CHAPTER 4 REACTIONS IN AQUEOUS SOLUTION Copyright The







- Slides: 21

CHAPTER 4 : REACTIONS IN AQUEOUS SOLUTION Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

CHAPTER 4 (P 147 -151) 4. 5 Concentration of solutions ( Molarity and dilution ).

4. 5 CONCENTRATION OF SOLUTIONS ( MOLARITY AND DILUTION ).

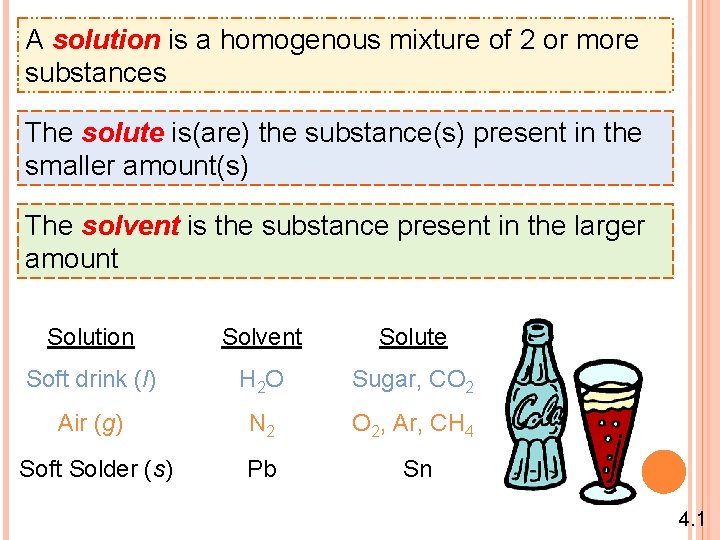
A solution is a homogenous mixture of 2 or more substances The solute is(are) the substance(s) present in the smaller amount(s) The solvent is the substance present in the larger amount Solution Solvent Solute Soft drink (l) H 2 O Sugar, CO 2 Air (g) N 2 O 2, Ar, CH 4 Soft Solder (s) Pb Sn 4. 1

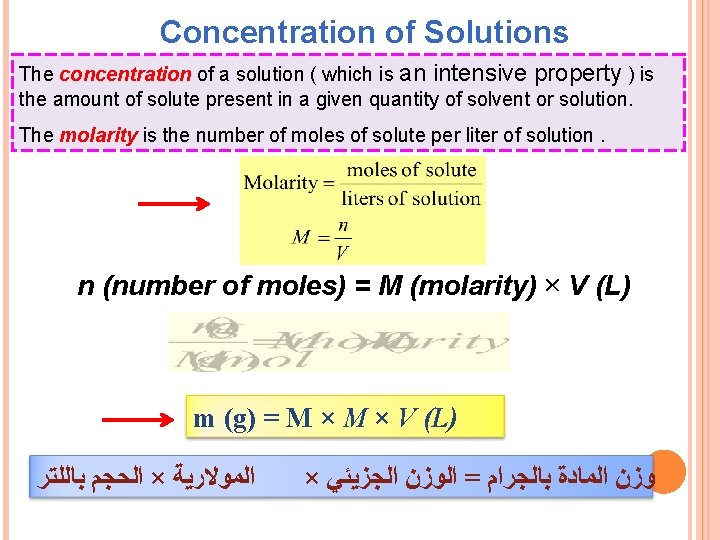
Concentration of Solutions The concentration of a solution ( which is an intensive property ) is the amount of solute present in a given quantity of solvent or solution. The molarity is the number of moles of solute per liter of solution. n (number of moles) = M (molarity) × V (L) m (g) = M × V (L) ﺍﻟﻤﻮﻻﺭﻳﺔ × ﺍﻟﺤﺠﻢ ﺑﺎﻟﻠﺘﺮ × ﻭﺯﻥ ﺍﻟﻤﺎﺩﺓ ﺑﺎﻟﺠﺮﺍﻡ = ﺍﻟﻮﺯﻥ ﺍﻟﺠﺰﻳﺌﻲ

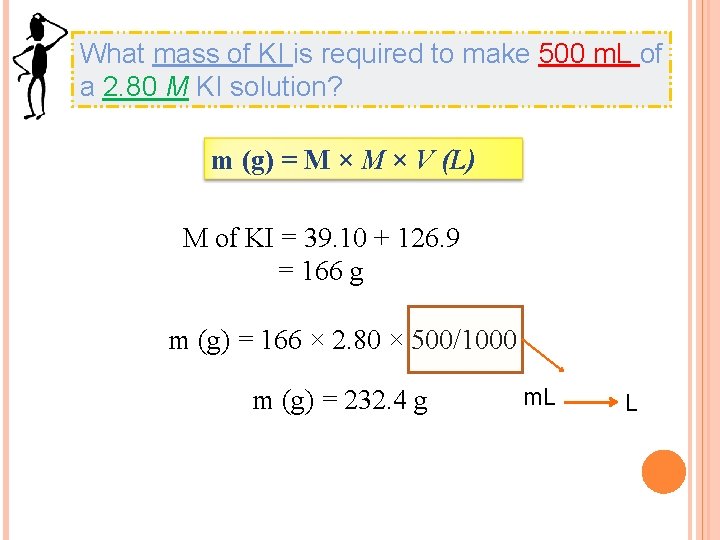
What mass of KI is required to make 500 m. L of a 2. 80 M KI solution? m (g) = M × V (L) M of KI = 39. 10 + 126. 9 = 166 g m (g) = 166 × 2. 80 × 500/1000 m (g) = 232. 4 g m. L L


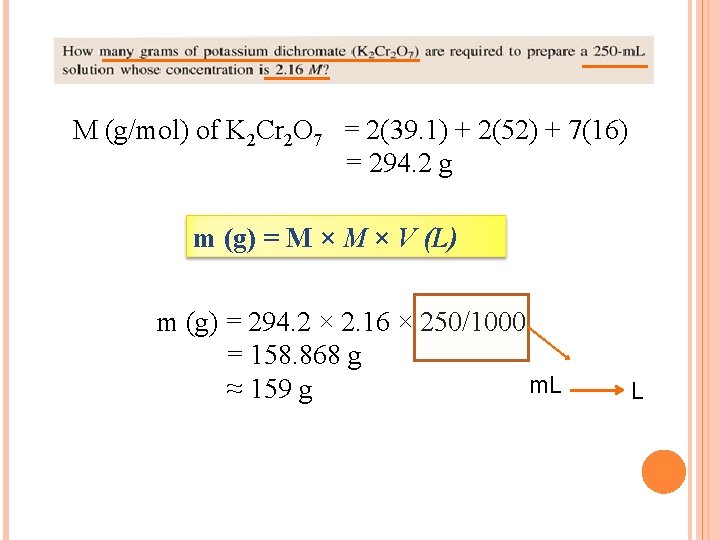
M (g/mol) of K 2 Cr 2 O 7 = 2(39. 1) + 2(52) + 7(16) = 294. 2 g m (g) = M × V (L) m (g) = 294. 2 × 2. 16 × 250/1000 = 158. 868 g m. L ≈ 159 g L

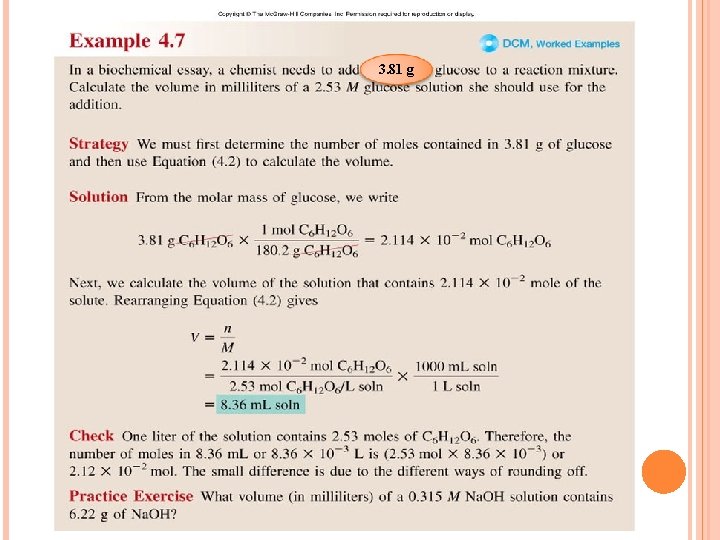
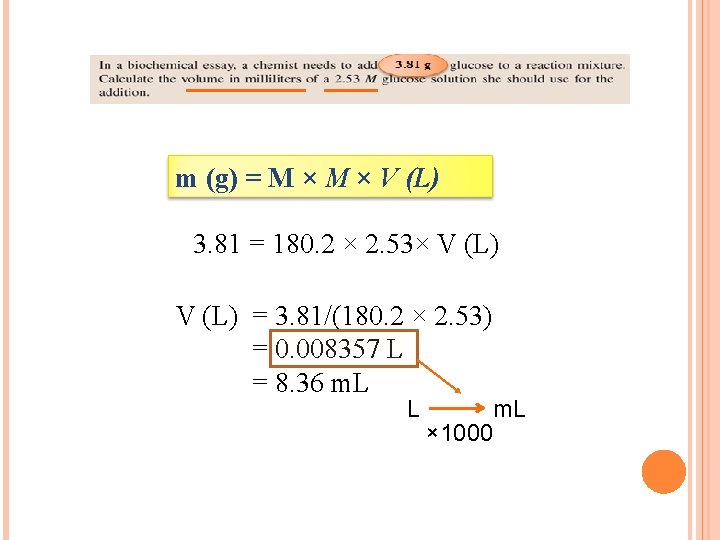
3. 81 g

m (g) = M × V (L) 3. 81 = 180. 2 × 2. 53× V (L) = 3. 81/(180. 2 × 2. 53) = 0. 008357 L = 8. 36 m. L L m. L × 1000

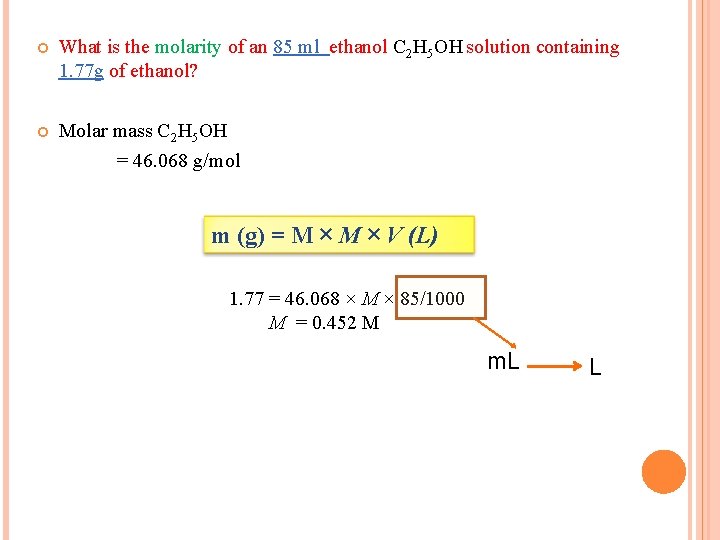
What is the molarity of an 85 ml ethanol C 2 H 5 OH solution containing 1. 77 g of ethanol? Molar mass C 2 H 5 OH = 46. 068 g/mol m (g) = M × V (L) 1. 77 = 46. 068 × M × 85/1000 M = 0. 452 M m. L L

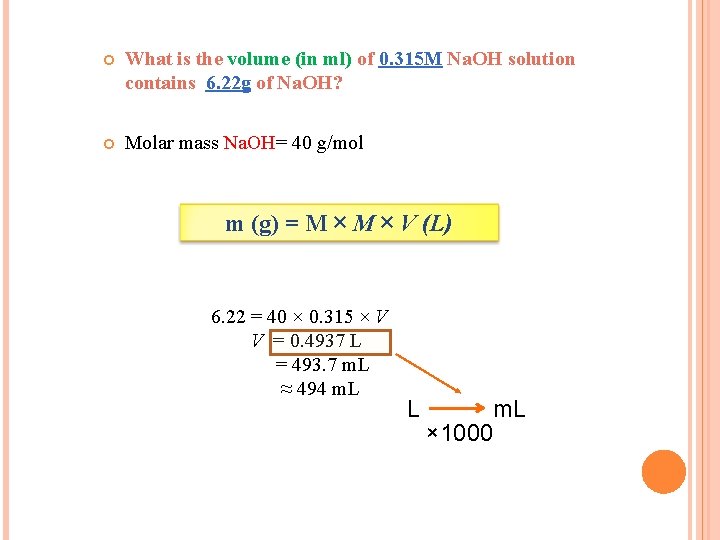
What is the volume (in ml) of 0. 315 M Na. OH solution contains 6. 22 g of Na. OH? Molar mass Na. OH= 40 g/mol m (g) = M × V (L) 6. 22 = 40 × 0. 315 × V V = 0. 4937 L = 493. 7 m. L ≈ 494 m. L L m. L × 1000

4. 5

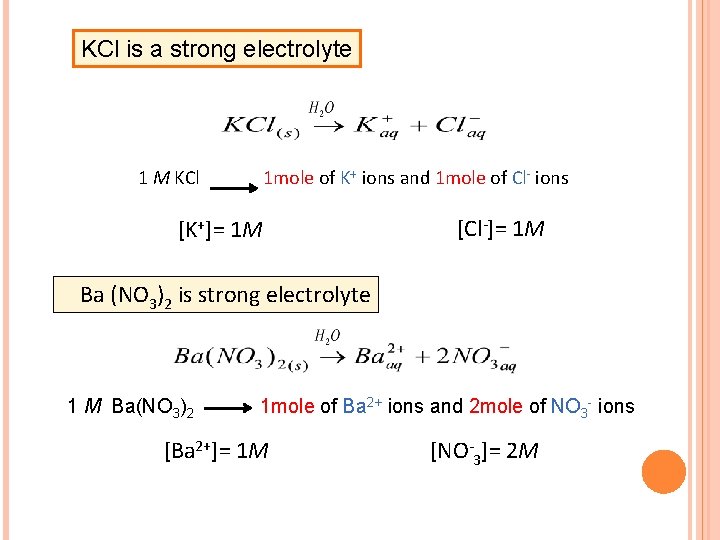
KCl is a strong electrolyte 1 M KCl 1 mole of K+ ions and 1 mole of Cl- ions [K+]= 1 M [Cl-]= 1 M Ba (NO 3)2 is strong electrolyte 1 M Ba(NO 3)2 1 mole of Ba 2+ ions and 2 mole of NO 3 - ions [Ba 2+]= 1 M [NO-3]= 2 M

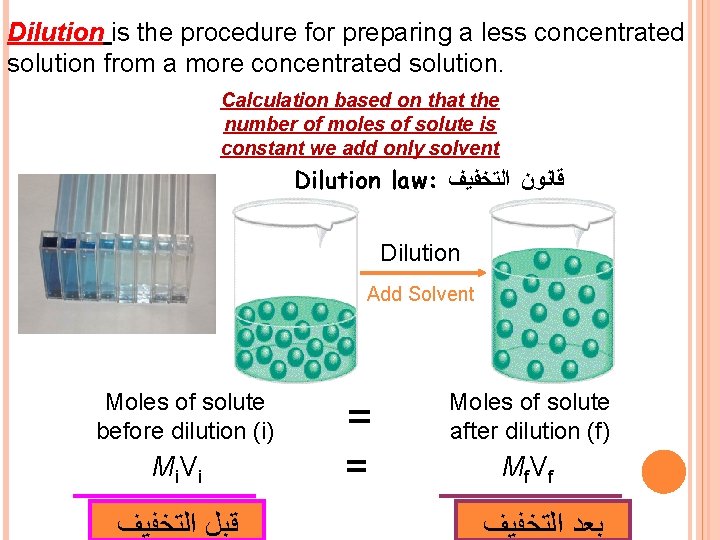
Dilution is the procedure for preparing a less concentrated solution from a more concentrated solution. Calculation based on that the number of moles of solute is constant we add only solvent Dilution law: ﻗﺎﻧﻮﻥ ﺍﻟﺘﺨﻔﻴﻒ Dilution Add Solvent Moles of solute before dilution (i) Mi V i ﺍﻟﺘﺨﻔﻴﻒ ﻗﺒﻞ = Moles of solute after dilution (f) = Mf V f ﺍﻟﺘﺨﻔﻴﻒ ﺑﻌﺪ

How would you prepare 60. 0 m. L of 0. 200 M HNO 3 from a stock solution of 4. 00 M HNO 3? Mi. Vi = Mf. Vf The units of Vi & Vf must be the same (ml or L) Mi = 4. 00 Vi = Mf = 0. 200 Mf V f Mi Vf = 0. 06 L Vi = ? L 0. 200 x 0. 06 = 0. 003 L = 3 m. L = 4. 00 3 m. L of acid + 57 m. L of water = 60 m. L of solution


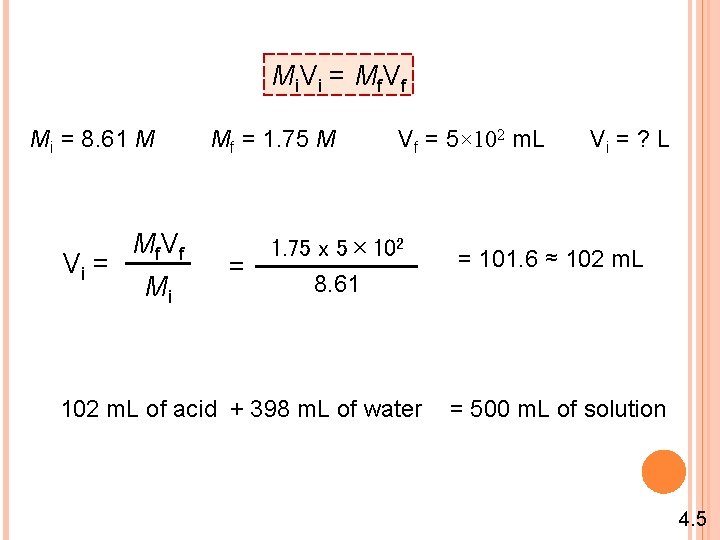
Mi. Vi = Mf. Vf Mi = 8. 61 M Vi = Mf V f Mi Mf = 1. 75 M = Vf = 5× 102 m. L 1. 75 x 5× 102 8. 61 102 m. L of acid + 398 m. L of water Vi = ? L = 101. 6 ≈ 102 m. L = 500 m. L of solution 4. 5

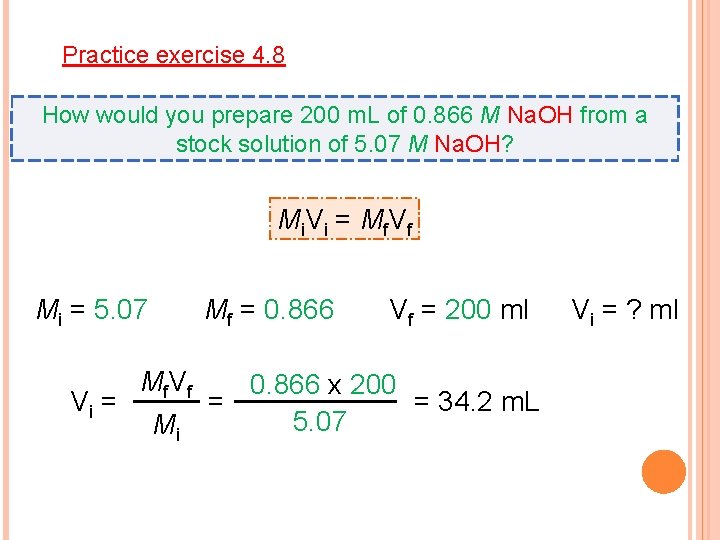
Practice exercise 4. 8 How would you prepare 200 m. L of 0. 866 M Na. OH from a stock solution of 5. 07 M Na. OH? Mi. Vi = Mf. Vf Mi = 5. 07 Vi = Mf = 0. 866 Mf V f Mi Vf = 200 ml 0. 866 x 200 = 34. 2 m. L = 5. 07 Vi = ? ml

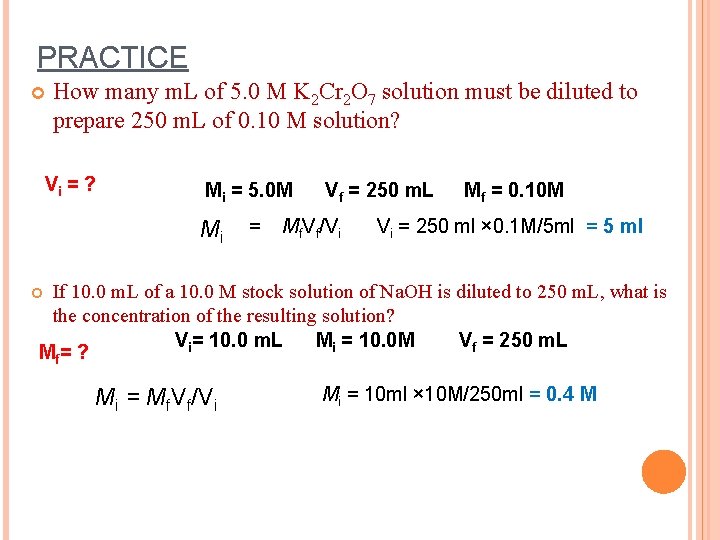
PRACTICE How many m. L of 5. 0 M K 2 Cr 2 O 7 solution must be diluted to prepare 250 m. L of 0. 10 M solution? Vi = ? Mi = 5. 0 M Mi = Vf = 250 m. L Mf. Vf/Vi Mf = 0. 10 M Vi = 250 ml × 0. 1 M/5 ml = 5 ml If 10. 0 m. L of a 10. 0 M stock solution of Na. OH is diluted to 250 m. L, what is the concentration of the resulting solution? Vi= 10. 0 m. L Mi = 10. 0 M Vf = 250 m. L Mf= ? Mi = Mf. Vf/Vi Mi = 10 ml × 10 M/250 ml = 0. 4 M

Problems 4. 60 – 4. 62 – 4. 64 – 4. 66 – 4. 70 – 4. 74