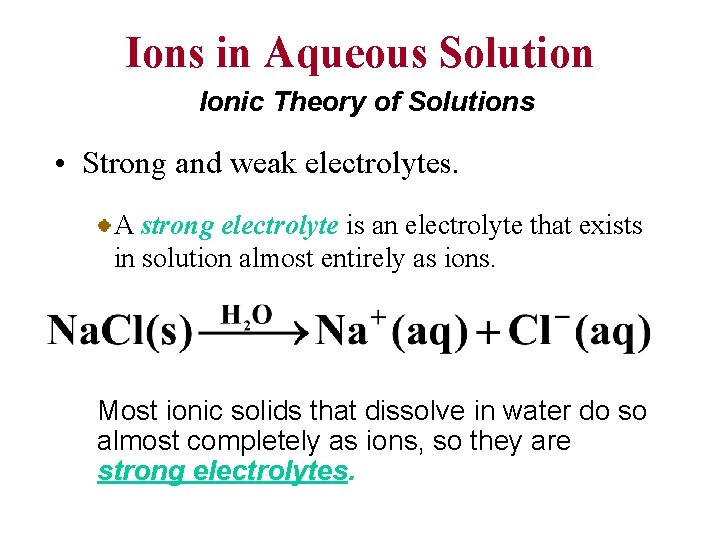
Ions in Aqueous Solution Ionic Theory of Solutions • Strong and weak electrolytes. A strong electrolyte is an electrolyte that exists in solution almost entirely as ions. Most ionic solids that dissolve in water do so almost completely as ions, so they are strong electrolytes.
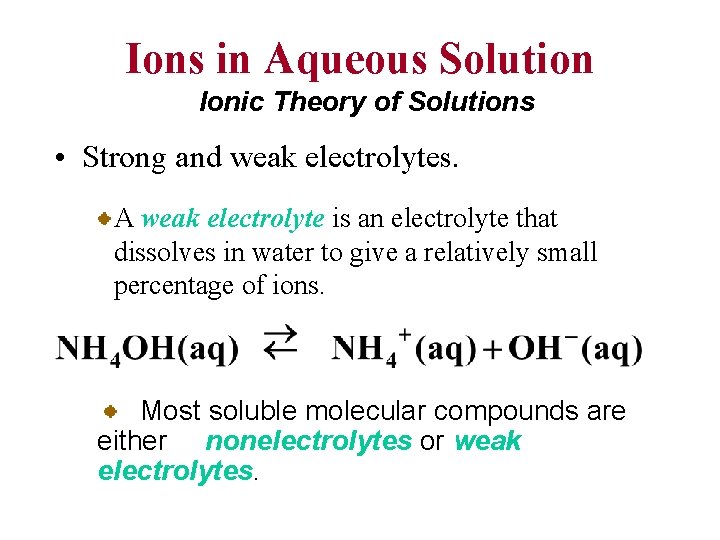
Ions in Aqueous Solution Ionic Theory of Solutions • Strong and weak electrolytes. A weak electrolyte is an electrolyte that dissolves in water to give a relatively small percentage of ions. Most soluble molecular compounds are either nonelectrolytes or weak electrolytes.

Ions in Aqueous Solution Ionic Theory of Solutions Strong and weak electrolytes. Solutions of weak electrolytes contain only a small percentage of ions. We denote this situation by writing the equation with a double arrow.

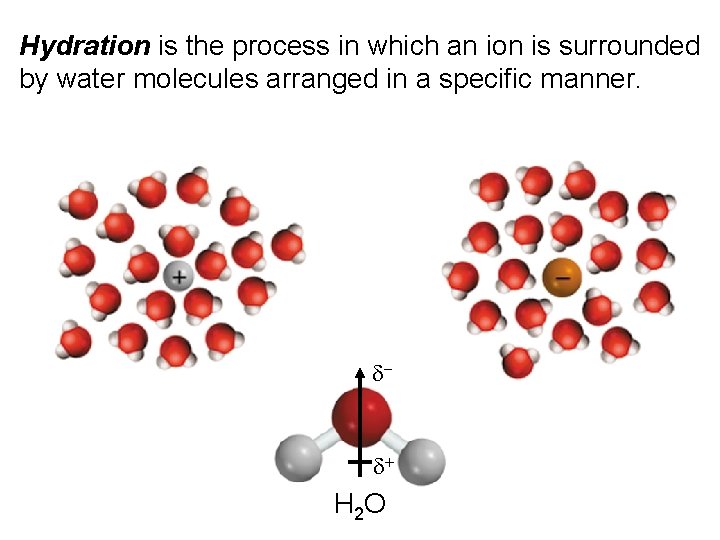
Hydration is the process in which an ion is surrounded by water molecules arranged in a specific manner. d- d+ H 2 O

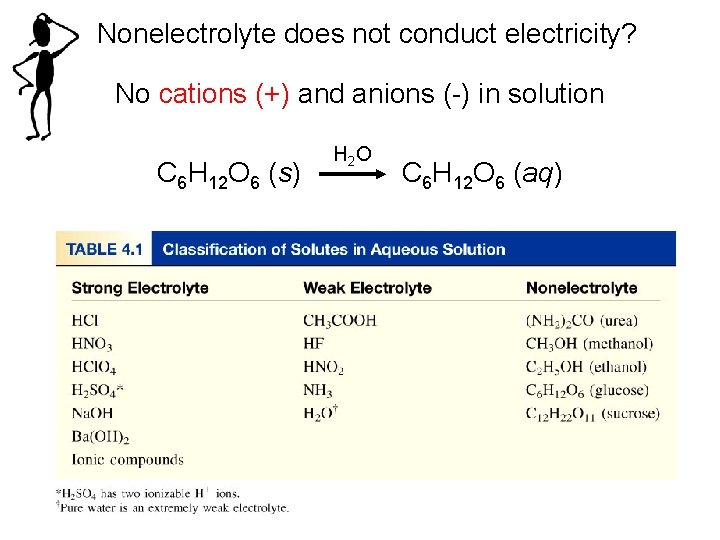
Nonelectrolyte does not conduct electricity? No cations (+) and anions (-) in solution C 6 H 12 O 6 (s) H 2 O C 6 H 12 O 6 (aq)

Types of Chemical Reactions Most of the reactions we will study fall into one of the following categories Precipitation Reactions Acid-Base Reactions Oxidation-Reduction Reactions

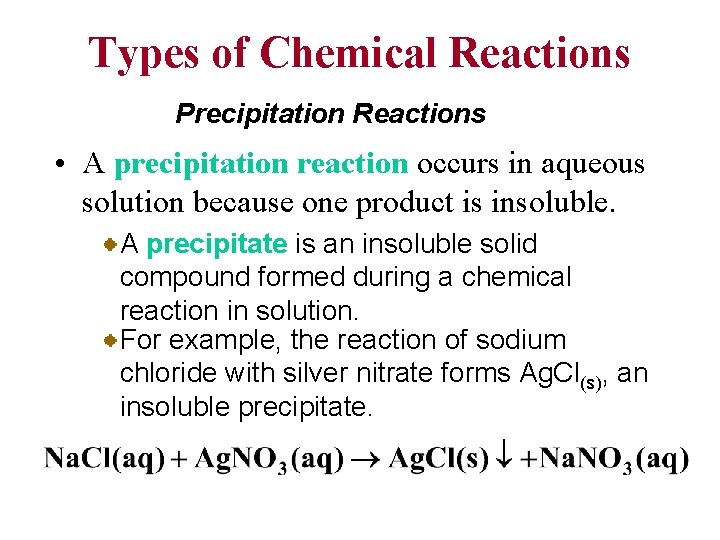
Types of Chemical Reactions Precipitation Reactions • A precipitation reaction occurs in aqueous solution because one product is insoluble. A precipitate is an insoluble solid compound formed during a chemical reaction in solution. For example, the reaction of sodium chloride with silver nitrate forms Ag. Cl(s), an insoluble precipitate.

Reaction of magnesium chloride and silver nitrate.

Figure 4. 4

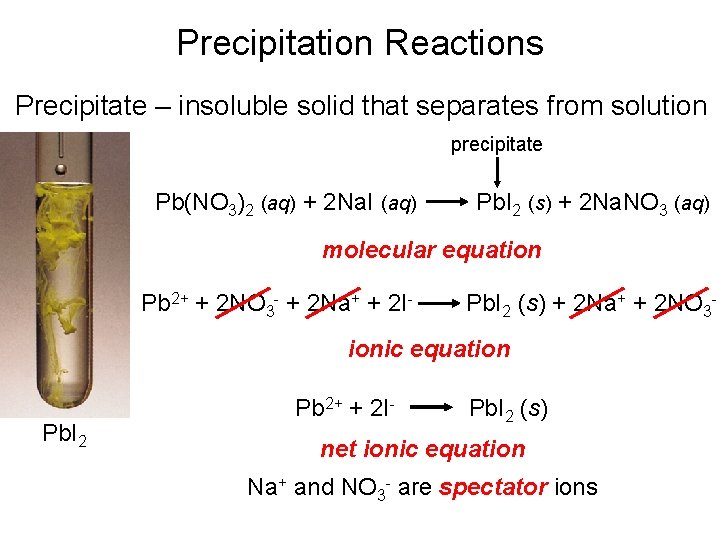
Precipitation Reactions Precipitate – insoluble solid that separates from solution precipitate Pb(NO 3)2 (aq) + 2 Na. I (aq) Pb. I 2 (s) + 2 Na. NO 3 (aq) molecular equation Pb 2+ + 2 NO 3 - + 2 Na+ + 2 I- Pb. I 2 (s) + 2 Na+ + 2 NO 3 - ionic equation Pb. I 2 Pb 2+ + 2 I- Pb. I 2 (s) net ionic equation Na+ and NO 3 - are spectator ions

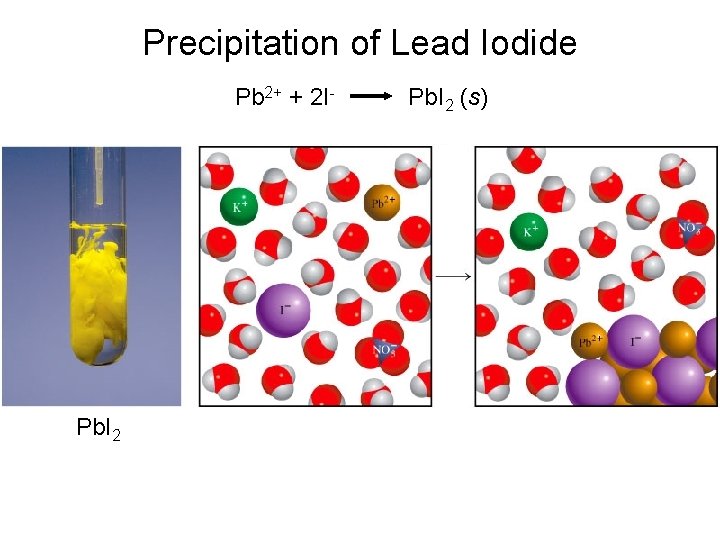
Precipitation of Lead Iodide Pb 2+ + 2 I- Pb. I 2 (s)

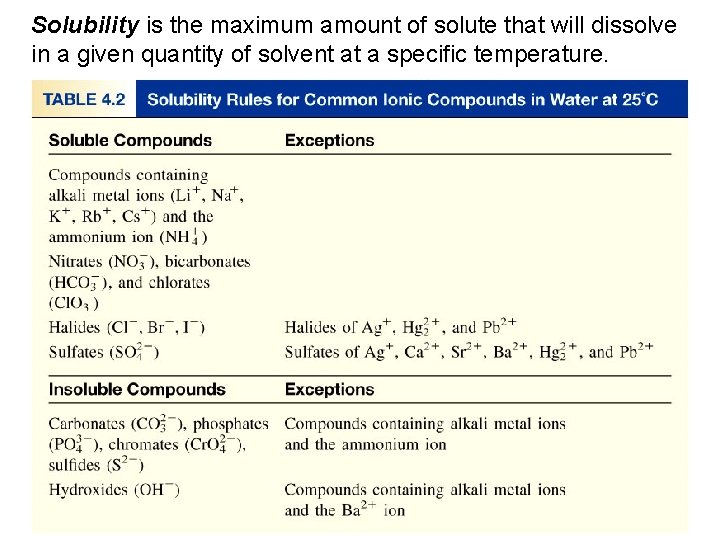
Types of Chemical Reactions Precipitation Reactions • Solubility rules Substances vary widely in their solubility, or ability to dissolve, in water. For example, Na. Cl is very soluble in water whereas calcium carbonate, Ca. CO 3, is insoluble in water.

Types of Chemical Reactions Precipitation Reactions • Predicting Precipitation Reactions. To predict whether a precipitate will form, we need to look at potential insoluble products. Next slide - Table lists eight solubility rules for ionic compounds. These rules apply to the most common ionic compounds.

Solubility is the maximum amount of solute that will dissolve in a given quantity of solvent at a specific temperature.

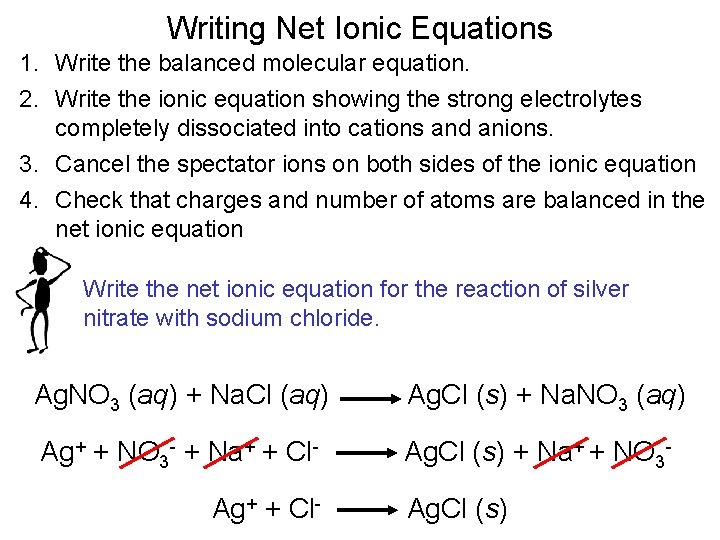
Writing Net Ionic Equations 1. Write the balanced molecular equation. 2. Write the ionic equation showing the strong electrolytes completely dissociated into cations and anions. 3. Cancel the spectator ions on both sides of the ionic equation 4. Check that charges and number of atoms are balanced in the net ionic equation Write the net ionic equation for the reaction of silver nitrate with sodium chloride. Ag. NO 3 (aq) + Na. Cl (aq) Ag. Cl (s) + Na. NO 3 (aq) Ag+ + NO 3 - + Na+ + Cl- Ag. Cl (s) + Na+ + NO 3 - Ag+ + Cl- Ag. Cl (s)

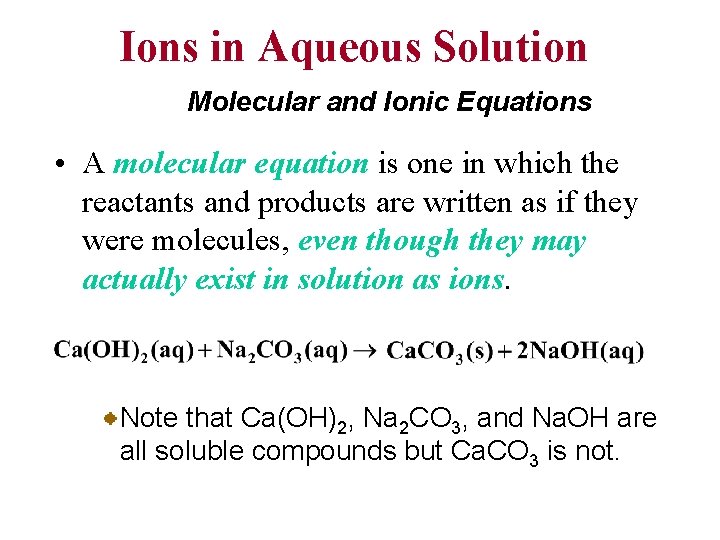
Ions in Aqueous Solution Molecular and Ionic Equations • A molecular equation is one in which the reactants and products are written as if they were molecules, even though they may actually exist in solution as ions. Note that Ca(OH)2, Na 2 CO 3, and Na. OH are all soluble compounds but Ca. CO 3 is not.
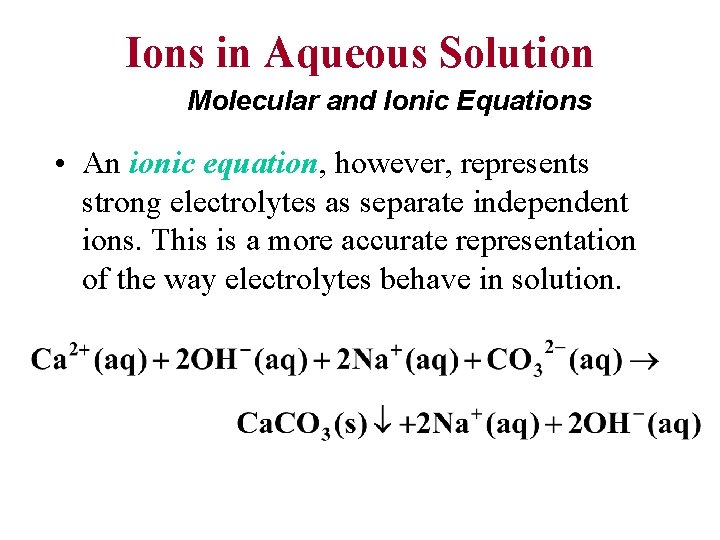
Ions in Aqueous Solution Molecular and Ionic Equations • An ionic equation, however, represents strong electrolytes as separate independent ions. This is a more accurate representation of the way electrolytes behave in solution.
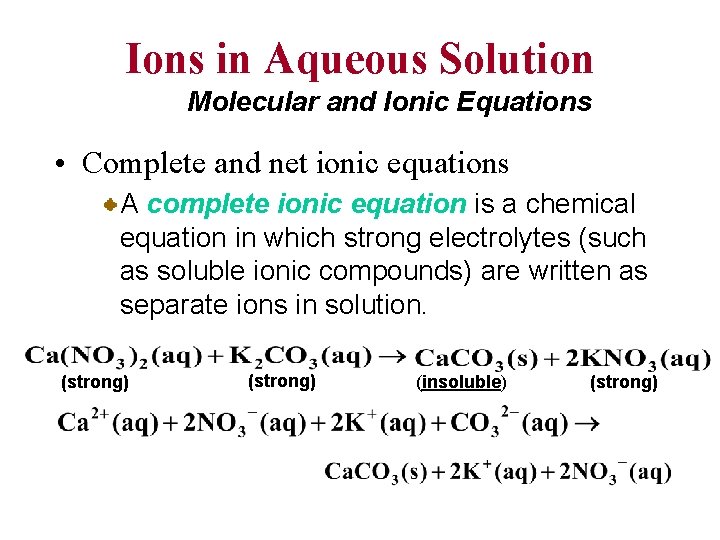
Ions in Aqueous Solution Molecular and Ionic Equations • Complete and net ionic equations A complete ionic equation is a chemical equation in which strong electrolytes (such as soluble ionic compounds) are written as separate ions in solution. (strong) (insoluble) (strong)
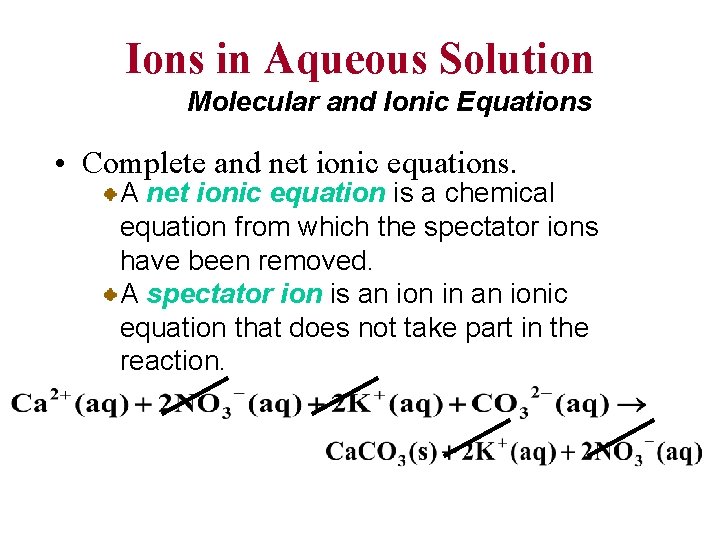
Ions in Aqueous Solution Molecular and Ionic Equations • Complete and net ionic equations. A net ionic equation is a chemical equation from which the spectator ions have been removed. A spectator ion is an ion in an ionic equation that does not take part in the reaction.

Ions in Aqueous Solution Molecular and Ionic Equations • Complete and net ionic equations Let’s try an example. First, we start with a molecular equation. Nitric acid, HNO 3, and magnesium nitrate, Mg(NO 3)2, are both strong electrolytes.
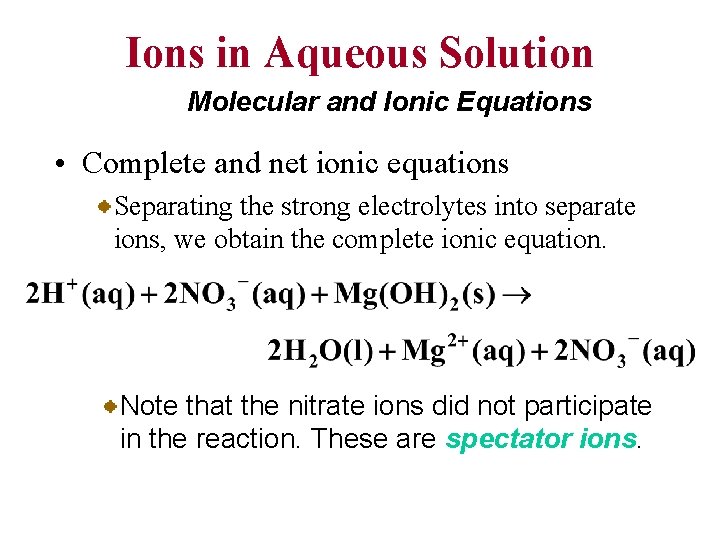
Ions in Aqueous Solution Molecular and Ionic Equations • Complete and net ionic equations Separating the strong electrolytes into separate ions, we obtain the complete ionic equation. Note that the nitrate ions did not participate in the reaction. These are spectator ions.
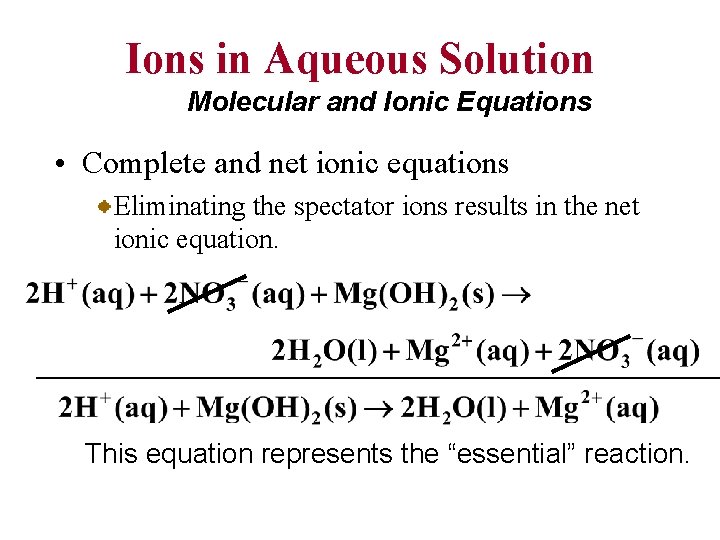
Ions in Aqueous Solution Molecular and Ionic Equations • Complete and net ionic equations Eliminating the spectator ions results in the net ionic equation. This equation represents the “essential” reaction.

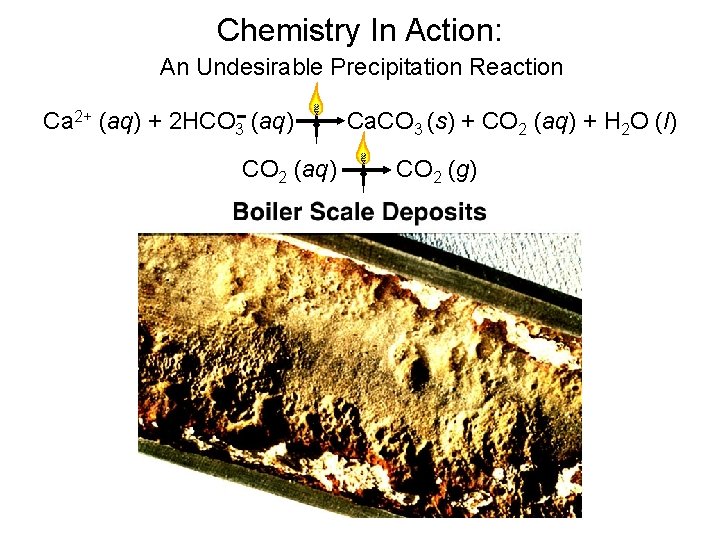
Chemistry In Action: An Undesirable Precipitation Reaction Ca 2+ (aq) + 2 HCO 3 - (aq) CO 2 (aq) Ca. CO 3 (s) + CO 2 (aq) + H 2 O (l) CO 2 (g)

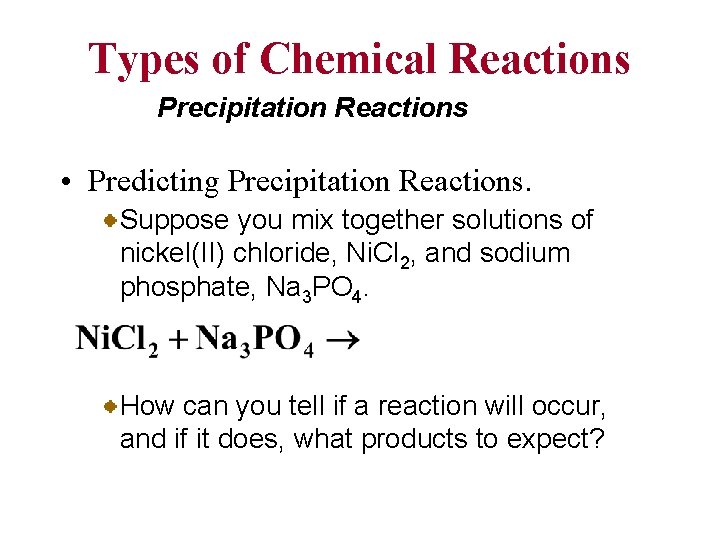
Types of Chemical Reactions Precipitation Reactions • Predicting Precipitation Reactions. Suppose you mix together solutions of nickel(II) chloride, Ni. Cl 2, and sodium phosphate, Na 3 PO 4. How can you tell if a reaction will occur, and if it does, what products to expect?

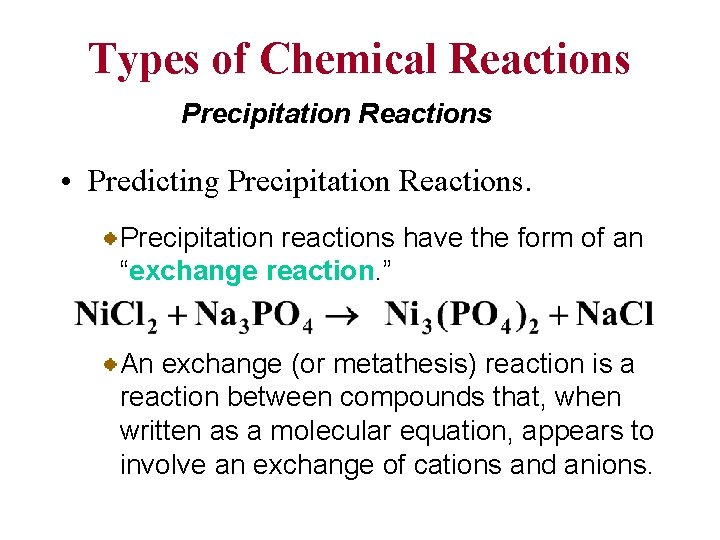
Types of Chemical Reactions Precipitation Reactions • Predicting Precipitation Reactions. Precipitation reactions have the form of an “exchange reaction. ” An exchange (or metathesis) reaction is a reaction between compounds that, when written as a molecular equation, appears to involve an exchange of cations and anions.

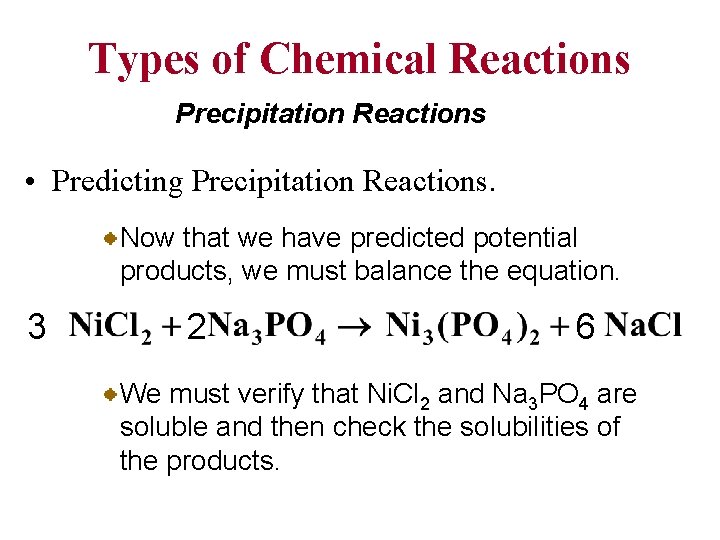
Types of Chemical Reactions Precipitation Reactions • Predicting Precipitation Reactions. Now that we have predicted potential products, we must balance the equation. 3 2 6 We must verify that Ni. Cl 2 and Na 3 PO 4 are soluble and then check the solubilities of the products.

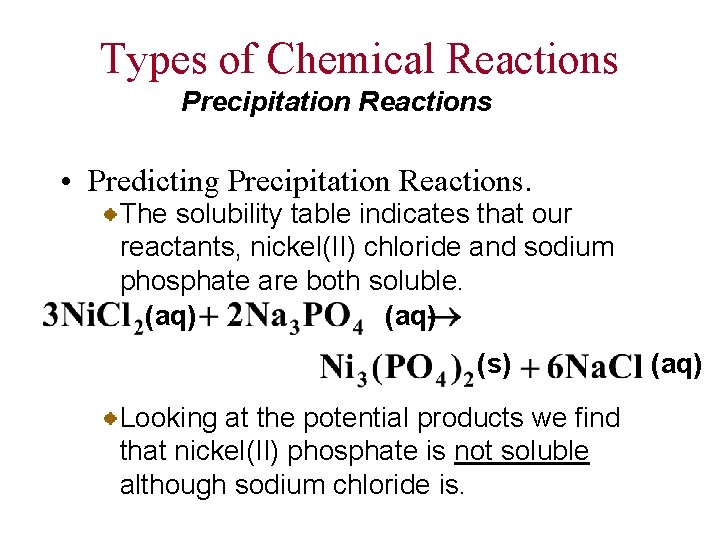
Types of Chemical Reactions Precipitation Reactions • Predicting Precipitation Reactions. The solubility table indicates that our reactants, nickel(II) chloride and sodium phosphate are both soluble. (aq) (s) Looking at the potential products we find that nickel(II) phosphate is not soluble although sodium chloride is. (aq)

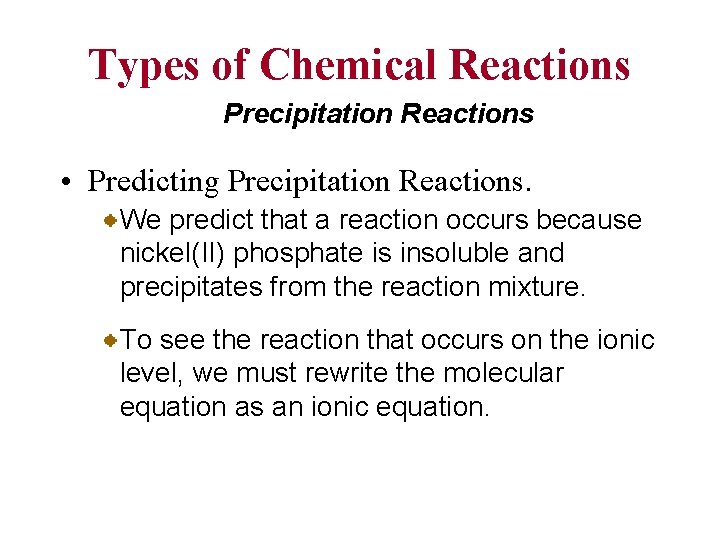
Types of Chemical Reactions Precipitation Reactions • Predicting Precipitation Reactions. We predict that a reaction occurs because nickel(II) phosphate is insoluble and precipitates from the reaction mixture. To see the reaction that occurs on the ionic level, we must rewrite the molecular equation as an ionic equation.

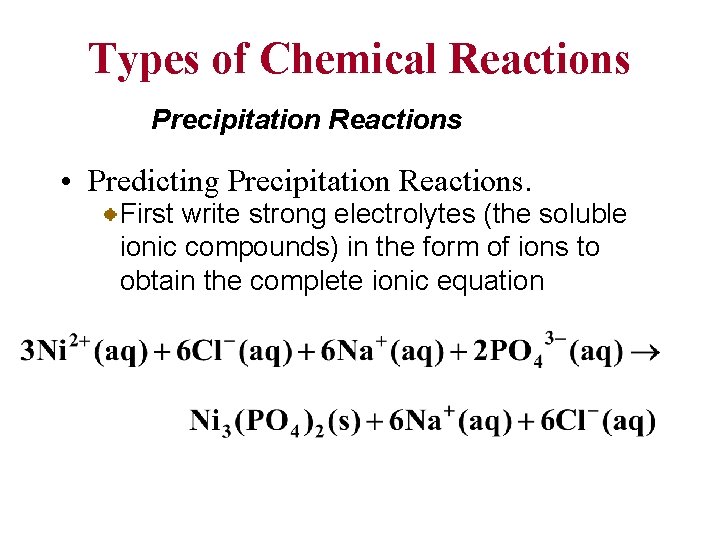
Types of Chemical Reactions Precipitation Reactions • Predicting Precipitation Reactions. First write strong electrolytes (the soluble ionic compounds) in the form of ions to obtain the complete ionic equation

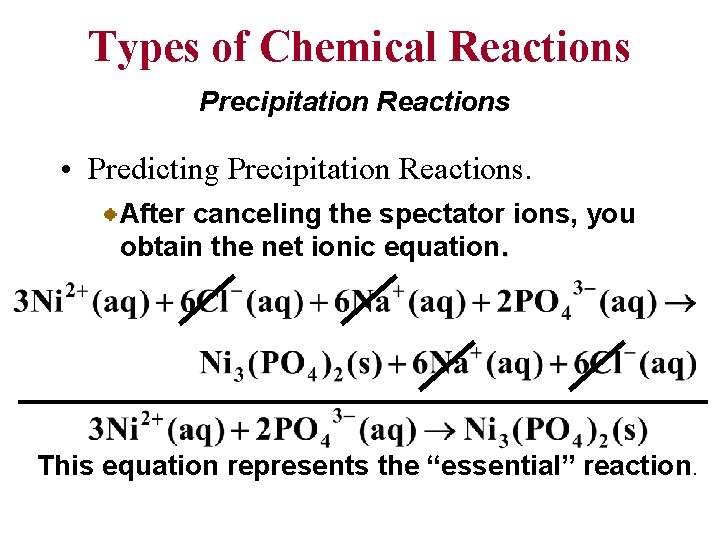
Types of Chemical Reactions Precipitation Reactions • Predicting Precipitation Reactions. After canceling the spectator ions, you obtain the net ionic equation. This equation represents the “essential” reaction.

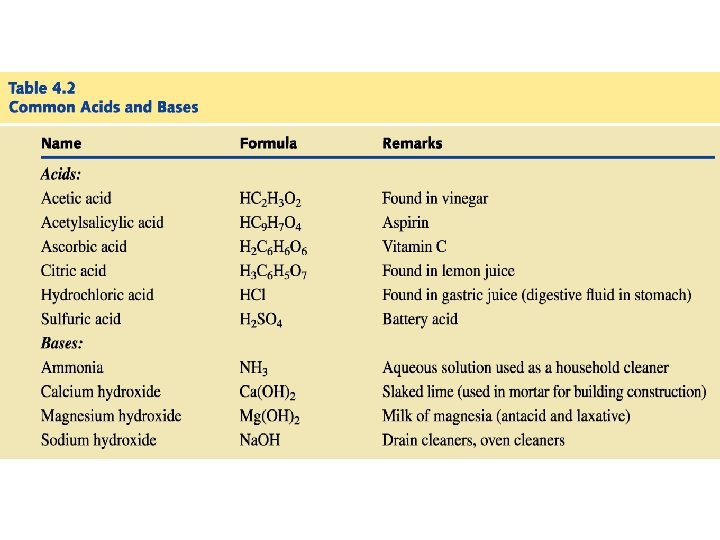
Acids Have a sour taste. Vinegar owes its taste to acetic acid. Citrus fruits contain citric acid. Cause color changes in plant dyes. React with certain metals to produce hydrogen gas. 2 HCl (aq) + Mg (s) Mg. Cl 2 (aq) + H 2 (g) React with carbonates and bicarbonates to produce carbon dioxide gas 2 HCl (aq) + Ca. CO 3 (s) Ca. Cl 2 (aq) + CO 2 (g) + H 2 O (l) Aqueous acid solutions conduct electricity.

Bases Have a bitter taste. Feel slippery. Many soaps contain bases. Cause color changes in plant dyes. Aqueous base solutions conduct electricity.

Pg 129


Household acids and bases. Photo courtesy of American Color.

Types of Chemical Reactions • Acid-Base Reactions Acids and bases are some of the most important electrolytes. They can cause color changes in certain dyes called acid-base indicators. Household acids and bases. Red cabbage juice as an acid-base indicator.

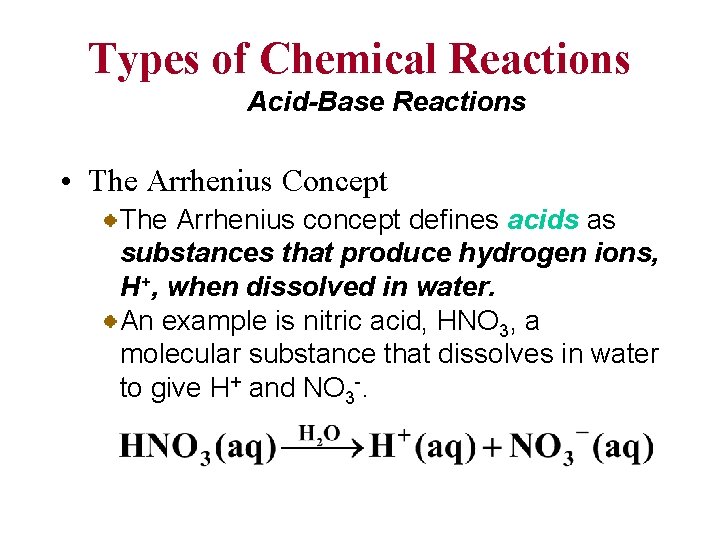
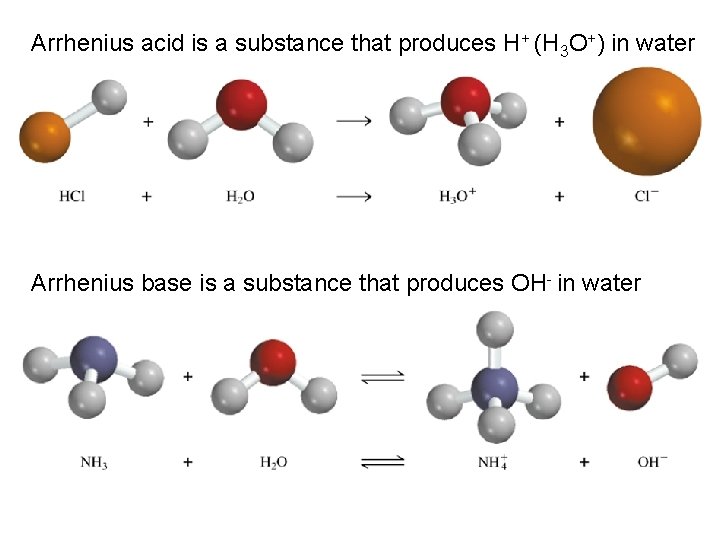
Types of Chemical Reactions Acid-Base Reactions • The Arrhenius Concept The Arrhenius concept defines acids as substances that produce hydrogen ions, H+, when dissolved in water. An example is nitric acid, HNO 3, a molecular substance that dissolves in water to give H+ and NO 3 -.

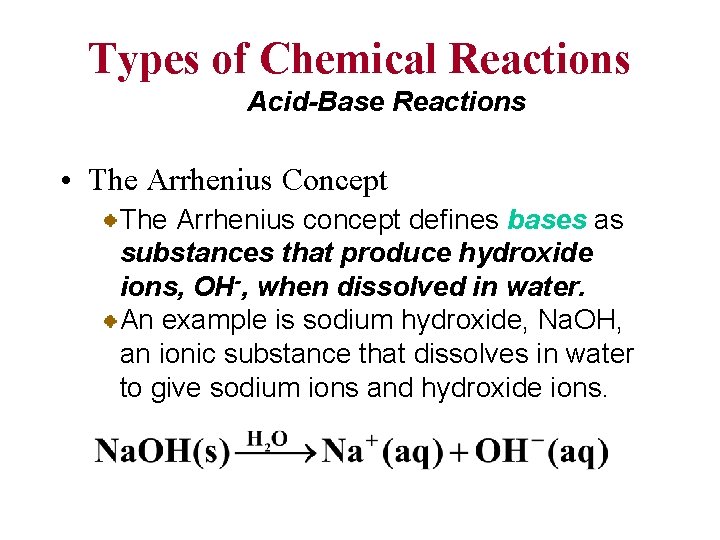
Types of Chemical Reactions Acid-Base Reactions • The Arrhenius Concept The Arrhenius concept defines bases as substances that produce hydroxide ions, OH-, when dissolved in water. An example is sodium hydroxide, Na. OH, an ionic substance that dissolves in water to give sodium ions and hydroxide ions.

Arrhenius acid is a substance that produces H+ (H 3 O+) in water Arrhenius base is a substance that produces OH- in water

Hydronium ion, hydrated proton, H 3 O+

Types of Chemical Reactions Acid-Base Reactions • The Arrhenius Concept The molecular substance ammonia, NH 3, is a base in the Arrhenius view, because it yields hydroxide ions when it reacts with water.

Types of Chemical Reactions Acid-Base Reactions • The Brønsted-Lowry Concept The Brønsted-Lowry concept of acids and bases involves the transfer of a proton (H+) from the acid to the base. In this view, acid-base reactions are proton-transfer reactions.

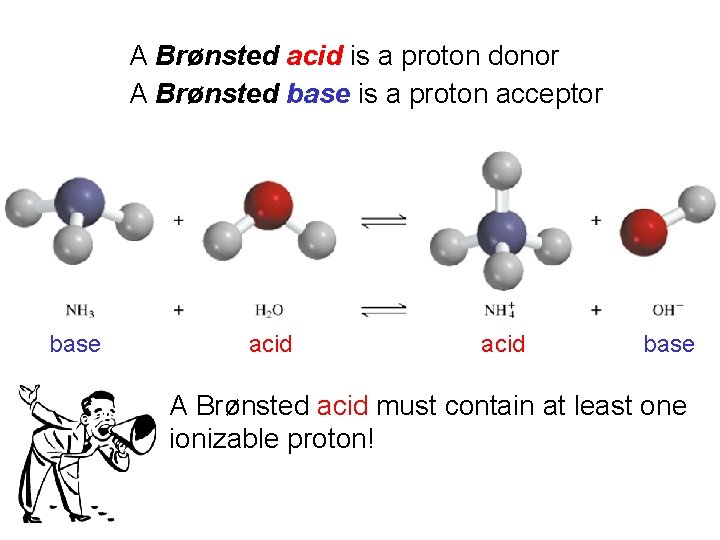
A Brønsted acid is a proton donor A Brønsted base is a proton acceptor base acid base A Brønsted acid must contain at least one ionizable proton!

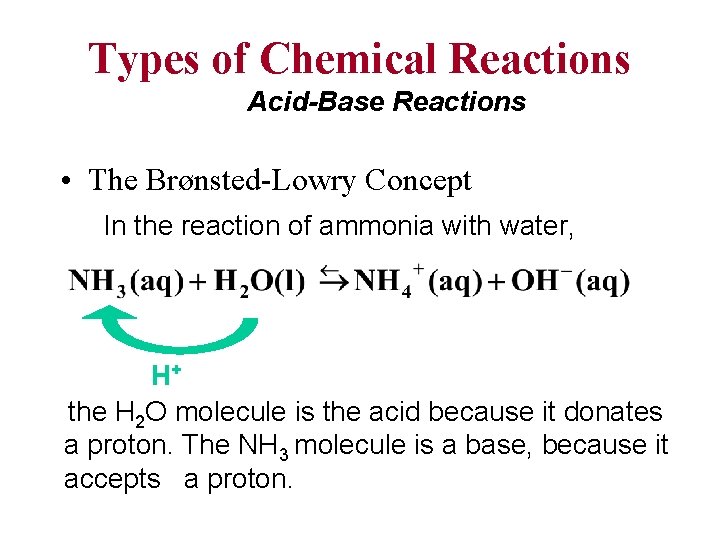
Types of Chemical Reactions Acid-Base Reactions • The Brønsted-Lowry Concept The Brønsted-Lowry concept defines an acid as the species (molecule or ion) that donates a proton (H+) to another species in a proton-transfer reaction. A base is defined as the species (molecule or ion) that accepts the proton (H+) in a proton-transfer reaction.

Types of Chemical Reactions Acid-Base Reactions • The Brønsted-Lowry Concept In the reaction of ammonia with water, H+ the H 2 O molecule is the acid because it donates a proton. The NH 3 molecule is a base, because it accepts a proton.

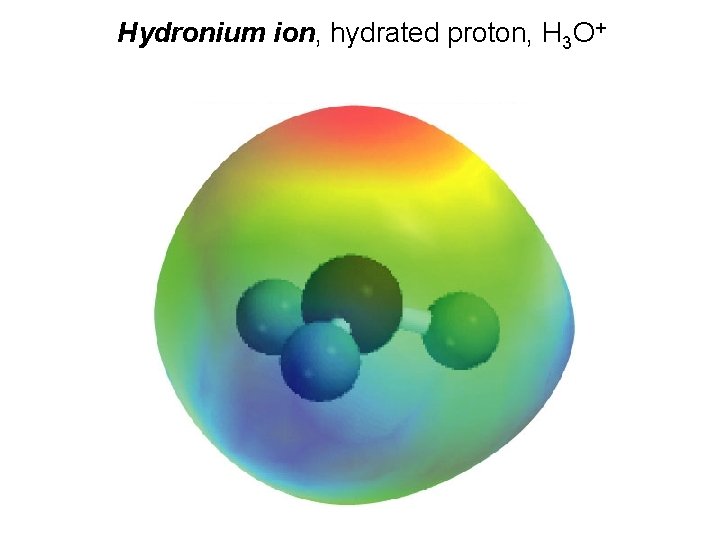
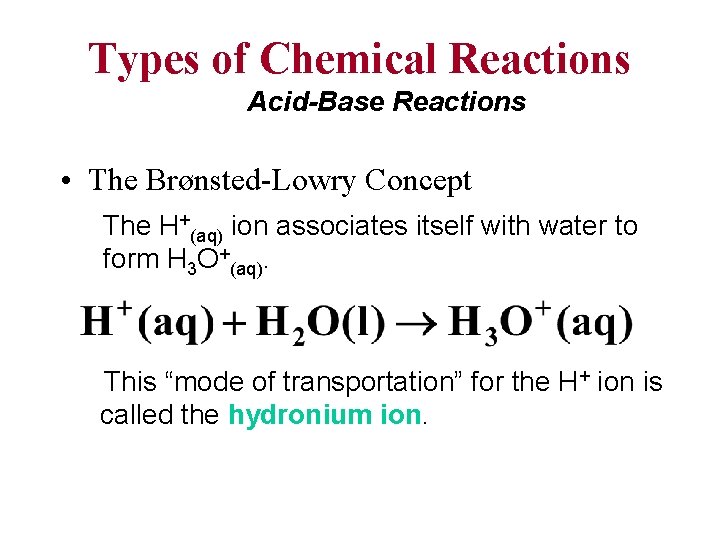
Types of Chemical Reactions Acid-Base Reactions • The Brønsted-Lowry Concept The H+(aq) ion associates itself with water to form H 3 O+(aq). This “mode of transportation” for the H+ ion is called the hydronium ion.

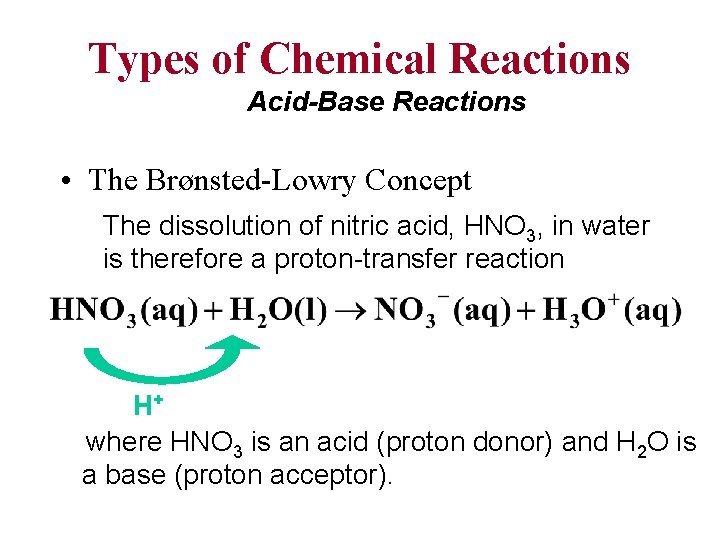
Types of Chemical Reactions Acid-Base Reactions • The Brønsted-Lowry Concept The dissolution of nitric acid, HNO 3, in water is therefore a proton-transfer reaction H+ where HNO 3 is an acid (proton donor) and H 2 O is a base (proton acceptor).

Types of Chemical Reactions Acid-Base Reactions • In summary, the Arrhenius concept and the Brønsted-Lowry concept are essentially the same in aqueous solution. – The Arrhenius concept acid: proton (H+) donor base: hydroxide ion (OH-) donor

Types of Chemical Reactions Acid-Base Reactions • In summary, the Arrhenius concept and the Brønsted-Lowry concept are essentially the same in aqueous solution. – The Brønsted-Lowry concept acid: proton (H+) donor base: proton (H+) acceptor

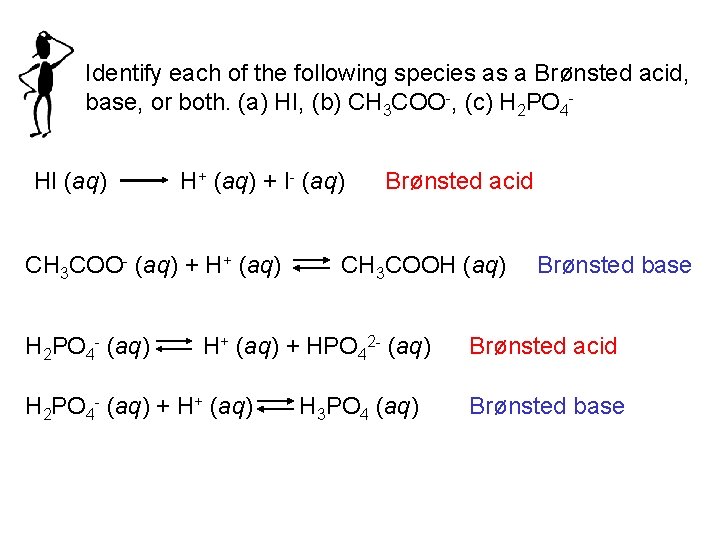
Identify each of the following species as a Brønsted acid, base, or both. (a) HI, (b) CH 3 COO-, (c) H 2 PO 4 HI (aq) H+ (aq) + I- (aq) CH 3 COO- (aq) + H+ (aq) H 2 PO 4 - (aq) Brønsted acid CH 3 COOH (aq) H+ (aq) + HPO 42 - (aq) H 2 PO 4 - (aq) + H+ (aq) H 3 PO 4 (aq) Brønsted base Brønsted acid Brønsted base

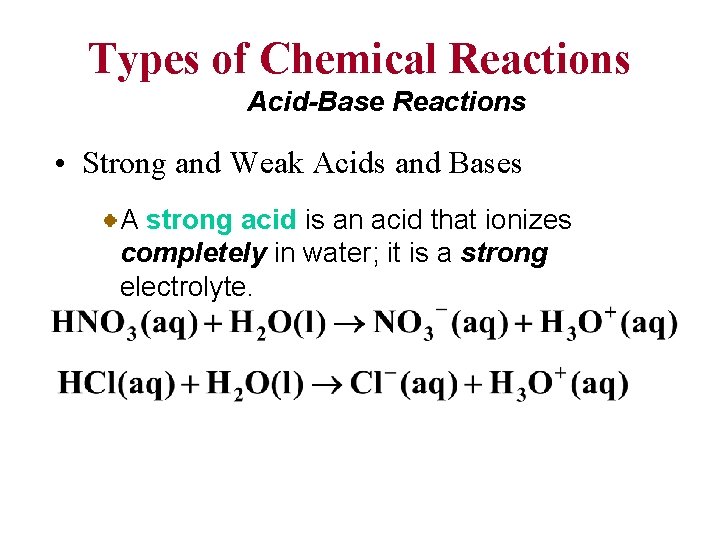
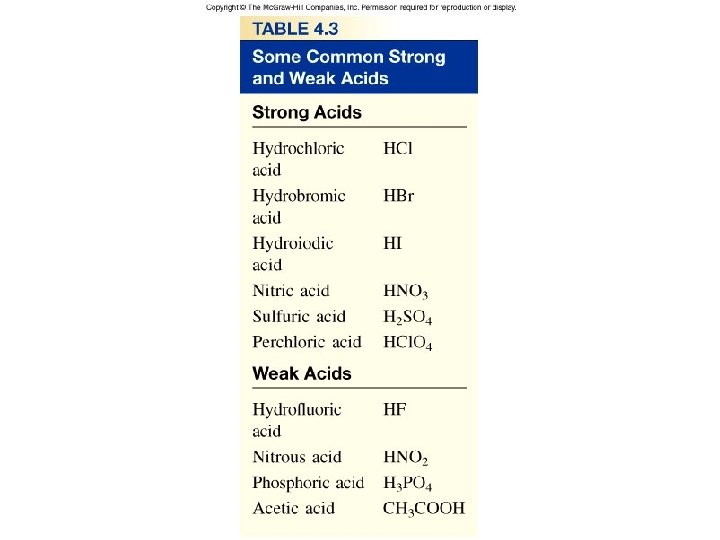
Types of Chemical Reactions Acid-Base Reactions • Strong and Weak Acids and Bases A strong acid is an acid that ionizes completely in water; it is a strong electrolyte.

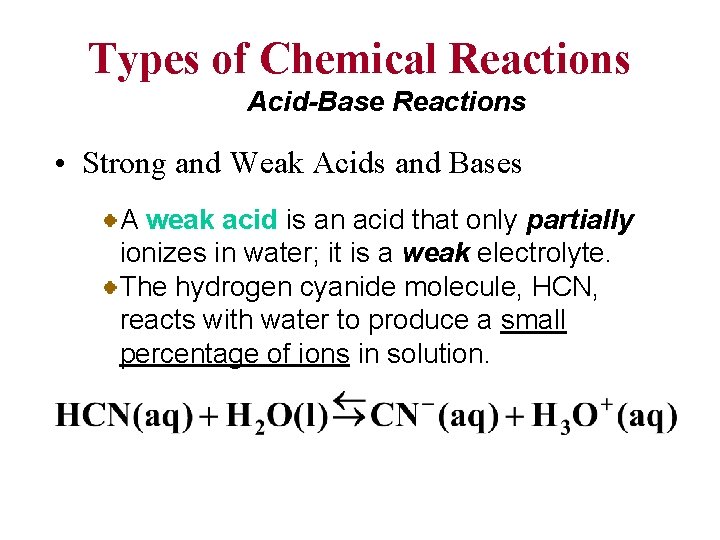
Types of Chemical Reactions Acid-Base Reactions • Strong and Weak Acids and Bases A weak acid is an acid that only partially ionizes in water; it is a weak electrolyte. The hydrogen cyanide molecule, HCN, reacts with water to produce a small percentage of ions in solution.

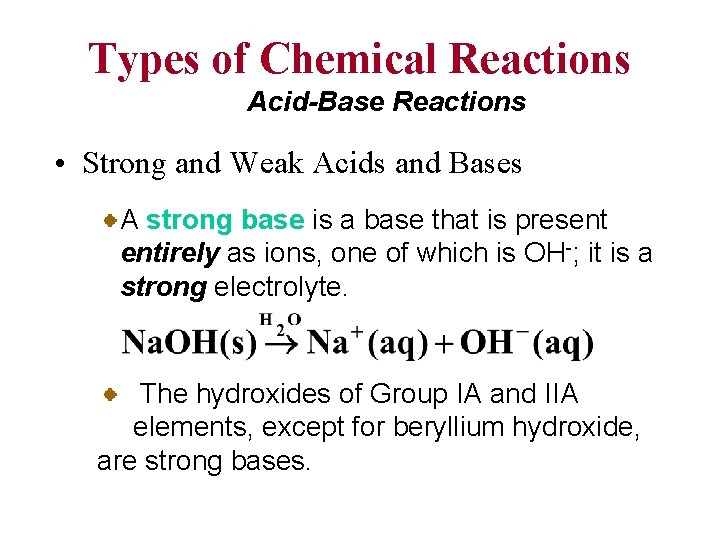
Types of Chemical Reactions Acid-Base Reactions • Strong and Weak Acids and Bases A strong base is a base that is present entirely as ions, one of which is OH-; it is a strong electrolyte. The hydroxides of Group IA and IIA elements, except for beryllium hydroxide, are strong bases.

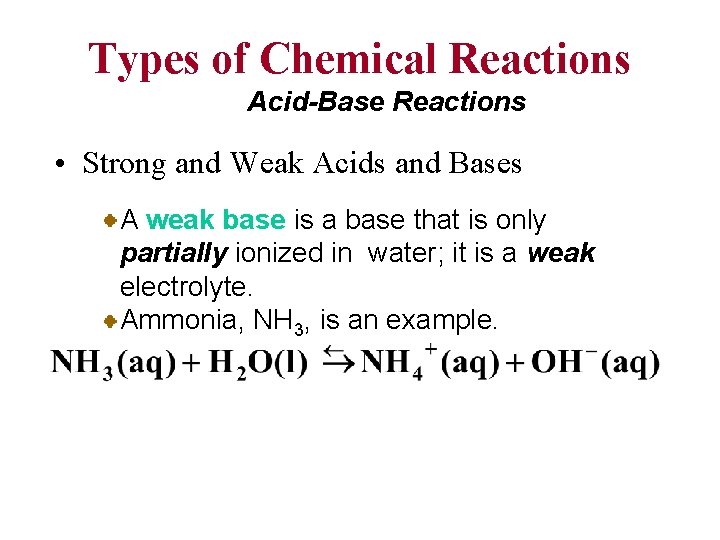
Types of Chemical Reactions Acid-Base Reactions • Strong and Weak Acids and Bases A weak base is a base that is only partially ionized in water; it is a weak electrolyte. Ammonia, NH 3, is an example.

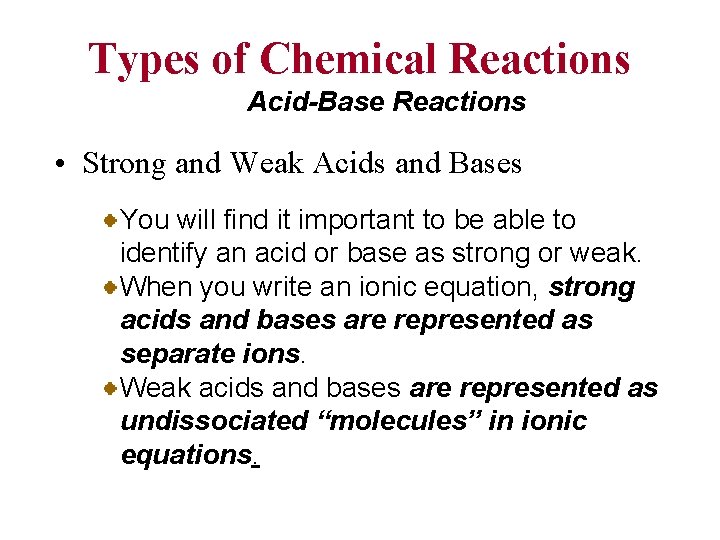
Types of Chemical Reactions Acid-Base Reactions • Strong and Weak Acids and Bases You will find it important to be able to identify an acid or base as strong or weak. When you write an ionic equation, strong acids and bases are represented as separate ions. Weak acids and bases are represented as undissociated “molecules” in ionic equations.

Table 4. 3

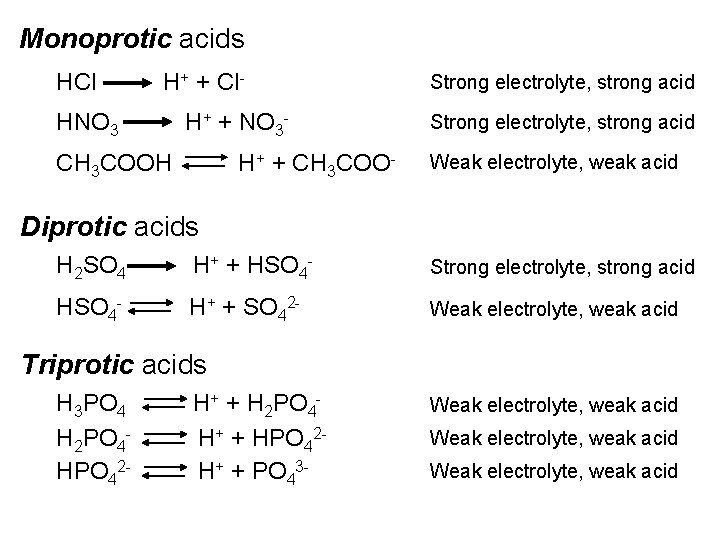
Monoprotic acids HCl H+ + Cl- HNO 3 H+ + NO 3 - CH 3 COOH H+ + CH 3 COO- Strong electrolyte, strong acid Weak electrolyte, weak acid Diprotic acids H 2 SO 4 H+ + HSO 4 - Strong electrolyte, strong acid HSO 4 - H+ + SO 42 - Weak electrolyte, weak acid Triprotic acids H 3 PO 4 H 2 PO 4 HPO 42 - H+ + H 2 PO 4 H+ + HPO 42 H+ + PO 43 - Weak electrolyte, weak acid

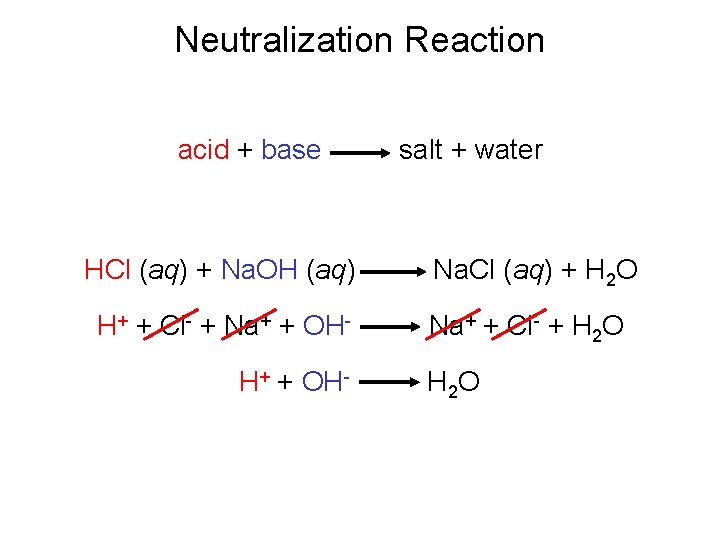
Neutralization Reaction acid + base HCl (aq) + Na. OH (aq) H+ + Cl- + Na+ + OHH+ + OH- salt + water Na. Cl (aq) + H 2 O Na+ + Cl- + H 2 O

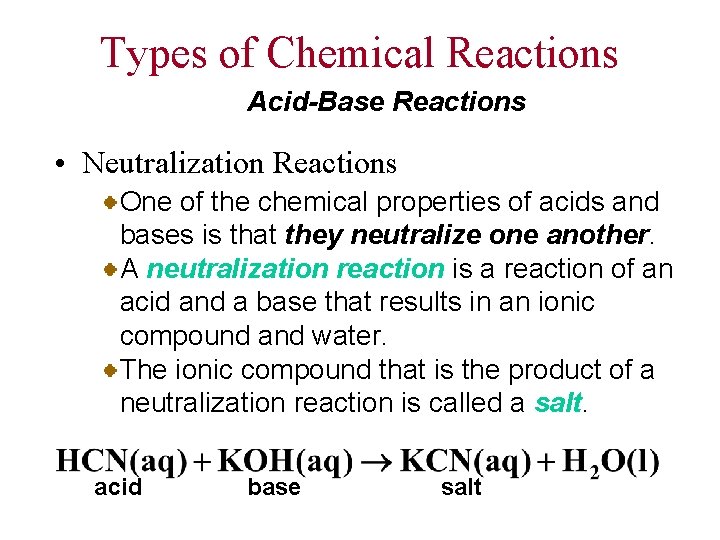
Types of Chemical Reactions Acid-Base Reactions • Neutralization Reactions One of the chemical properties of acids and bases is that they neutralize one another. A neutralization reaction is a reaction of an acid and a base that results in an ionic compound and water. The ionic compound that is the product of a neutralization reaction is called a salt. acid base salt

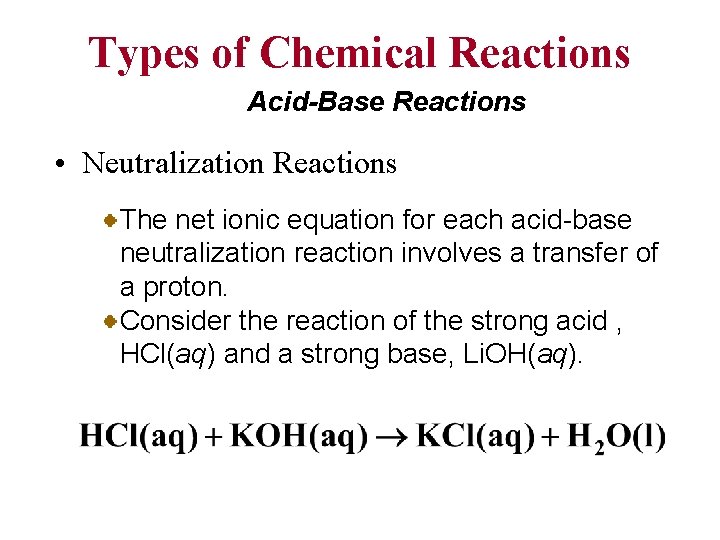
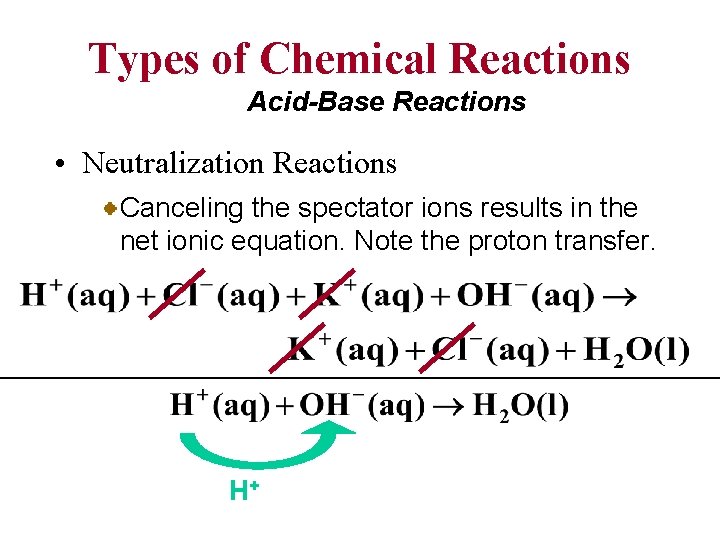
Types of Chemical Reactions Acid-Base Reactions • Neutralization Reactions The net ionic equation for each acid-base neutralization reaction involves a transfer of a proton. Consider the reaction of the strong acid , HCl(aq) and a strong base, Li. OH(aq).

Types of Chemical Reactions Acid-Base Reactions • Neutralization Reactions Writing the strong electrolytes in the form of ions gives the following complete ionic equation.

Types of Chemical Reactions Acid-Base Reactions • Neutralization Reactions Canceling the spectator ions results in the net ionic equation. Note the proton transfer. H+

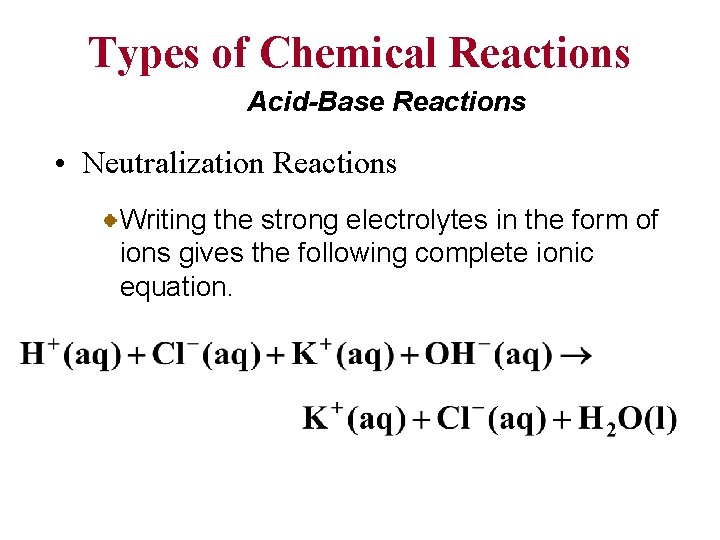
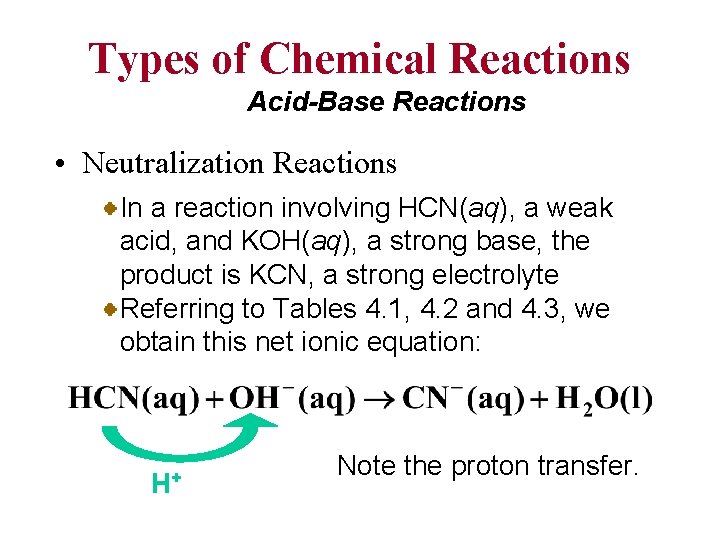
Types of Chemical Reactions Acid-Base Reactions • Neutralization Reactions In a reaction involving HCN(aq), a weak acid, and KOH(aq), a strong base, the product is KCN, a strong electrolyte Referring to Tables 4. 1, 4. 2 and 4. 3, we obtain this net ionic equation: H+ Note the proton transfer.

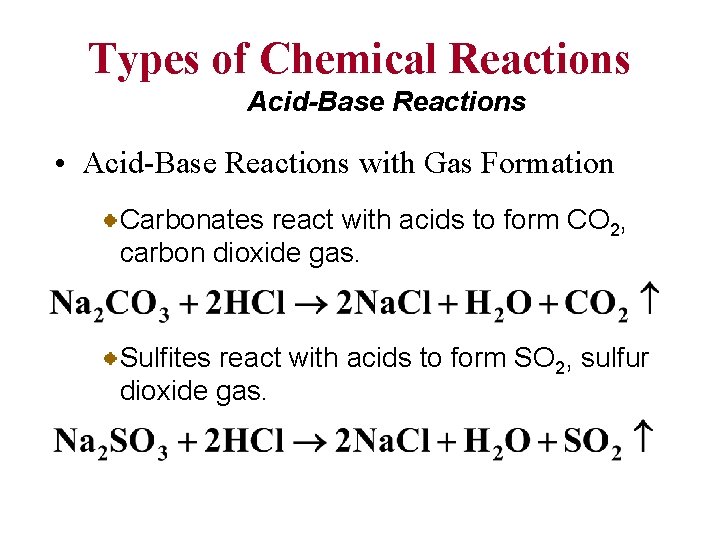
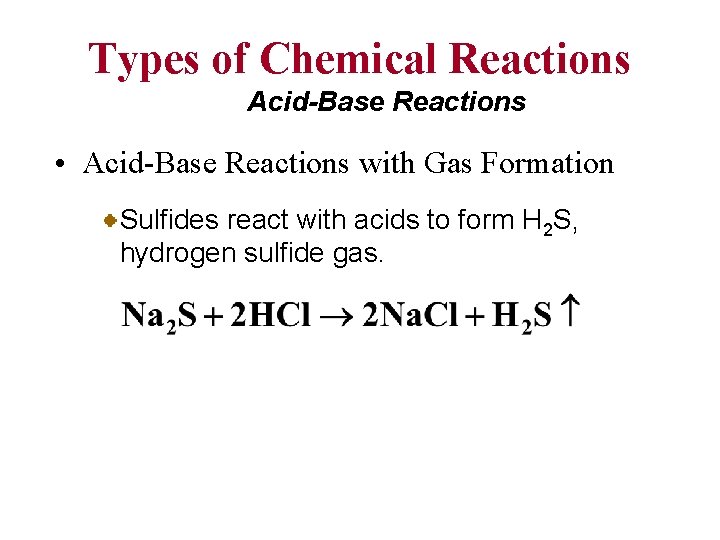
Types of Chemical Reactions Acid-Base Reactions • Acid-Base Reactions with Gas Formation Carbonates react with acids to form CO 2, carbon dioxide gas. Sulfites react with acids to form SO 2, sulfur dioxide gas.

Types of Chemical Reactions Acid-Base Reactions • Acid-Base Reactions with Gas Formation Sulfides react with acids to form H 2 S, hydrogen sulfide gas.

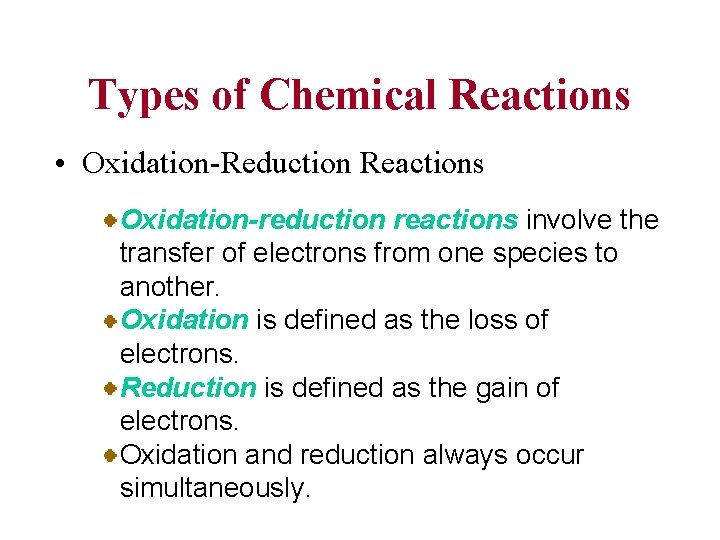
Types of Chemical Reactions • Oxidation-Reduction Reactions Oxidation-reduction reactions involve the transfer of electrons from one species to another. Oxidation is defined as the loss of electrons. Reduction is defined as the gain of electrons. Oxidation and reduction always occur simultaneously.

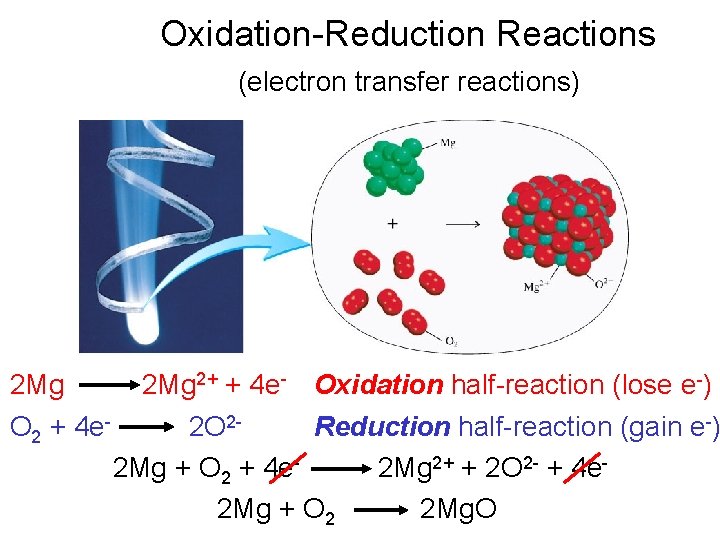
Oxidation-Reduction Reactions (electron transfer reactions) 2 Mg 2+ + 4 e- Oxidation half-reaction (lose e-) O 2 + 4 e 2 O 2 Reduction half-reaction (gain e-) 2 Mg + O 2 + 4 e 2 Mg 2+ + 2 O 2 - + 4 e 2 Mg + O 2 2 Mg. O


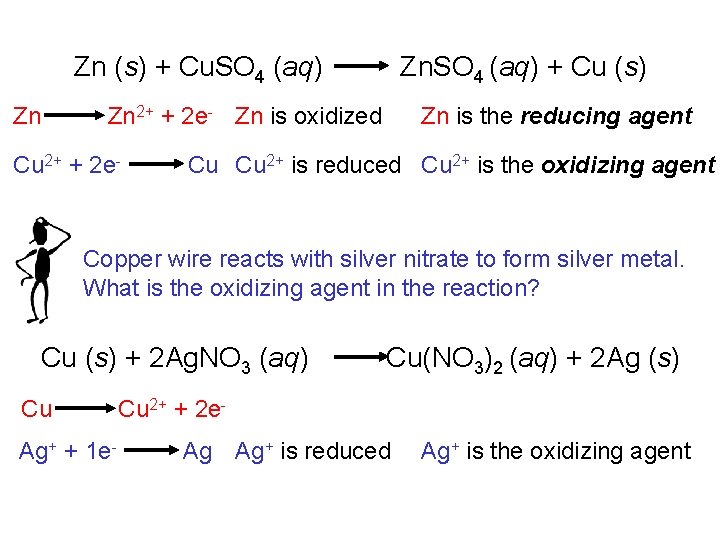
Zn (s) + Cu. SO 4 (aq) Zn Zn. SO 4 (aq) + Cu (s) Zn is the reducing agent Zn 2+ + 2 e- Zn is oxidized Cu 2+ + 2 e- Cu Cu 2+ is reduced Cu 2+ is the oxidizing agent Copper wire reacts with silver nitrate to form silver metal. What is the oxidizing agent in the reaction? Cu (s) + 2 Ag. NO 3 (aq) Cu Ag+ + 1 e- Cu(NO 3)2 (aq) + 2 Ag (s) Cu 2+ + 2 e. Ag Ag+ is reduced Ag+ is the oxidizing agent

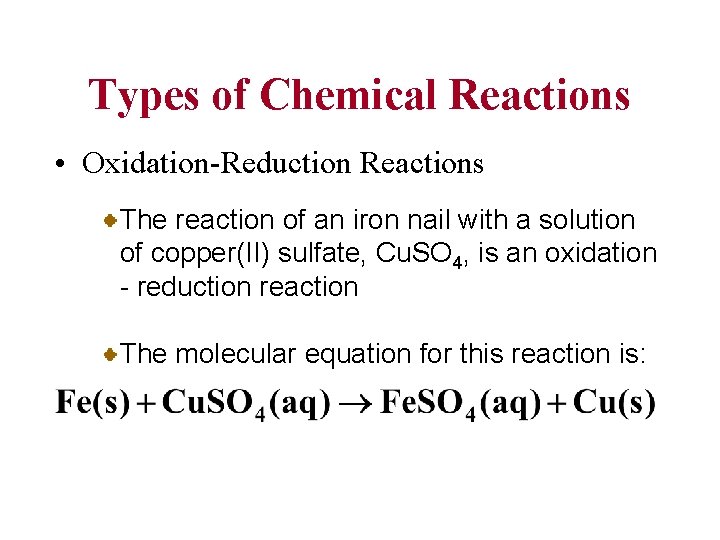
Types of Chemical Reactions • Oxidation-Reduction Reactions The reaction of an iron nail with a solution of copper(II) sulfate, Cu. SO 4, is an oxidation - reduction reaction The molecular equation for this reaction is:

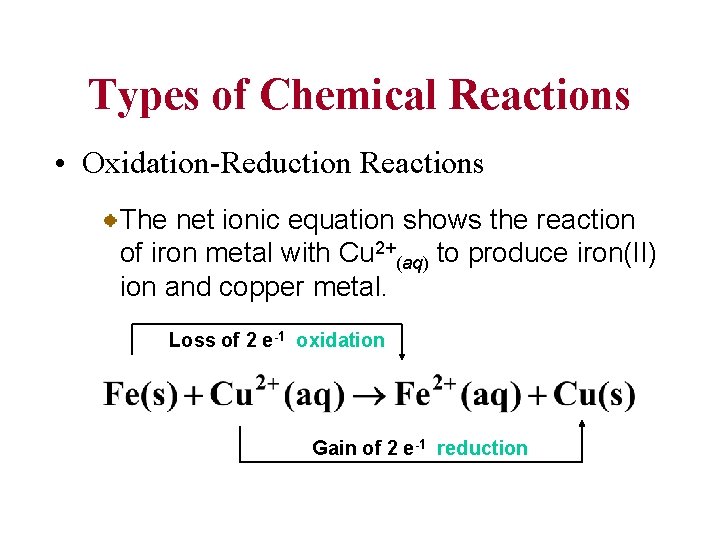
Types of Chemical Reactions • Oxidation-Reduction Reactions The net ionic equation shows the reaction of iron metal with Cu 2+(aq) to produce iron(II) ion and copper metal. Loss of 2 e-1 oxidation Gain of 2 e-1 reduction

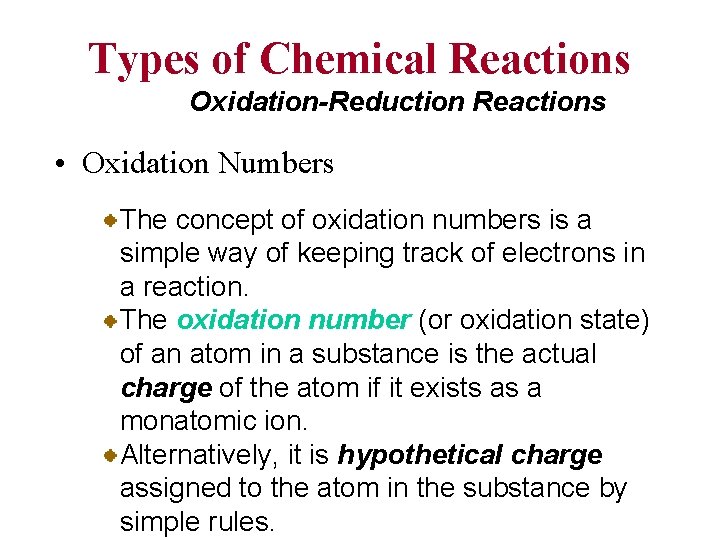
Types of Chemical Reactions Oxidation-Reduction Reactions • Oxidation Numbers The concept of oxidation numbers is a simple way of keeping track of electrons in a reaction. The oxidation number (or oxidation state) of an atom in a substance is the actual charge of the atom if it exists as a monatomic ion. Alternatively, it is hypothetical charge assigned to the atom in the substance by simple rules.

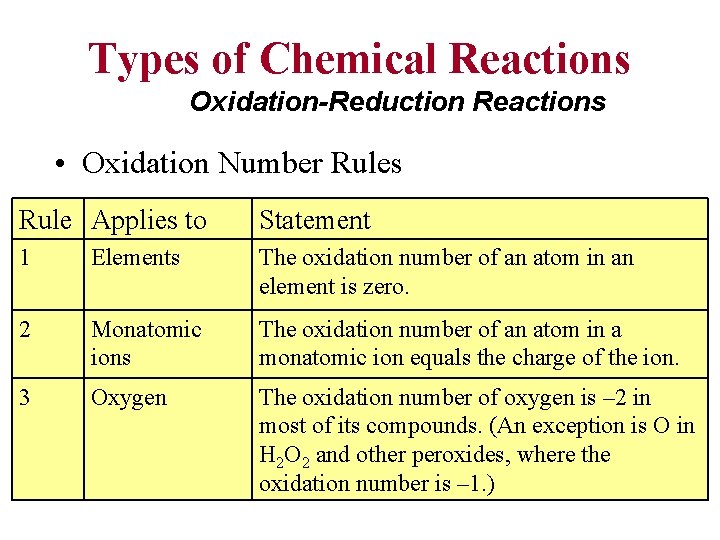
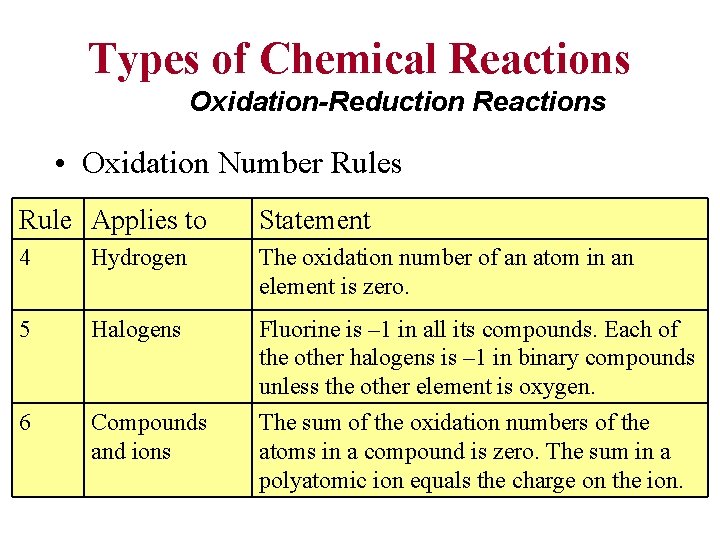
Types of Chemical Reactions Oxidation-Reduction Reactions • Oxidation Number Rules Rule Applies to Statement 1 Elements The oxidation number of an atom in an element is zero. 2 Monatomic ions The oxidation number of an atom in a monatomic ion equals the charge of the ion. 3 Oxygen The oxidation number of oxygen is – 2 in most of its compounds. (An exception is O in H 2 O 2 and other peroxides, where the oxidation number is – 1. )

Types of Chemical Reactions Oxidation-Reduction Reactions • Oxidation Number Rules Rule Applies to Statement 4 Hydrogen The oxidation number of an atom in an element is zero. 5 Halogens 6 Compounds and ions Fluorine is – 1 in all its compounds. Each of the other halogens is – 1 in binary compounds unless the other element is oxygen. The sum of the oxidation numbers of the atoms in a compound is zero. The sum in a polyatomic ion equals the charge on the ion.

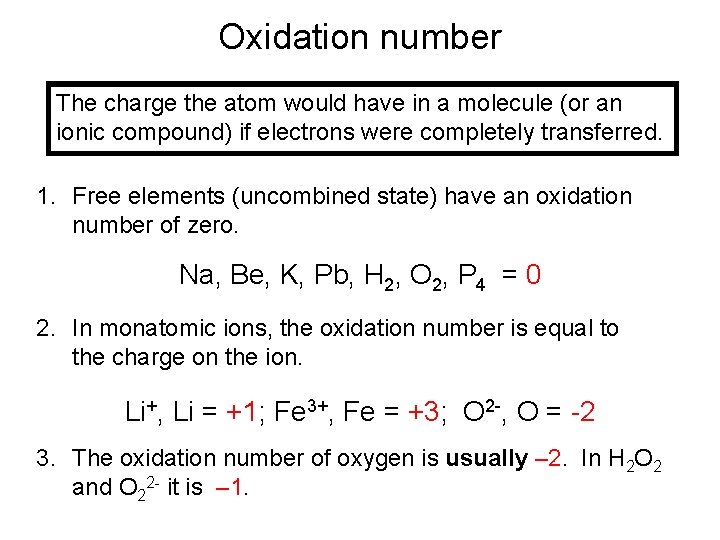
Oxidation number The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred. 1. Free elements (uncombined state) have an oxidation number of zero. Na, Be, K, Pb, H 2, O 2, P 4 = 0 2. In monatomic ions, the oxidation number is equal to the charge on the ion. Li+, Li = +1; Fe 3+, Fe = +3; O 2 -, O = -2 3. The oxidation number of oxygen is usually – 2. In H 2 O 2 and O 22 - it is – 1.

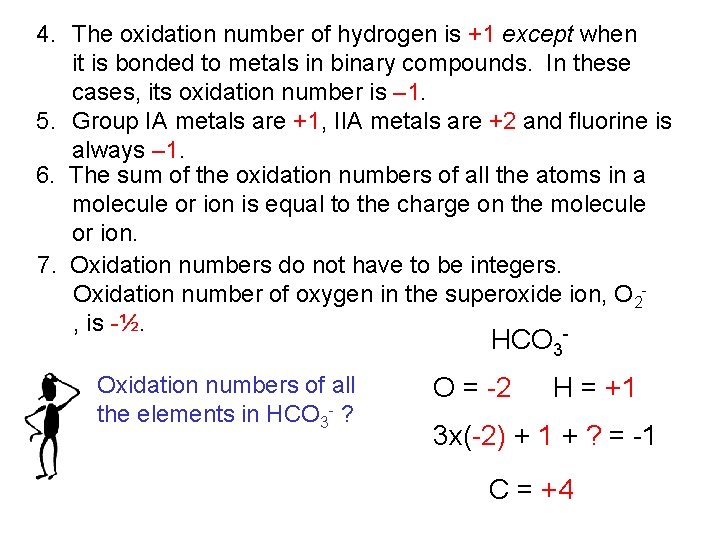
4. The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds. In these cases, its oxidation number is – 1. 5. Group IA metals are +1, IIA metals are +2 and fluorine is always – 1. 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O 2, is -½. - HCO 3 Oxidation numbers of all the elements in HCO 3 - ? O = -2 H = +1 3 x(-2) + 1 + ? = -1 C = +4

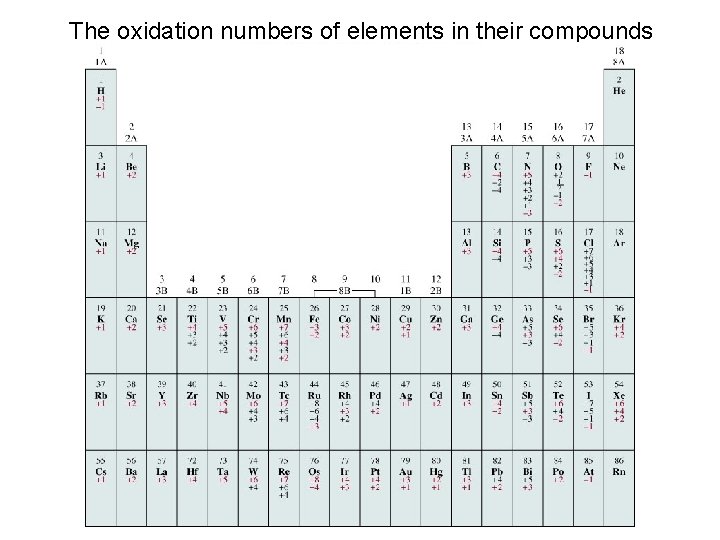
The oxidation numbers of elements in their compounds

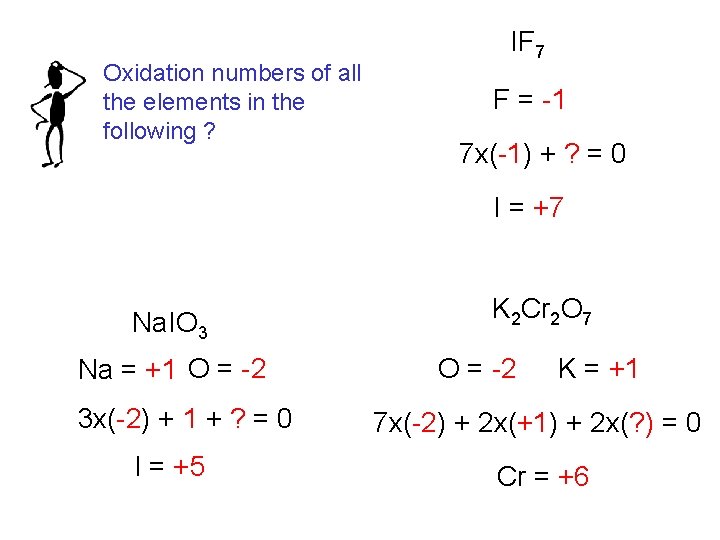
Oxidation numbers of all the elements in the following ? IF 7 F = -1 7 x(-1) + ? = 0 I = +7 Na. IO 3 Na = +1 O = -2 3 x(-2) + 1 + ? = 0 I = +5 K 2 Cr 2 O 7 O = -2 K = +1 7 x(-2) + 2 x(+1) + 2 x(? ) = 0 Cr = +6

Types of Chemical Reactions Oxidation-Reduction Reactions • Describing Oxidation-Reduction Reactions Look again at the reaction of iron with copper(II) sulfate. We can write this reaction in terms of two half-reactions.

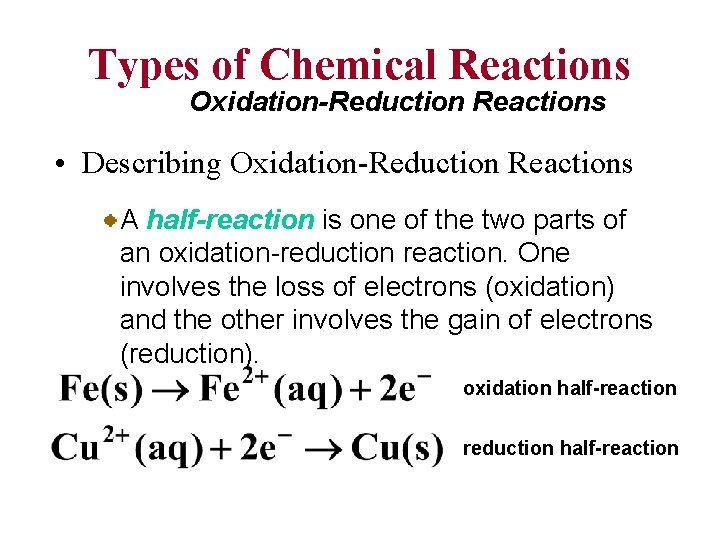
Types of Chemical Reactions Oxidation-Reduction Reactions • Describing Oxidation-Reduction Reactions A half-reaction is one of the two parts of an oxidation-reduction reaction. One involves the loss of electrons (oxidation) and the other involves the gain of electrons (reduction). oxidation half-reaction reduction half-reaction

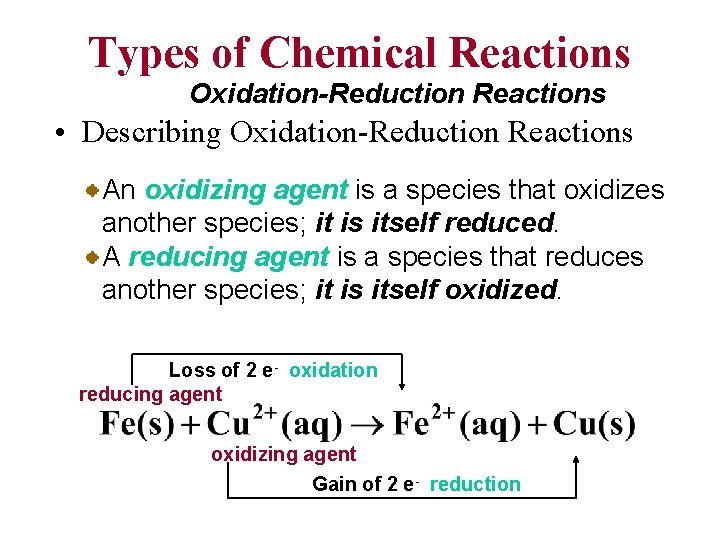
Types of Chemical Reactions Oxidation-Reduction Reactions • Describing Oxidation-Reduction Reactions An oxidizing agent is a species that oxidizes another species; it is itself reduced. A reducing agent is a species that reduces another species; it is itself oxidized. Loss of 2 e- oxidation reducing agent oxidizing agent Gain of 2 e- reduction

Types of Chemical Reactions Oxidation-Reduction Reactions • Some Common Oxidation-Reduction Reactions Most of the oxidation-reduction reactions fall into one of the following simple categories: Combination Reactions Decomposition Reactions Combustion Reactions Displacement Reactions

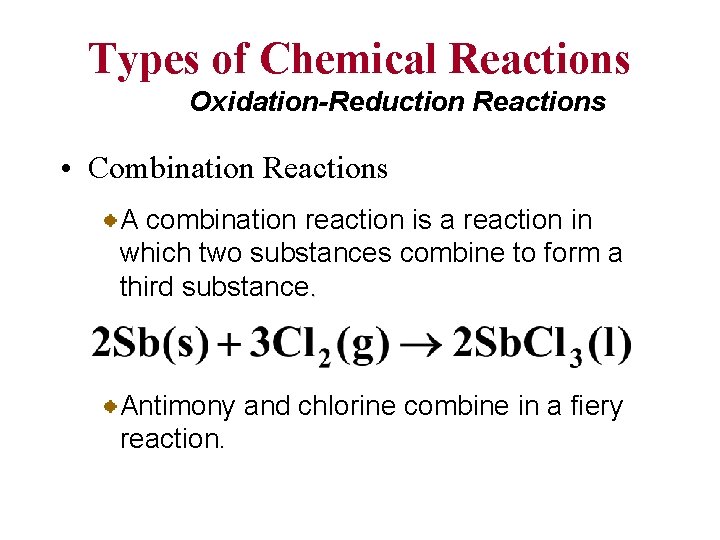
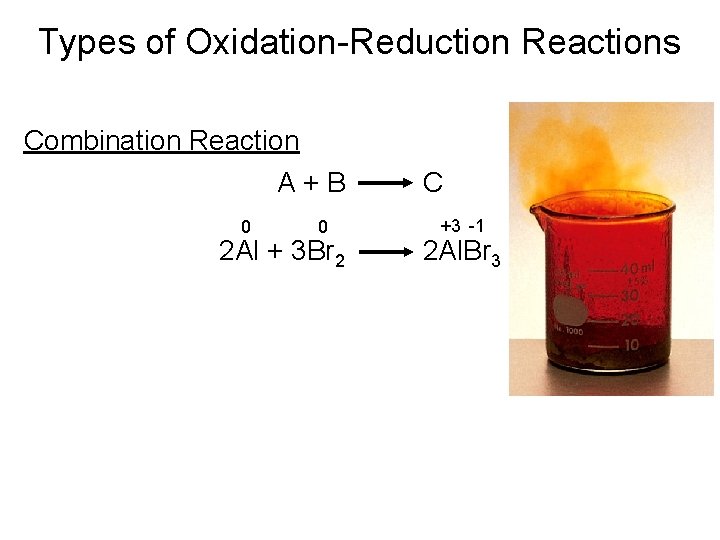
Types of Chemical Reactions Oxidation-Reduction Reactions • Combination Reactions A combination reaction is a reaction in which two substances combine to form a third substance. Antimony and chlorine combine in a fiery reaction.

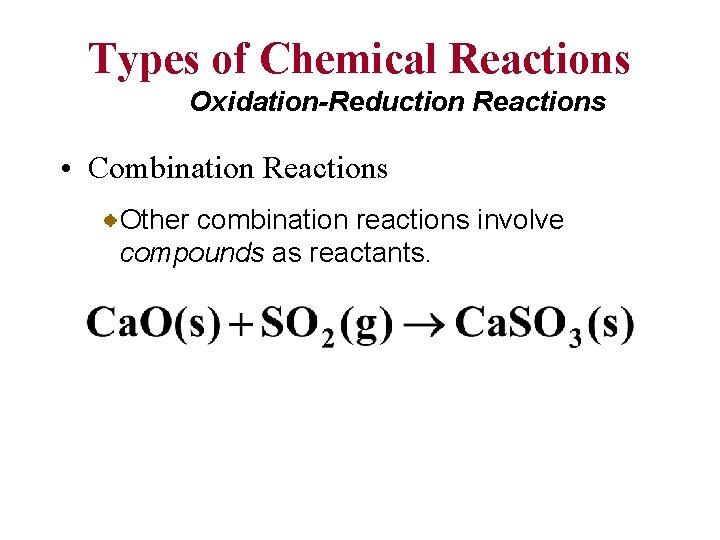
Types of Chemical Reactions Oxidation-Reduction Reactions • Combination Reactions Other combination reactions involve compounds as reactants.

Types of Oxidation-Reduction Reactions Combination Reaction A+B 0 0 2 Al + 3 Br 2 C +3 -1 2 Al. Br 3

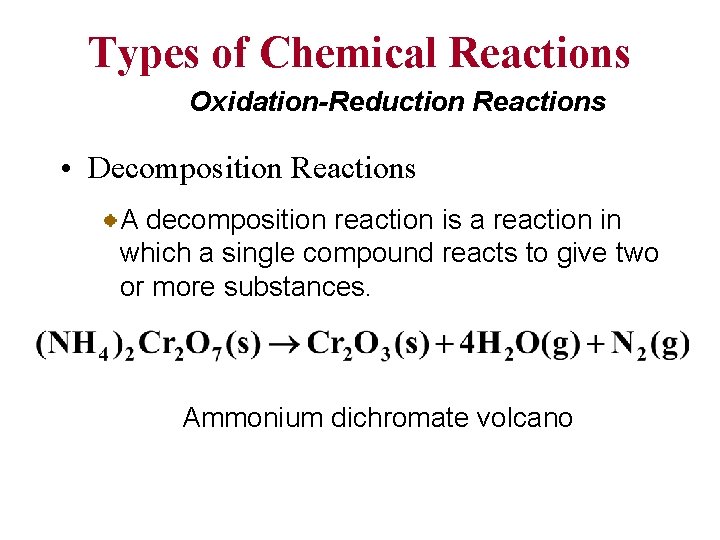
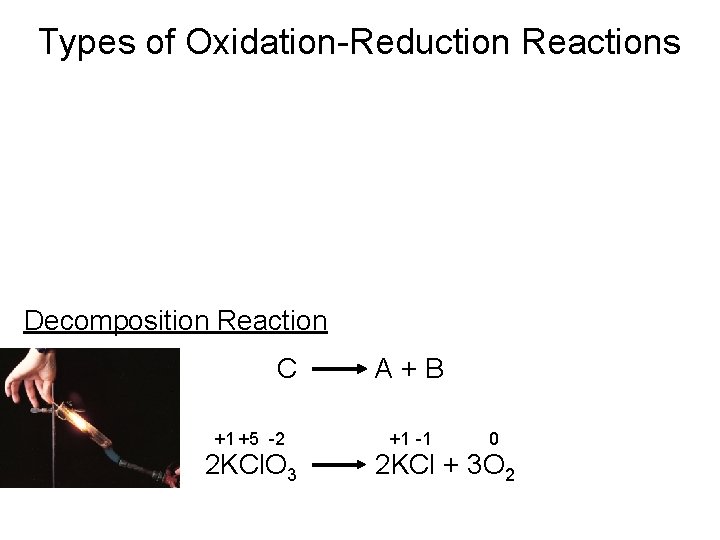
Types of Chemical Reactions Oxidation-Reduction Reactions • Decomposition Reactions A decomposition reaction is a reaction in which a single compound reacts to give two or more substances. Ammonium dichromate volcano

Types of Oxidation-Reduction Reactions Decomposition Reaction C +1 +5 -2 2 KCl. O 3 A+B +1 -1 0 2 KCl + 3 O 2

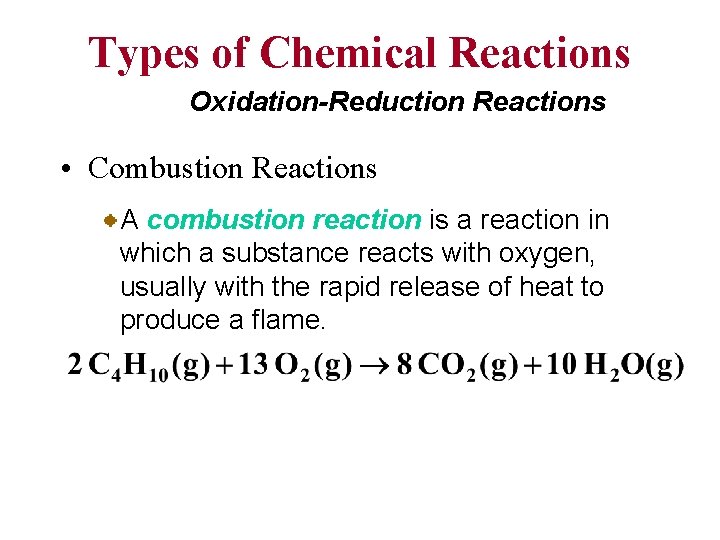
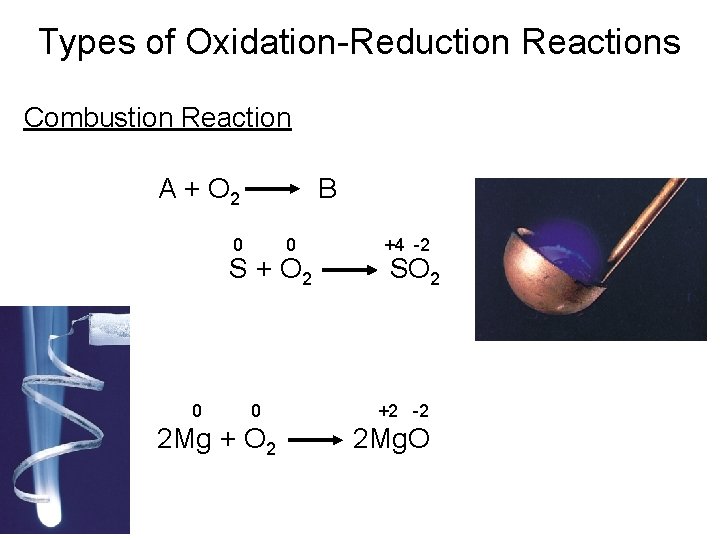
Types of Chemical Reactions Oxidation-Reduction Reactions • Combustion Reactions A combustion reaction is a reaction in which a substance reacts with oxygen, usually with the rapid release of heat to produce a flame.

Types of Oxidation-Reduction Reactions Combustion Reaction A + O 2 B 0 0 S + O 2 0 0 2 Mg + O 2 +4 -2 SO 2 +2 -2 2 Mg. O

The burning of calcium metal in oxygen.

The burning of calcium metal in chlorine.

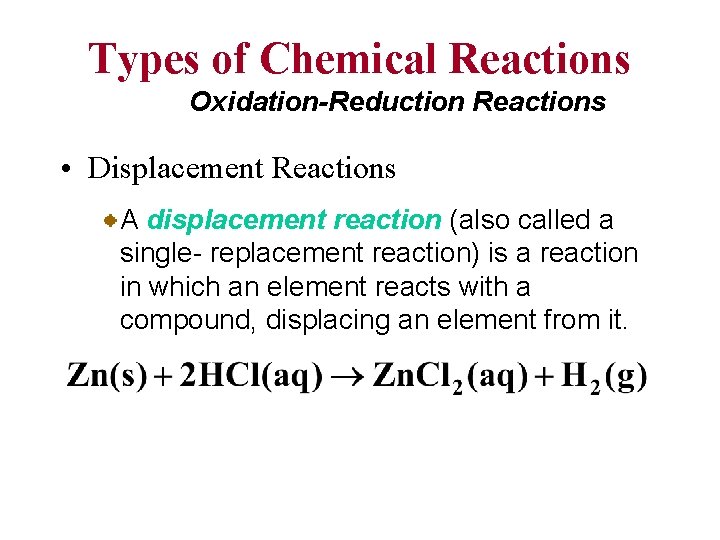
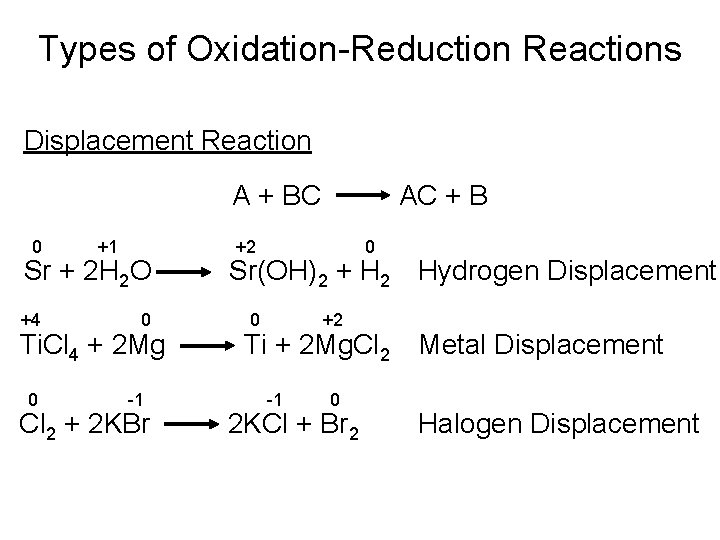
Types of Chemical Reactions Oxidation-Reduction Reactions • Displacement Reactions A displacement reaction (also called a single- replacement reaction) is a reaction in which an element reacts with a compound, displacing an element from it.

Types of Oxidation-Reduction Reactions Displacement Reaction A + BC 0 +1 Sr + 2 H 2 O +4 0 Ti. Cl 4 + 2 Mg 0 -1 Cl 2 + 2 KBr AC + B +2 0 Sr(OH)2 + H 2 Hydrogen Displacement 0 +2 Ti + 2 Mg. Cl 2 Metal Displacement -1 0 2 KCl + Br 2 Halogen Displacement

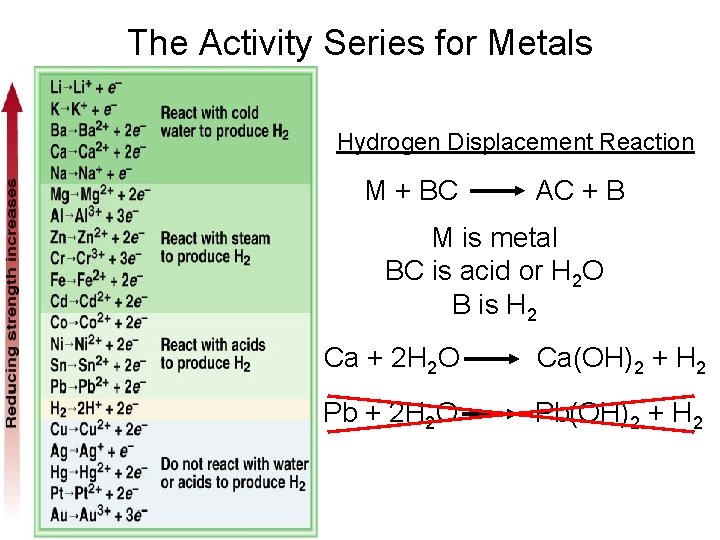
The Activity Series for Metals Hydrogen Displacement Reaction M + BC AC + B M is metal BC is acid or H 2 O B is H 2 Ca + 2 H 2 O Ca(OH)2 + H 2 Pb + 2 H 2 O Pb(OH)2 + H 2

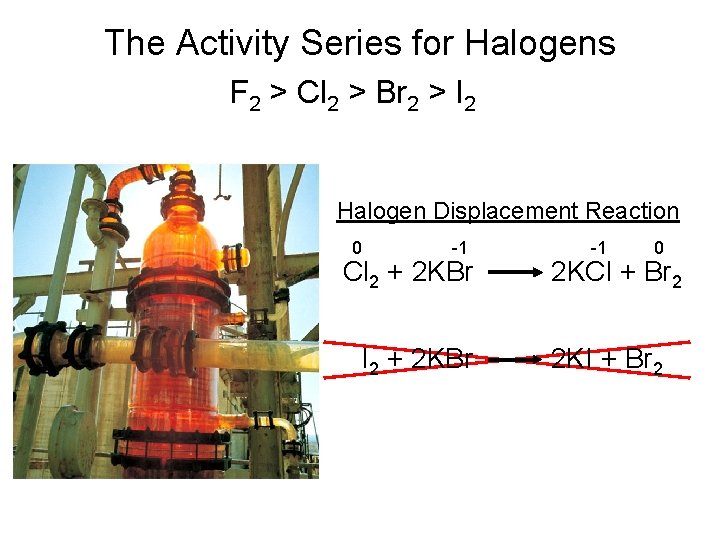
The Activity Series for Halogens F 2 > Cl 2 > Br 2 > I 2 Halogen Displacement Reaction 0 -1 Cl 2 + 2 KBr I 2 + 2 KBr -1 0 2 KCl + Br 2 2 KI + Br 2

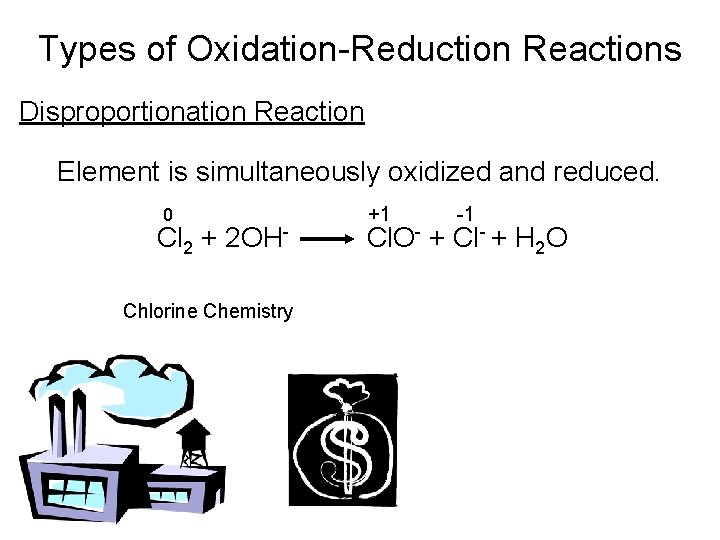
Types of Oxidation-Reduction Reactions Disproportionation Reaction Element is simultaneously oxidized and reduced. 0 Cl 2 + 2 OHChlorine Chemistry +1 -1 Cl. O- + Cl- + H 2 O

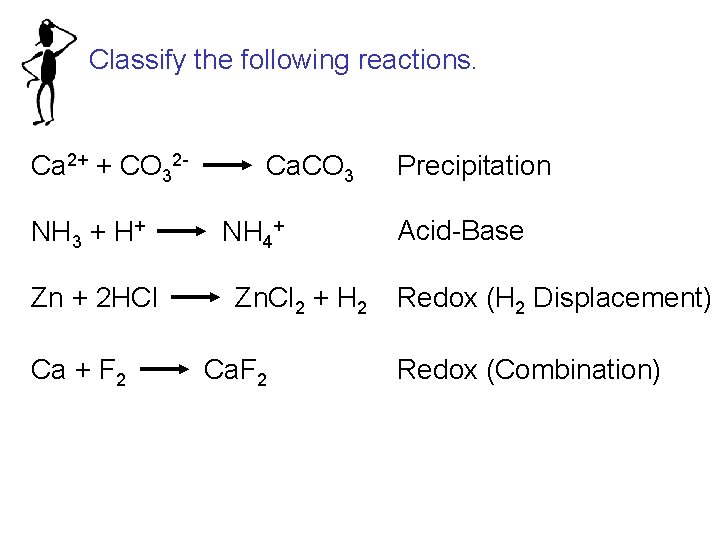
Classify the following reactions. Ca 2+ + CO 32 NH 3 + H+ Zn + 2 HCl Ca + F 2 Ca. CO 3 NH 4+ Zn. Cl 2 + H 2 Ca. F 2 Precipitation Acid-Base Redox (H 2 Displacement) Redox (Combination)

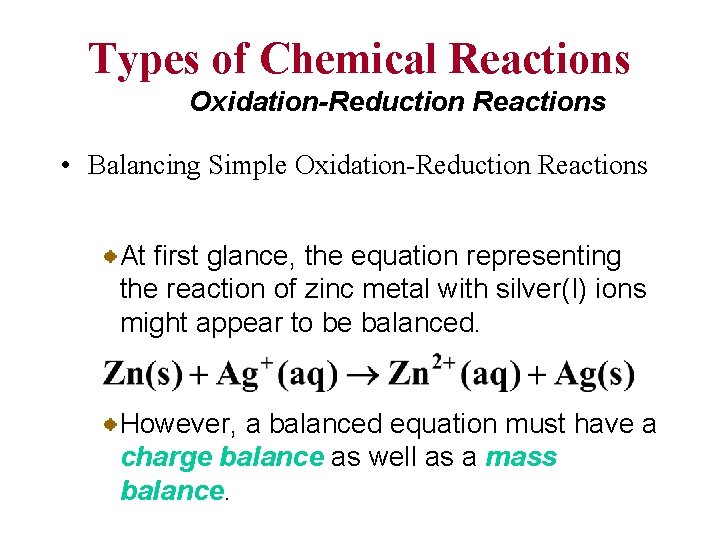
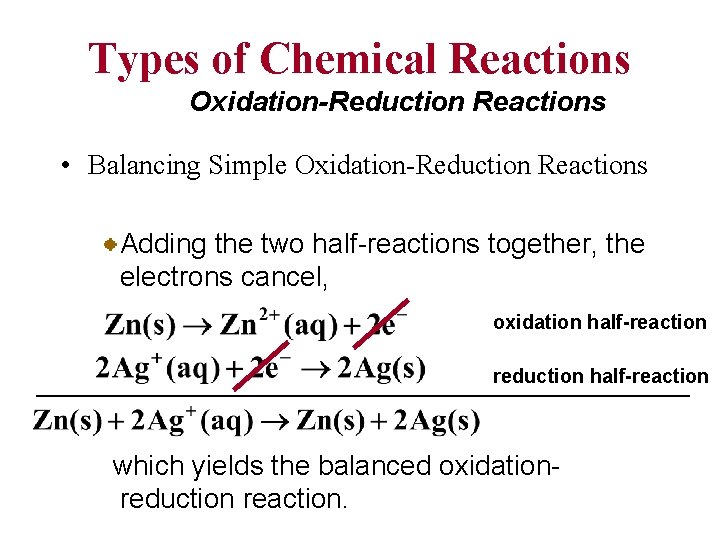
Types of Chemical Reactions Oxidation-Reduction Reactions • Balancing Simple Oxidation-Reduction Reactions At first glance, the equation representing the reaction of zinc metal with silver(I) ions might appear to be balanced. However, a balanced equation must have a charge balance as well as a mass balance.

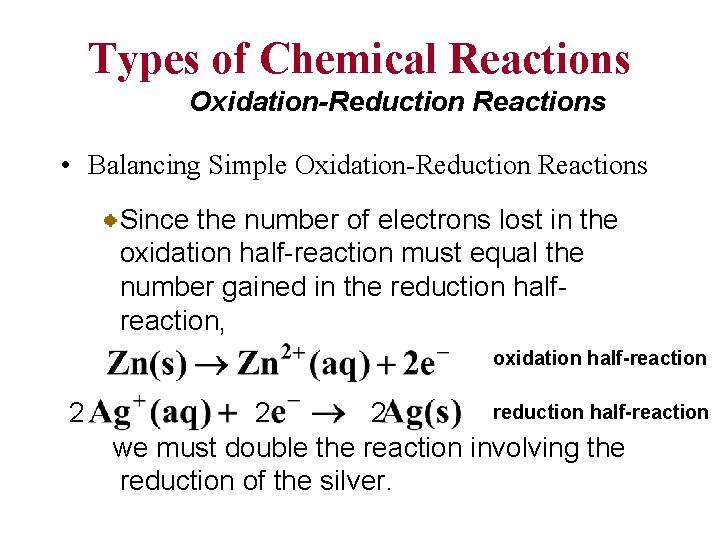
Types of Chemical Reactions Oxidation-Reduction Reactions • Balancing Simple Oxidation-Reduction Reactions Since the number of electrons lost in the oxidation half-reaction must equal the number gained in the reduction halfreaction, oxidation half-reaction 2 reduction half-reaction 2 2 we must double the reaction involving the reduction of the silver.

Types of Chemical Reactions Oxidation-Reduction Reactions • Balancing Simple Oxidation-Reduction Reactions Adding the two half-reactions together, the electrons cancel, oxidation half-reaction reduction half-reaction which yields the balanced oxidationreduction reaction.

Working with Solutions • The majority of chemical reactions discussed here occur in aqueous solution. When you run reactions in liquid solutions, it is convenient to dispense the amounts of reactants by measuring out volumes of reactant solutions.

Working with Solutions Molar Concentration • When we dissolve a substance in a liquid, we call the substance the solute and the liquid the solvent. The general term concentration refers to the quantity of solute in a standard quantity of solution.

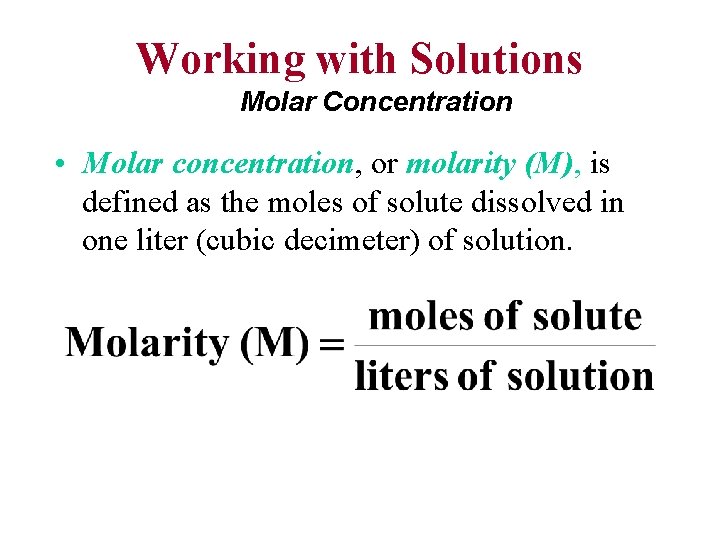
Working with Solutions Molar Concentration • Molar concentration, or molarity (M), is defined as the moles of solute dissolved in one liter (cubic decimeter) of solution.

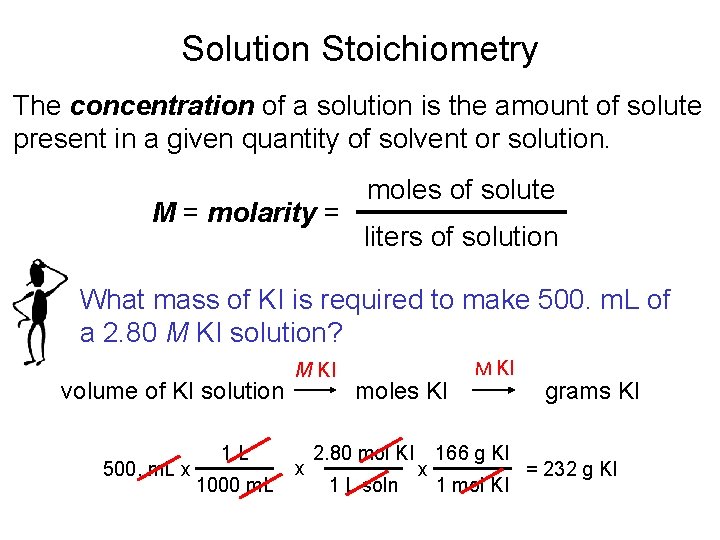
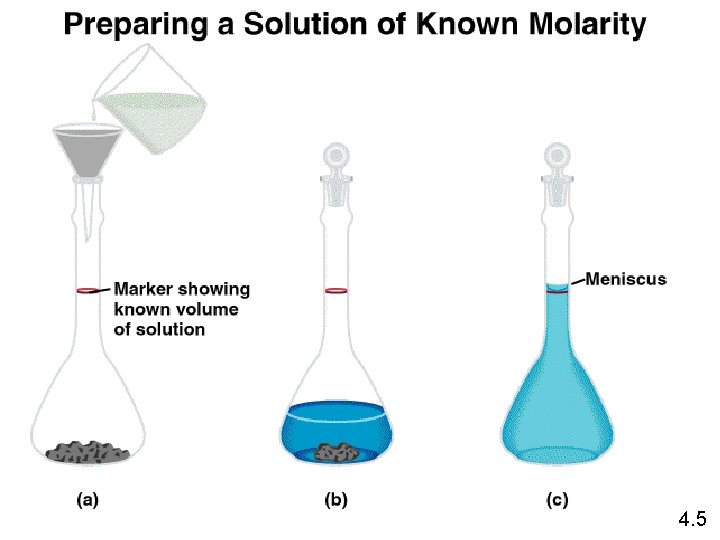
Solution Stoichiometry The concentration of a solution is the amount of solute present in a given quantity of solvent or solution. M = molarity = moles of solute liters of solution What mass of KI is required to make 500. m. L of a 2. 80 M KI solution? volume of KI solution 500. m. L x 1 L 1000 m. L M KI x moles KI 2. 80 mol KI 1 L soln x M KI 166 g KI 1 mol KI grams KI = 232 g KI

4. 5

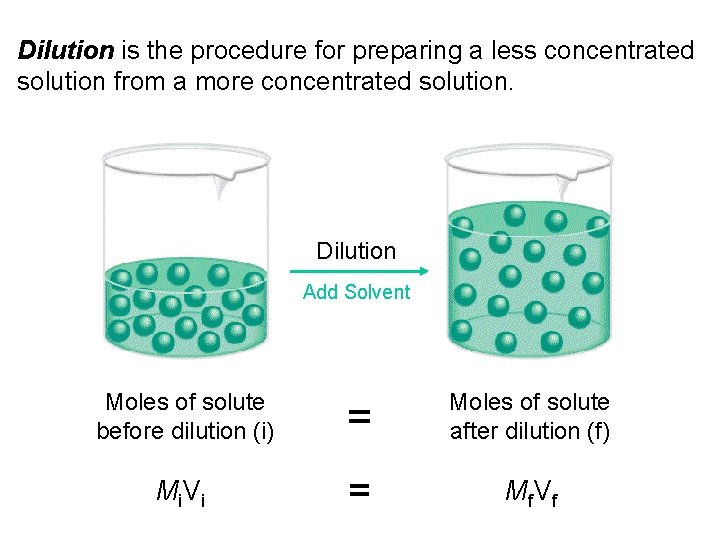
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Dilution Add Solvent Moles of solute before dilution (i) = Moles of solute after dilution (f) Mi V i = Mf V f

Working with Solutions Diluting Solutions • The molarity of a solution and its volume are inversely proportional. Therefore, adding water makes the solution less concentrated. This inverse relationship takes the form of: So, as water is added, increasing the final volume, Vf, the final molarity, Mf, decreases.

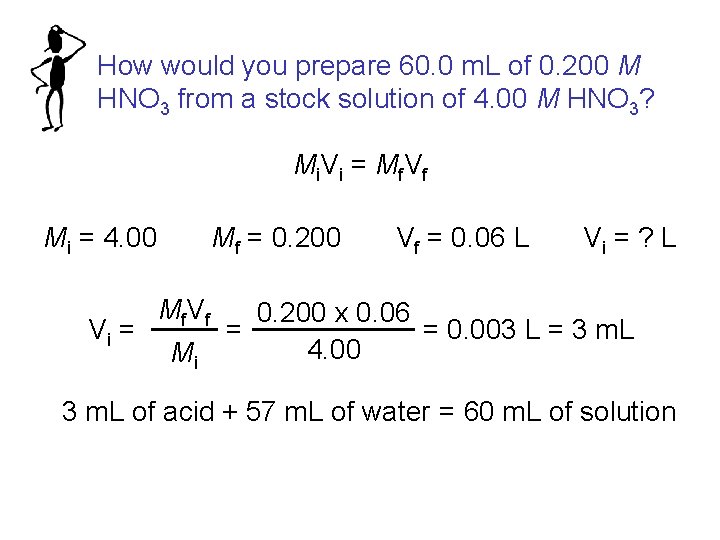
How would you prepare 60. 0 m. L of 0. 200 M HNO 3 from a stock solution of 4. 00 M HNO 3? Mi V i = Mf V f Mi = 4. 00 Vi = Mf = 0. 200 Mf V f Mi Vf = 0. 06 L Vi = ? L 0. 200 x 0. 06 = 0. 003 L = 3 m. L = 4. 00 3 m. L of acid + 57 m. L of water = 60 m. L of solution

Quantitative Analysis • Analytical chemistry deals with the determination of composition of materialsthat is, the analysis of materials. Quantitative analysis involves the determination of the amount of a substance or species present in a material.

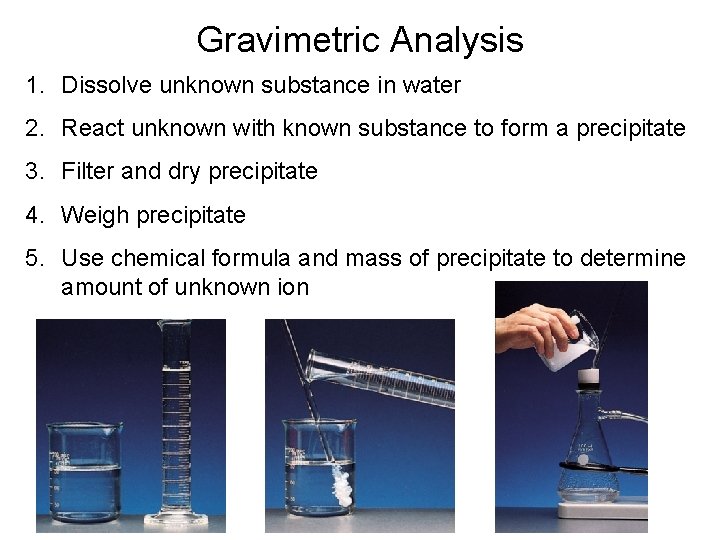
Quantitative Analysis Gravimetric Analysis • Gravimetric analysis is a type of quantitative analysis in which the amount of a species in a material is determined by converting the species into a product that can be isolated and weighed. Precipitation reactions are often used in gravimetric analysis. The precipitate from these reactions is then filtered, dried, and weighed.

Gravimetric Analysis 1. Dissolve unknown substance in water 2. React unknown with known substance to form a precipitate 3. Filter and dry precipitate 4. Weigh precipitate 5. Use chemical formula and mass of precipitate to determine amount of unknown ion

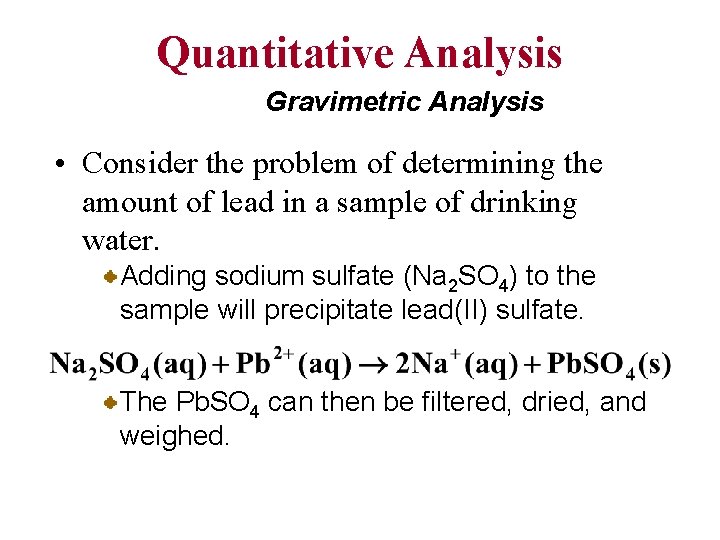
Quantitative Analysis Gravimetric Analysis • Consider the problem of determining the amount of lead in a sample of drinking water. Adding sodium sulfate (Na 2 SO 4) to the sample will precipitate lead(II) sulfate. The Pb. SO 4 can then be filtered, dried, and weighed.

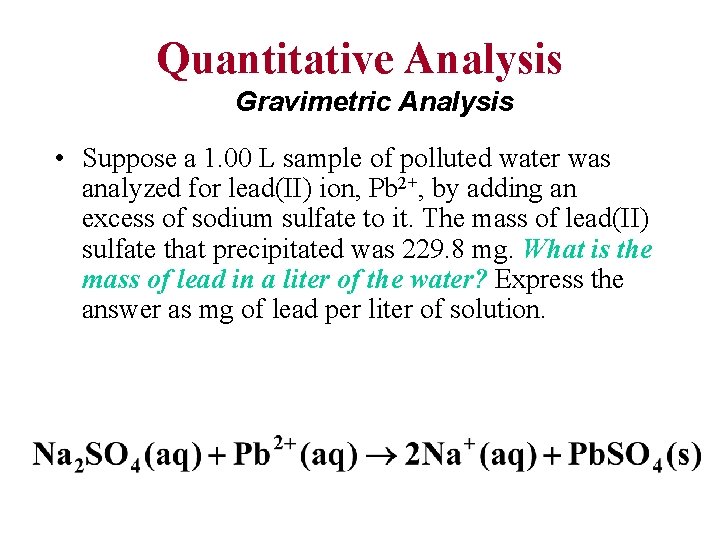
Quantitative Analysis Gravimetric Analysis • Suppose a 1. 00 L sample of polluted water was analyzed for lead(II) ion, Pb 2+, by adding an excess of sodium sulfate to it. The mass of lead(II) sulfate that precipitated was 229. 8 mg. What is the mass of lead in a liter of the water? Express the answer as mg of lead per liter of solution.

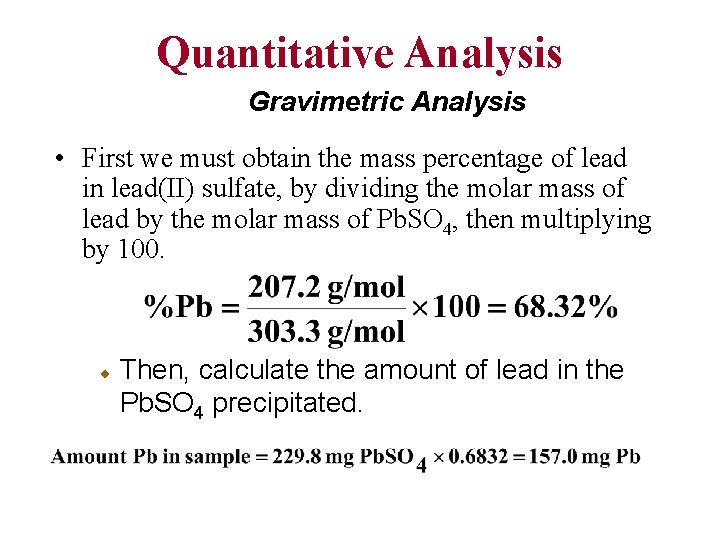
Quantitative Analysis Gravimetric Analysis • First we must obtain the mass percentage of lead in lead(II) sulfate, by dividing the molar mass of lead by the molar mass of Pb. SO 4, then multiplying by 100. Then, calculate the amount of lead in the Pb. SO 4 precipitated.

Quantitative Analysis Volumetric Analysis • An important method for determining the amount of a particular substance is based on measuring the volume of the reactant solution. Titration is a procedure for determining the amount of substance A by adding a carefully measured volume of a solution with known concentration of B until the reaction of A and B is just complete. Volumetric analysis is a method of analysis based on titration.

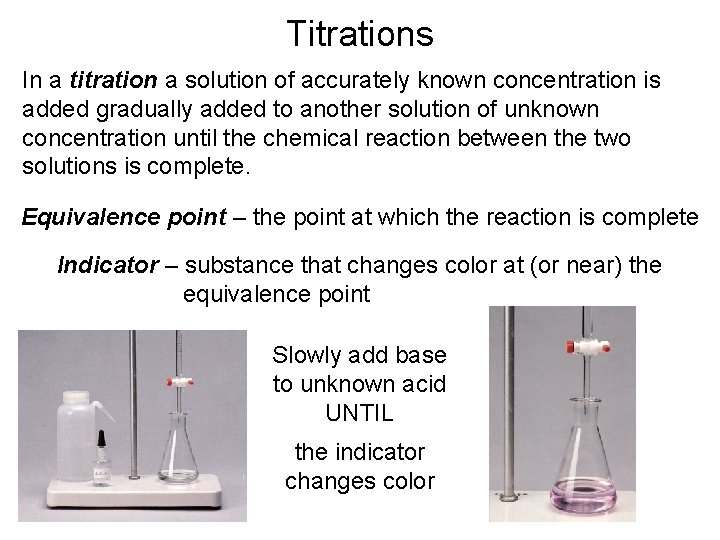
Titrations In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the equivalence point Slowly add base to unknown acid UNTIL the indicator changes color


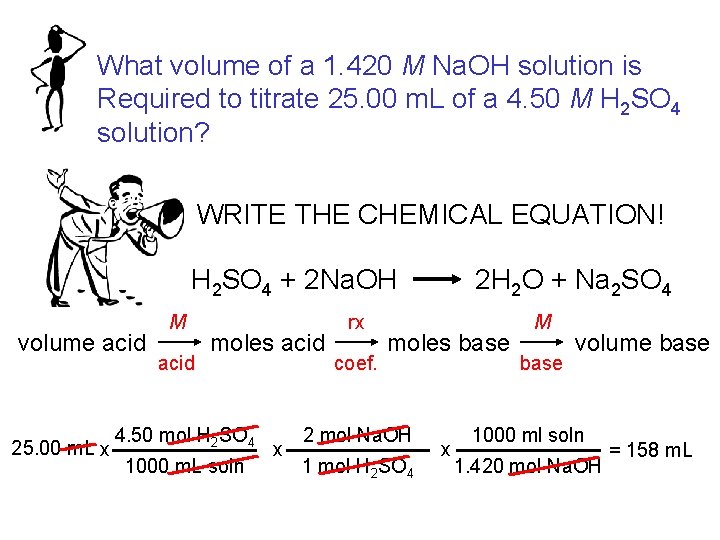
What volume of a 1. 420 M Na. OH solution is Required to titrate 25. 00 m. L of a 4. 50 M H 2 SO 4 solution? WRITE THE CHEMICAL EQUATION! H 2 SO 4 + 2 Na. OH volume acid 25. 00 m. L x M acid moles acid 4. 50 mol H 2 SO 4 1000 m. L soln x rx coef. 2 H 2 O + Na 2 SO 4 moles base 2 mol Na. OH 1 mol H 2 SO 4 x M base volume base 1000 ml soln 1. 420 mol Na. OH = 158 m. L

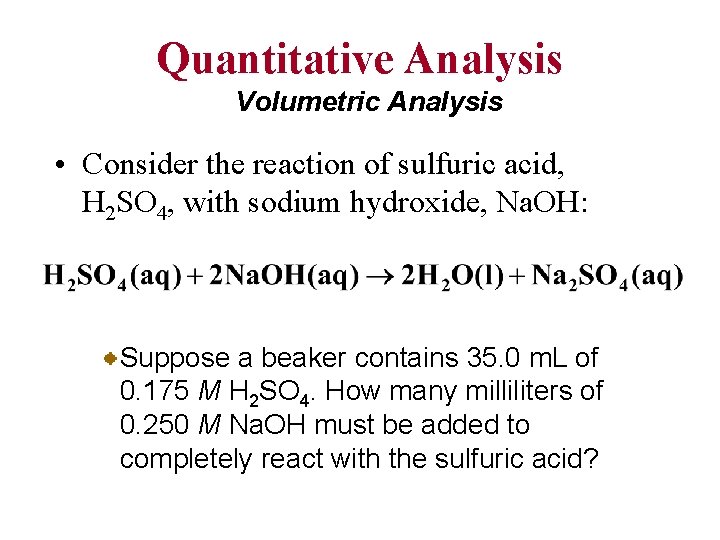
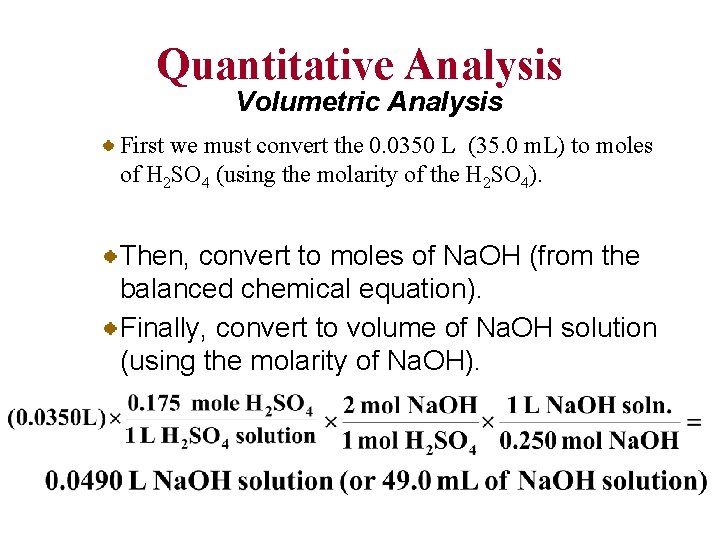
Quantitative Analysis Volumetric Analysis • Consider the reaction of sulfuric acid, H 2 SO 4, with sodium hydroxide, Na. OH: Suppose a beaker contains 35. 0 m. L of 0. 175 M H 2 SO 4. How many milliliters of 0. 250 M Na. OH must be added to completely react with the sulfuric acid?

Quantitative Analysis Volumetric Analysis First we must convert the 0. 0350 L (35. 0 m. L) to moles of H 2 SO 4 (using the molarity of the H 2 SO 4). Then, convert to moles of Na. OH (from the balanced chemical equation). Finally, convert to volume of Na. OH solution (using the molarity of Na. OH).

Chemical Reactions Summary • Reactions often involve ions in aqueous solution. Many of these compounds are electrolytes. We can represent these reactions as molecular equations, complete ionic equations (with strong electrolytes represented as ions), or net ionic equations (where spectator ions have been canceled). Most reactions are either precipitation reactions, acid-base reactions, or oxidation-reduction reactions. Acid-base reactions are proton-transfer reactions. Oxidation-reduction reactions involve a transfer of electrons from one species to another.

Chemical Reactions Summary • Oxidation-reduction reactions usually fall into the following categories: combination reactions, decomposition reactions, displacement reactions, and combustion reactions. Molarity is defined as the number of moles of solute per liter of solution. Knowing the molarity allows you to calculate the amount of solute in a given volume of solution. Quantitative analysis involves the determination of the amount of a species in a material.

Chemical Reactions Summary • In gravimetric analysis, you determine the amount of a species by converting it to a product you can weigh. In volumetric analysis, you determine the amount of a species by titration.

Operational Skills Using solubility rules. Writing net ionic equations. Deciding whether precipitation occurs. Classifying acids and bases as weak or strong. Writing an equation for a neutralization. Writing an equation for a reaction with gas formation. Assigning oxidation numbers. Balancing simple oxidation-reduction reactions. Calculating molarity from mass and volume. Using molarity as a conversion factor. Diluting a solution. Determining the amount of a substance by gravimetric analysis. Calculating the volume of reactant solution needed. Calculating the quantity of a substance by titration.

Worked Example 4. 1

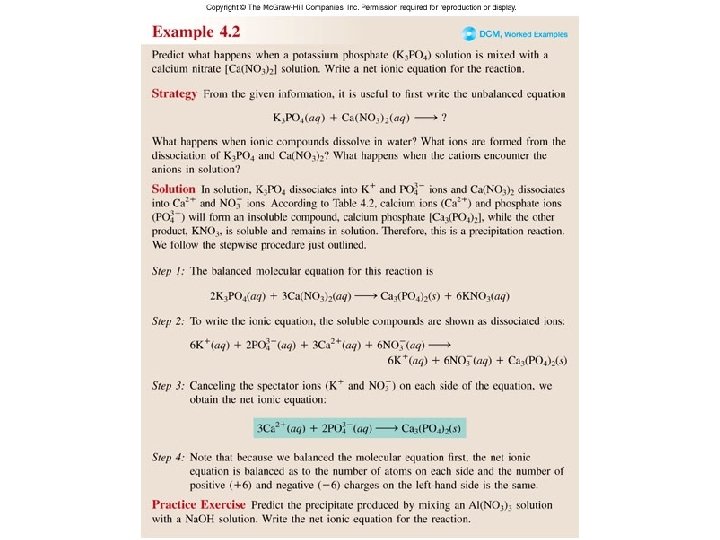
Worked Example 4. 2

Worked Example 4. 3

Worked Example 4. 4

Worked Example 4. 5

Worked Example 4. 6

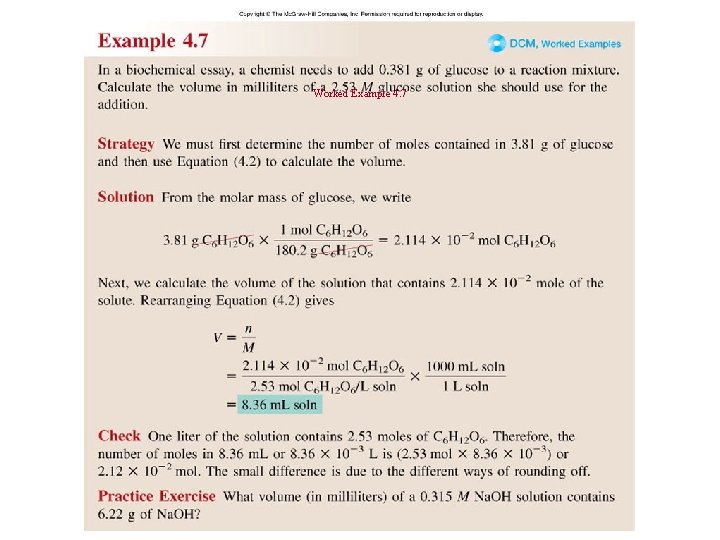
Worked Example 4. 7

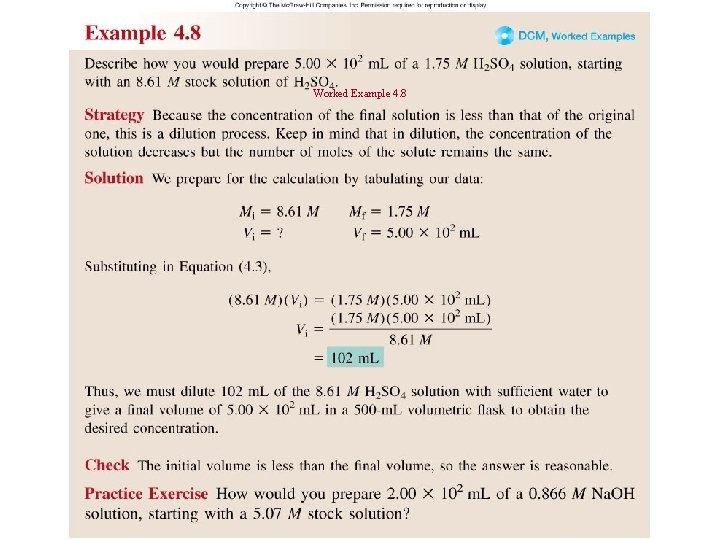
Worked Example 4. 8

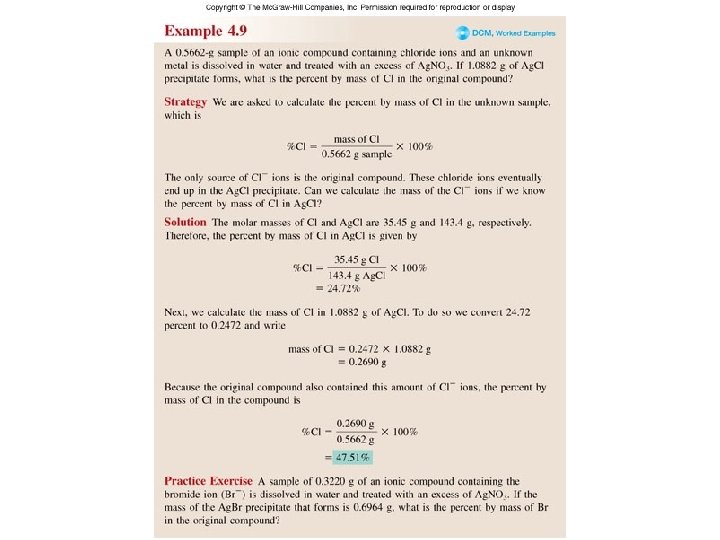
Worked Example 4. 9

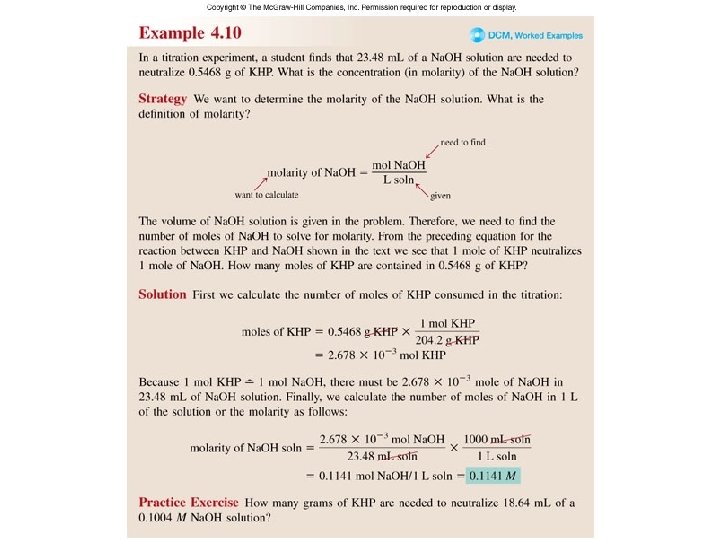
Worked Example 4. 10

Worked Example 4. 11

Worked Example 4. 12