PTT 155 Aldehydes and Ketones JOHAN ARIFF MOHTAR




- Slides: 49

PTT 155 Aldehydes and Ketones JOHAN ARIFF MOHTAR johanariff@unimap. edu. my

Course Outcomes Involved n Ability to APPLY the structure, nomenclature and naming of aldehydes and ketones n Ability to ILLUSTRATE and PROPOSE the reaction of aldehydes and ketones n Ability to ANALYZE the knowledge or organic chemistry in the chemical process industry especially in aldehydes and ketones 2

Outlines n Naming of Aldehydes and Ketones n Relative Reactivity of Carbonyl Group n Nucleophilc Addition Reaction n n n n Water H-Y HCN Gignard Reagent Hydride Amines Hydrazines Alcohols Phosphorus Ylid n Oxidation Reaction of Aldehydes and Ketones 3

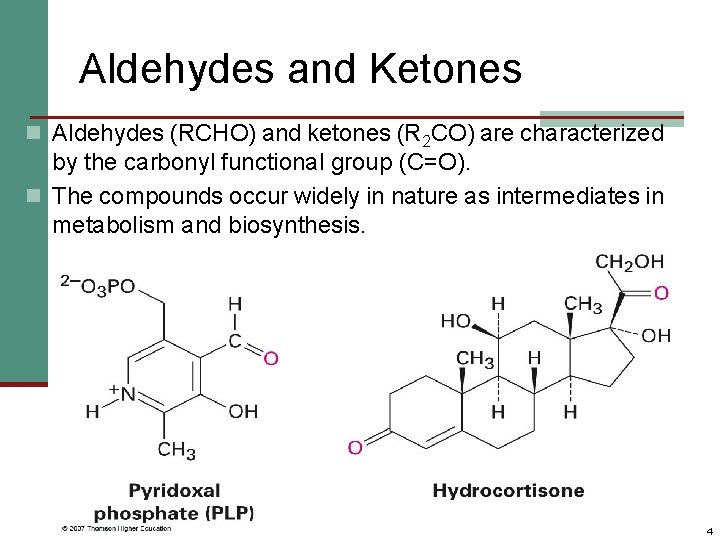
Aldehydes and Ketones n Aldehydes (RCHO) and ketones (R 2 CO) are characterized by the carbonyl functional group (C=O). n The compounds occur widely in nature as intermediates in metabolism and biosynthesis. 4

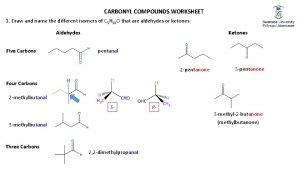
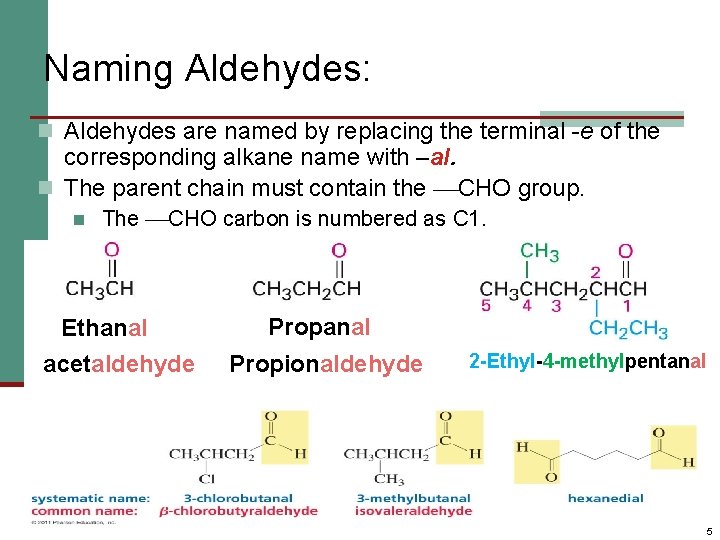
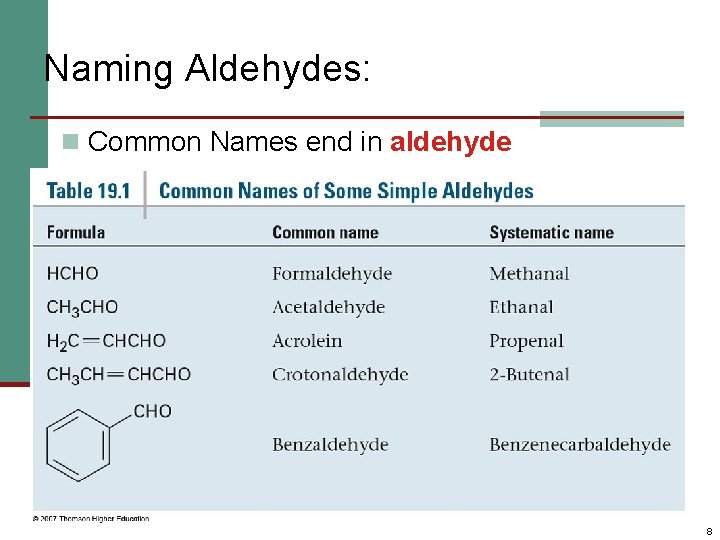
Naming Aldehydes: n Aldehydes are named by replacing the terminal -e of the corresponding alkane name with –al. n The parent chain must contain the CHO group. n The CHO carbon is numbered as C 1. Ethanal acetaldehyde Propanal Propionaldehyde 2 -Ethyl-4 -methylpentanal 5

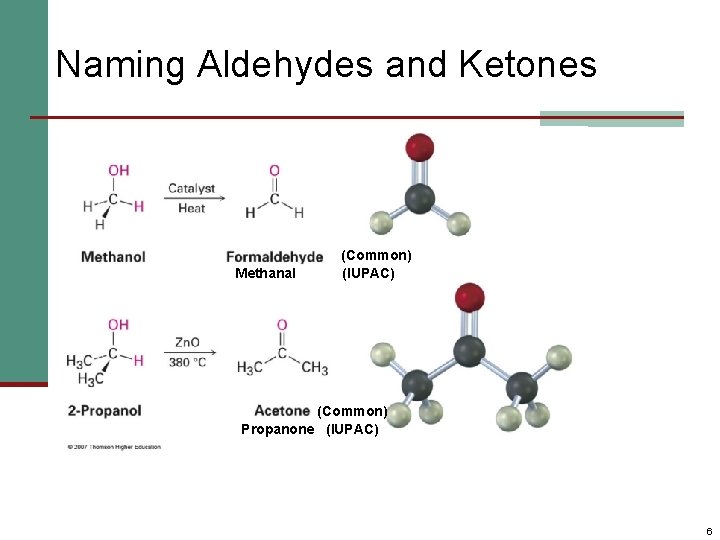
Naming Aldehydes and Ketones Methanal (Common) (IUPAC) (Common) Propanone (IUPAC) 6

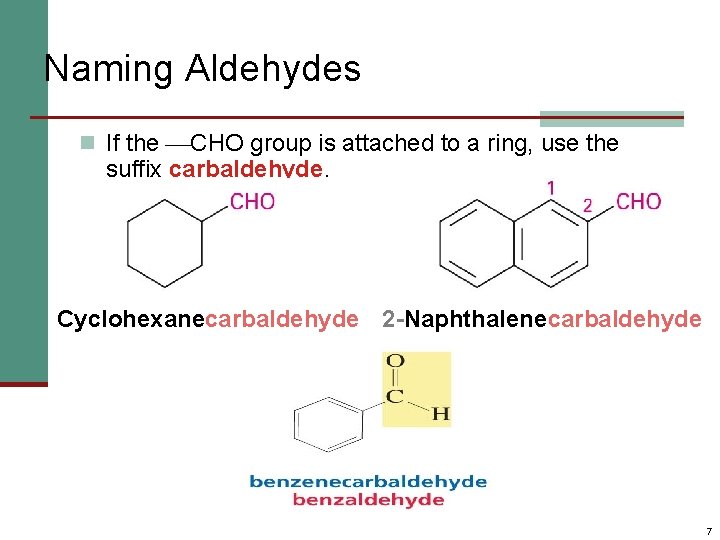
Naming Aldehydes n If the CHO group is attached to a ring, use the suffix carbaldehyde. Cyclohexanecarbaldehyde 2 -Naphthalenecarbaldehyde 7

Naming Aldehydes: n Common Names end in aldehyde 8

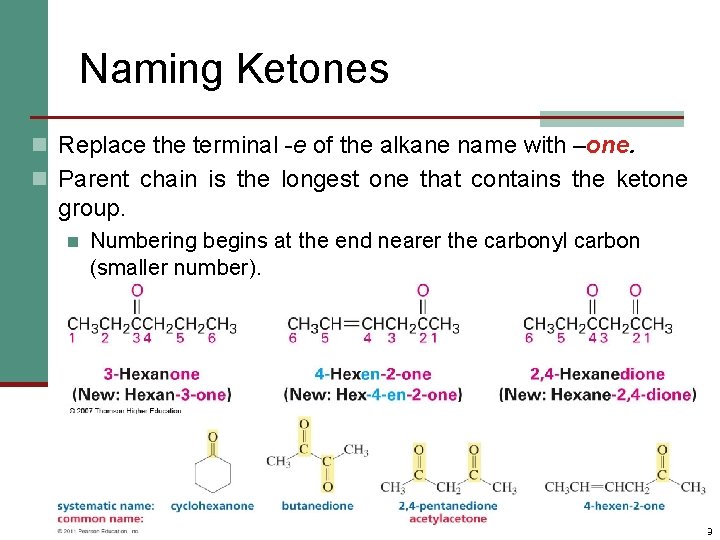
Naming Ketones n Replace the terminal -e of the alkane name with –one. n Parent chain is the longest one that contains the ketone group. n Numbering begins at the end nearer the carbonyl carbon (smaller number). 9

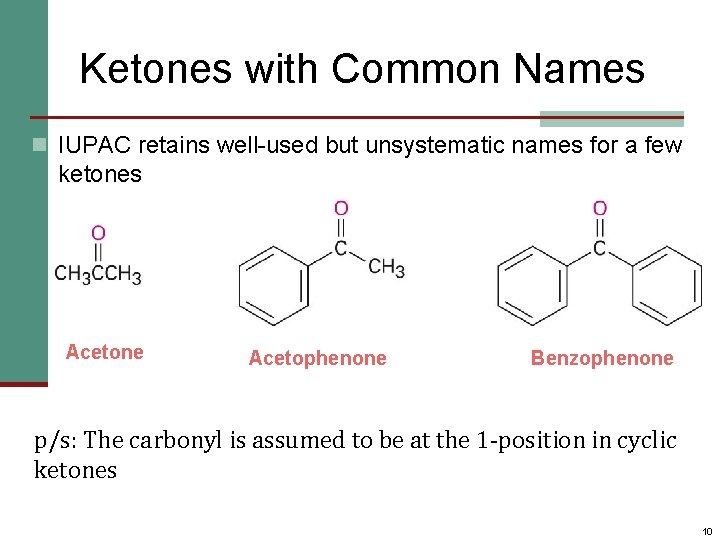
Ketones with Common Names n IUPAC retains well-used but unsystematic names for a few ketones Acetone Acetophenone Benzophenone p/s: The carbonyl is assumed to be at the 1 -position in cyclic ketones 10

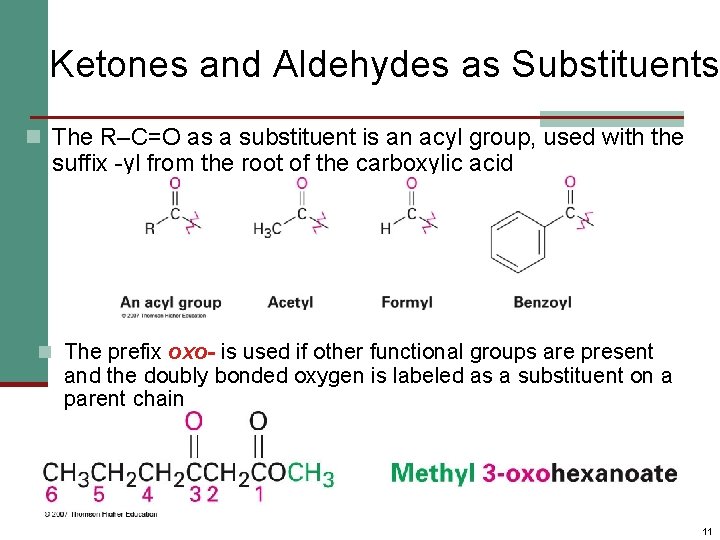
Ketones and Aldehydes as Substituents n The R–C=O as a substituent is an acyl group, used with the suffix -yl from the root of the carboxylic acid n The prefix oxo- is used if other functional groups are present and the doubly bonded oxygen is labeled as a substituent on a parent chain 11

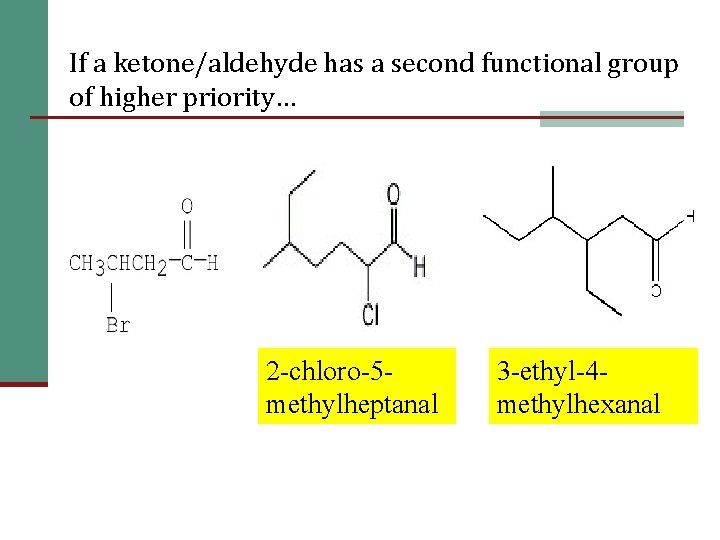
12 If a ketone/aldehyde has a second functional group of higher priority… 2 -chloro-5 methylheptanal 3 -ethyl-4 methylhexanal

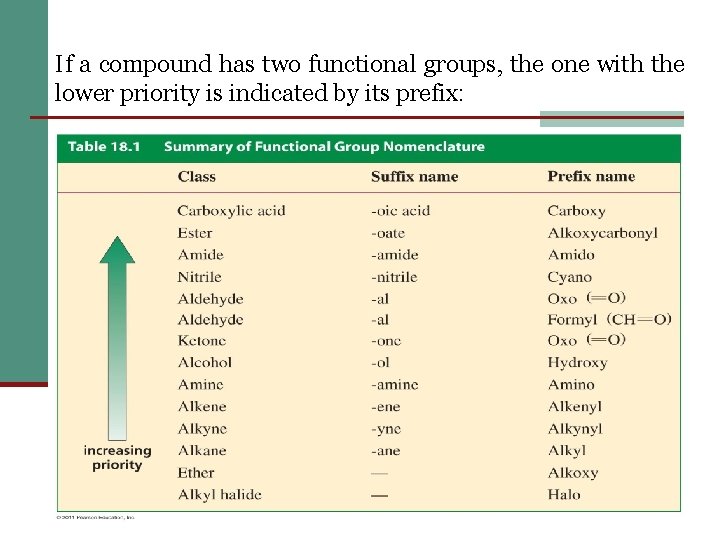
13 If a compound has two functional groups, the one with the lower priority is indicated by its prefix:

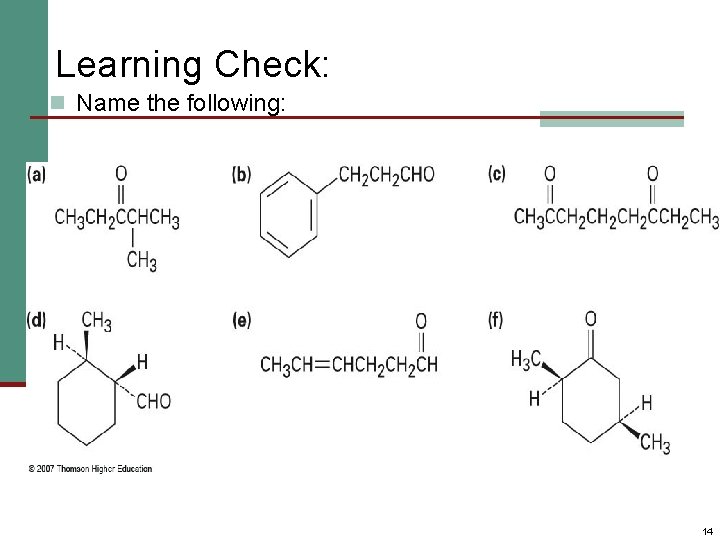
Learning Check: n Name the following: 14

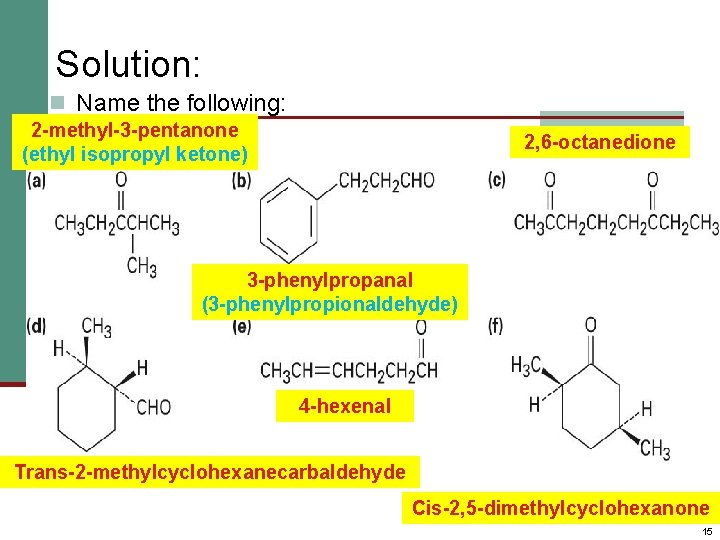
Solution: n Name the following: 2 -methyl-3 -pentanone (ethyl isopropyl ketone) 2, 6 -octanedione 3 -phenylpropanal (3 -phenylpropionaldehyde) 4 -hexenal Trans-2 -methylcyclohexanecarbaldehyde Cis-2, 5 -dimethylcyclohexanone 15

Physical Properties Have higher boiling points than hydrocarbon because they are more polar and the forces between molecules are stronger. They have lower boiling point than alcohols? , why? They are more soluble than hydrocarbons but less soluble than alcohols in water.

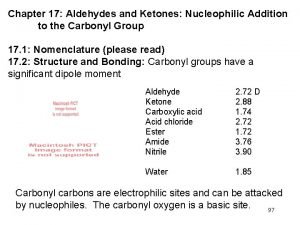
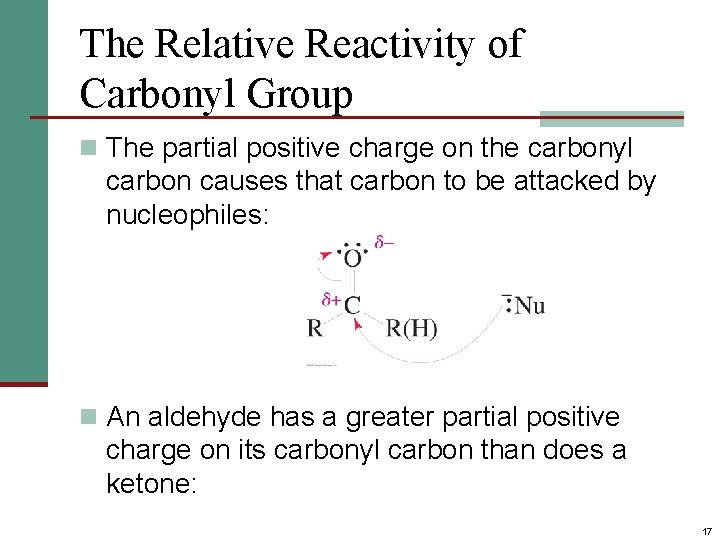
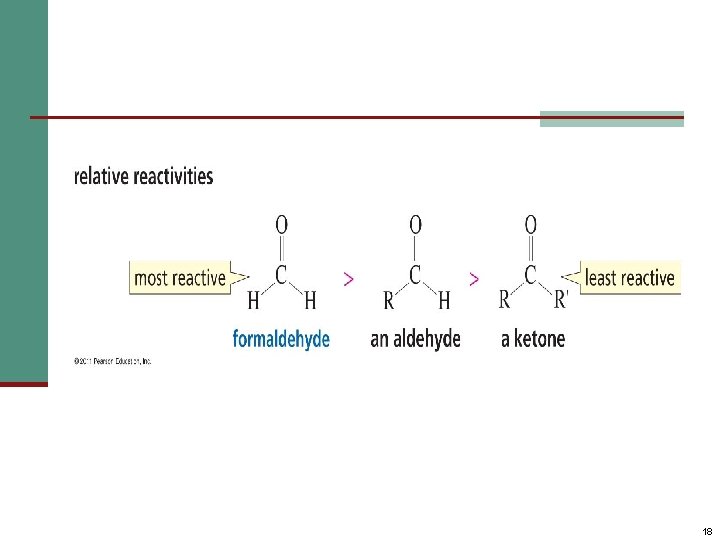
The Relative Reactivity of Carbonyl Group n The partial positive charge on the carbonyl carbon causes that carbon to be attacked by nucleophiles: n An aldehyde has a greater partial positive charge on its carbonyl carbon than does a ketone: 17

18

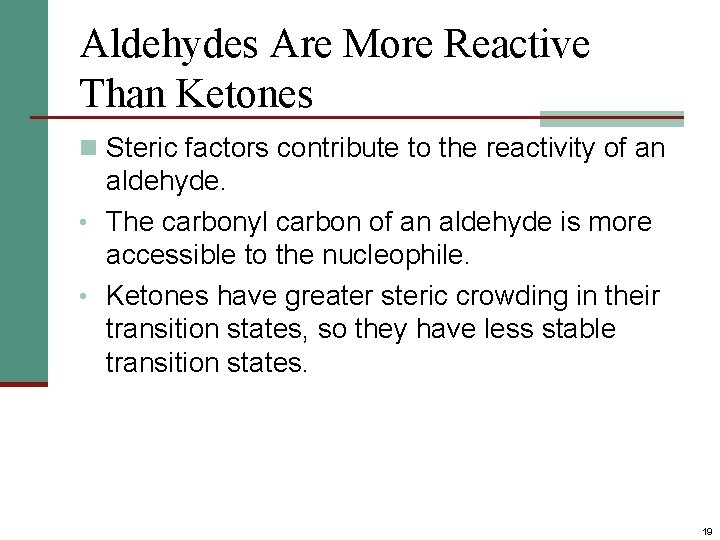
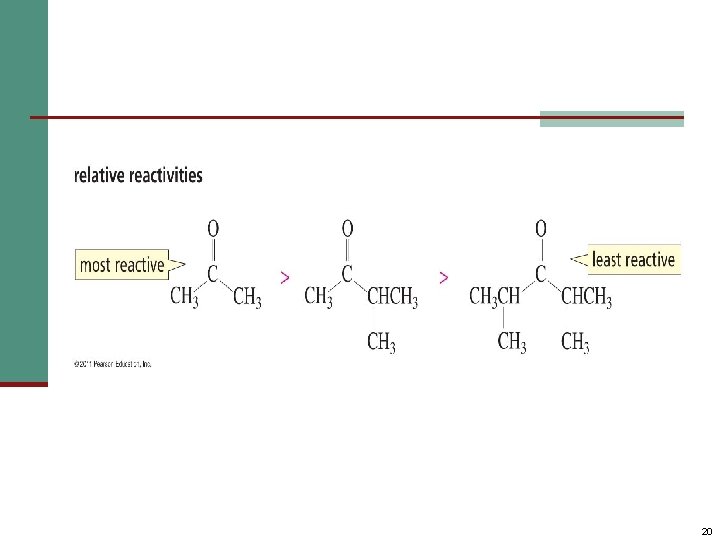
Aldehydes Are More Reactive Than Ketones n Steric factors contribute to the reactivity of an aldehyde. • The carbonyl carbon of an aldehyde is more accessible to the nucleophile. • Ketones have greater steric crowding in their transition states, so they have less stable transition states. 19

20

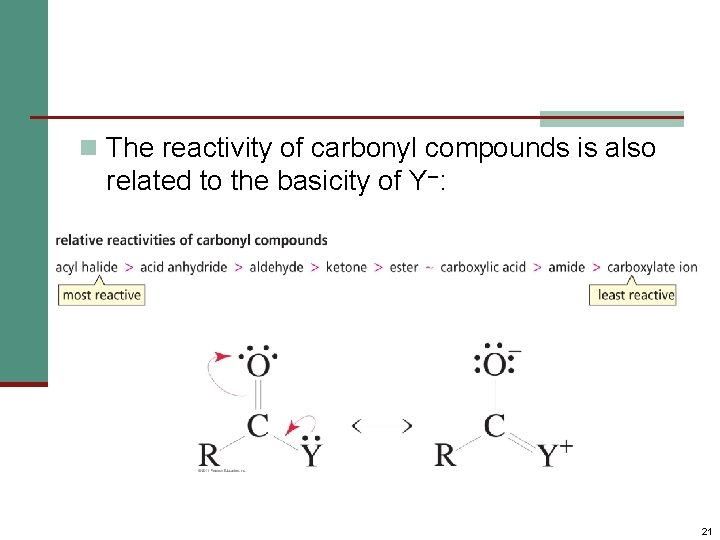
n The reactivity of carbonyl compounds is also related to the basicity of Y–: 21

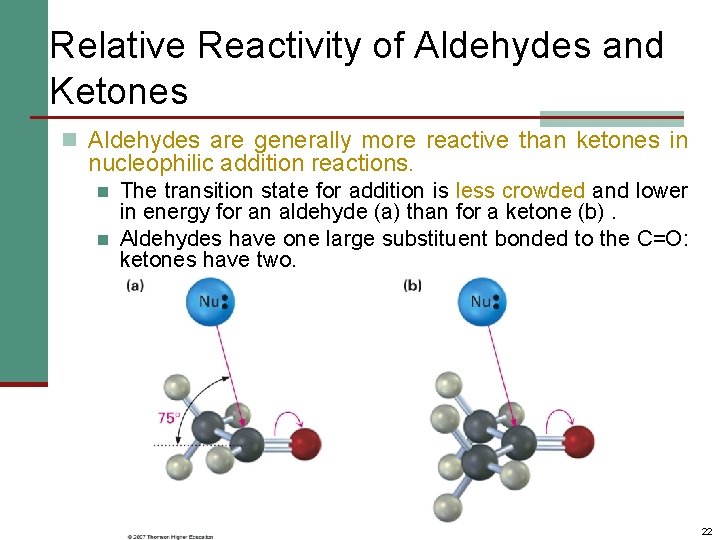
Relative Reactivity of Aldehydes and Ketones n Aldehydes are generally more reactive than ketones in nucleophilic addition reactions. n n The transition state for addition is less crowded and lower in energy for an aldehyde (a) than for a ketone (b). Aldehydes have one large substituent bonded to the C=O: ketones have two. 22

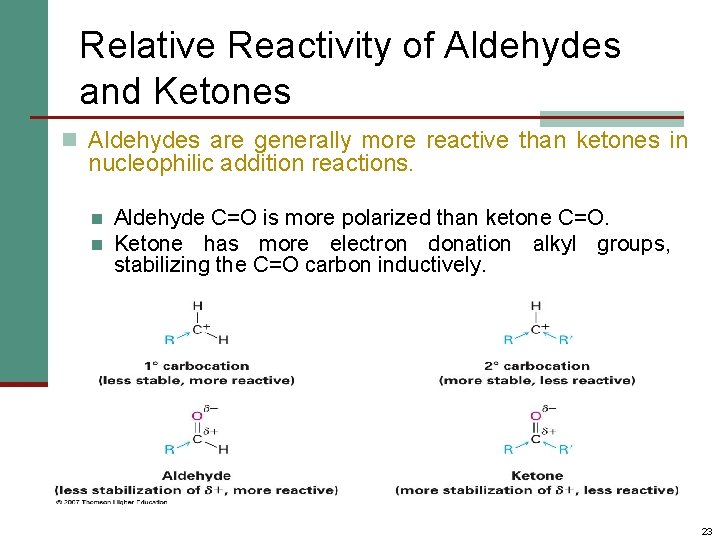
Relative Reactivity of Aldehydes and Ketones n Aldehydes are generally more reactive than ketones in nucleophilic addition reactions. n n Aldehyde C=O is more polarized than ketone C=O. Ketone has more electron donation alkyl groups, stabilizing the C=O carbon inductively. 23

How aldehydes and ketones react? 24

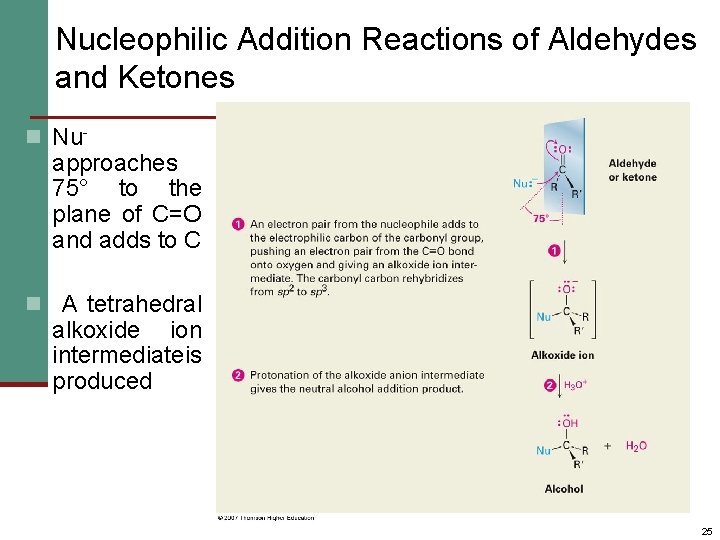
Nucleophilic Addition Reactions of Aldehydes and Ketones n Nu- approaches 75° to the plane of C=O and adds to C n A tetrahedral alkoxide ion intermediateis produced 25

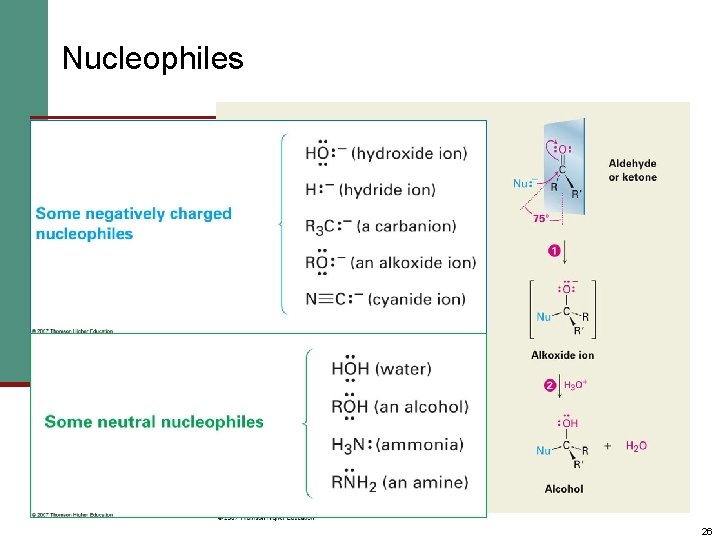
Nucleophiles 26

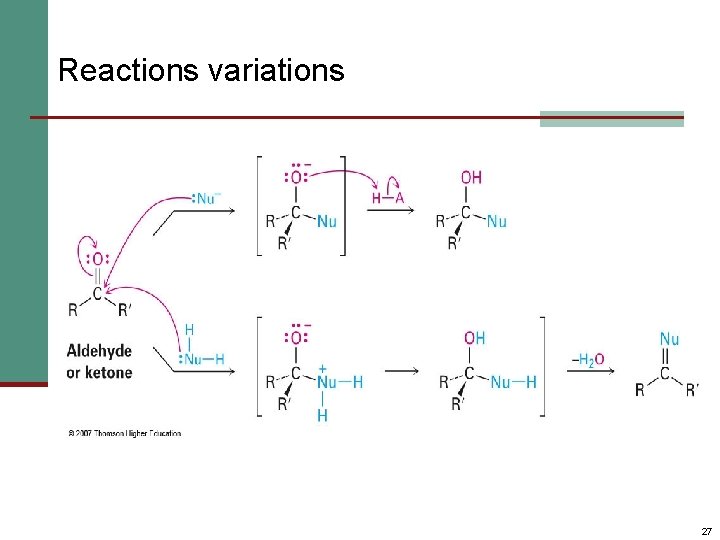
Reactions variations 27

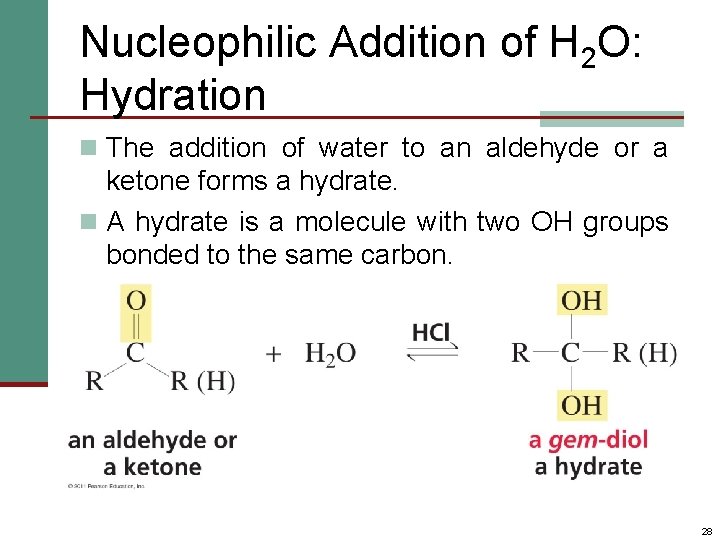
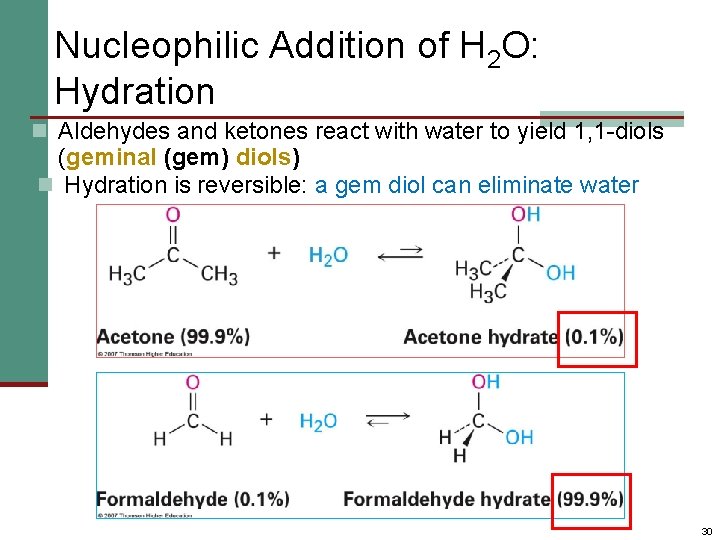
Nucleophilic Addition of H 2 O: Hydration n The addition of water to an aldehyde or a ketone forms a hydrate. n A hydrate is a molecule with two OH groups bonded to the same carbon. 28

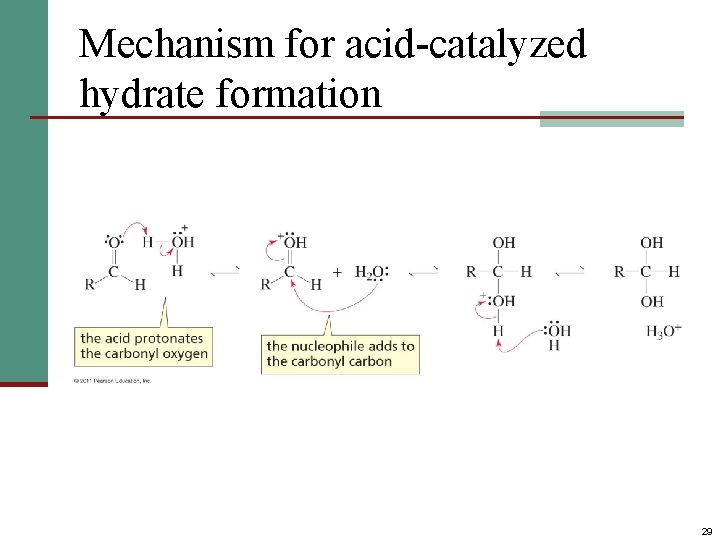
Mechanism for acid-catalyzed hydrate formation 29

Nucleophilic Addition of H 2 O: Hydration n Aldehydes and ketones react with water to yield 1, 1 -diols (geminal (gem) diols) n Hydration is reversible: a gem diol can eliminate water 30

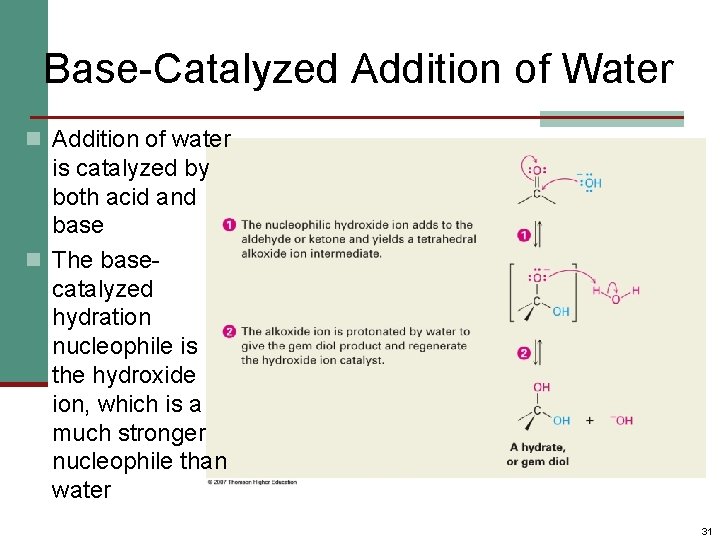
Base-Catalyzed Addition of Water n Addition of water is catalyzed by both acid and base n The basecatalyzed hydration nucleophile is the hydroxide ion, which is a much stronger nucleophile than water 31

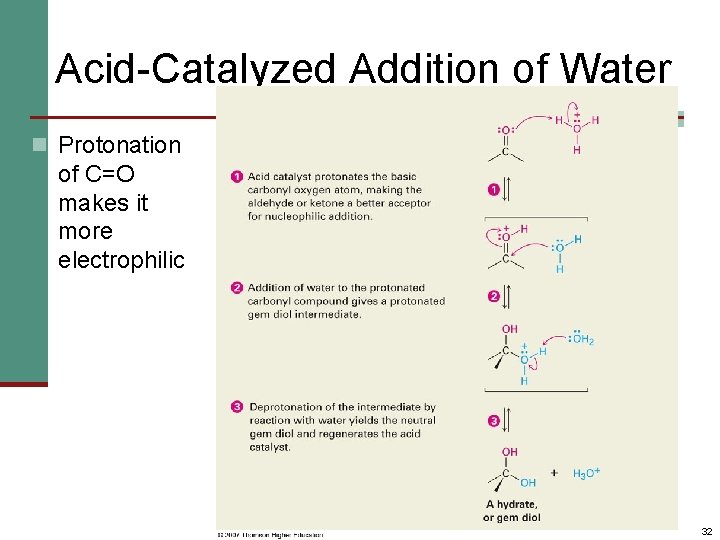
Acid-Catalyzed Addition of Water n Protonation of C=O makes it more electrophilic 32

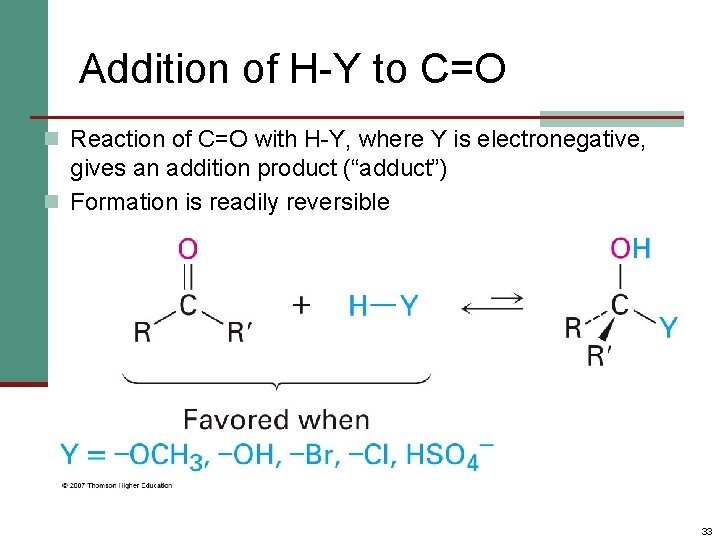
Addition of H-Y to C=O n Reaction of C=O with H-Y, where Y is electronegative, gives an addition product (“adduct”) n Formation is readily reversible 33

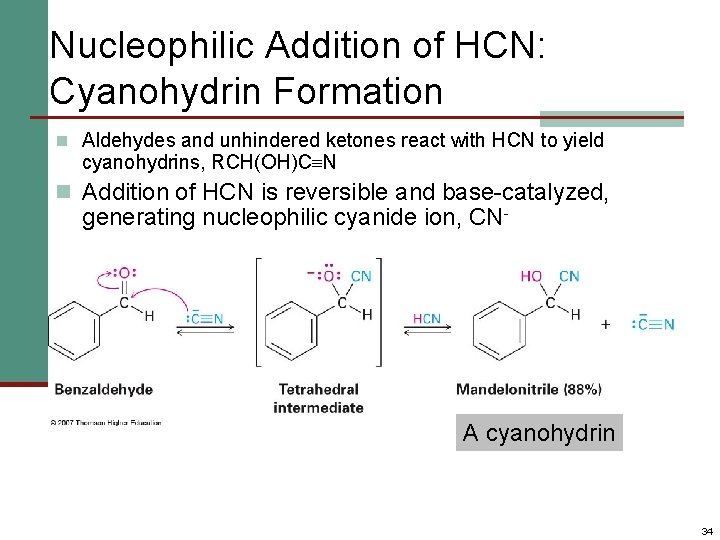
Nucleophilic Addition of HCN: Cyanohydrin Formation n Aldehydes and unhindered ketones react with HCN to yield cyanohydrins, RCH(OH)C N n Addition of HCN is reversible and base-catalyzed, generating nucleophilic cyanide ion, CN- A cyanohydrin 34

Nucleophilic Addition of Grignard Reagents and Hydride Reagents: Alcohol Formation n Grignard reagents react with aldehydes, ketones, and carboxylic acid derivatives. 35

Nucleophilic Addition of Grignard Reagents and Hydride Reagents: Alcohol Formation n Treatment of aldehydes or ketones with Grignard reagents yields an alcohol n Nucleophilic addition of the equivalent of a carbon anion, or carbanion. A carbon–magnesium bond is strongly polarized, so a Grignard reagent reacts for all practical purposes as R : Mg. X +. 36

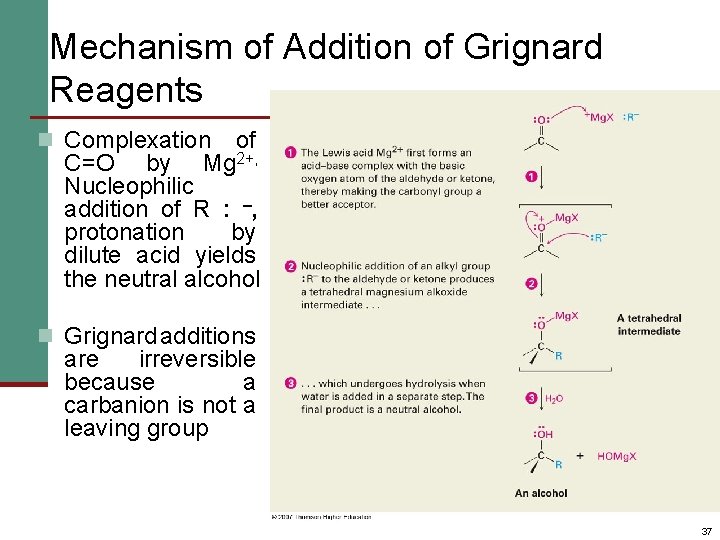
Mechanism of Addition of Grignard Reagents n Complexation of C=O by Mg 2+, Nucleophilic addition of R : , protonation by dilute acid yields the neutral alcohol n Grignard additions are irreversible because a carbanion is not a leaving group 37

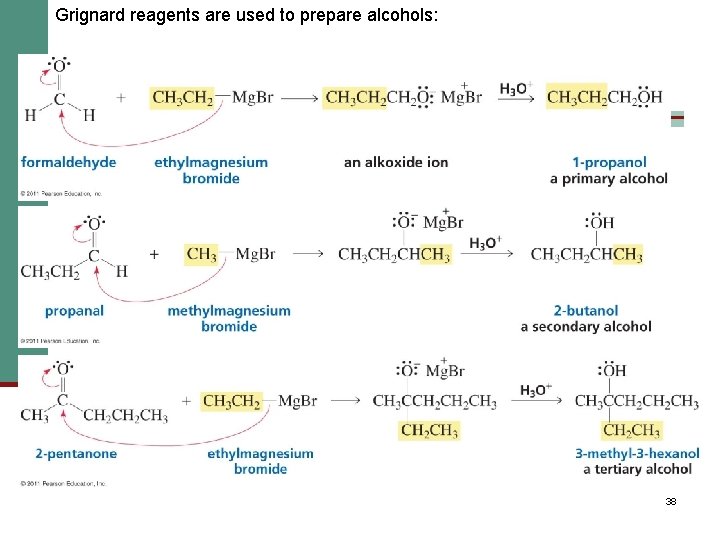
Grignard reagents are used to prepare alcohols: 38

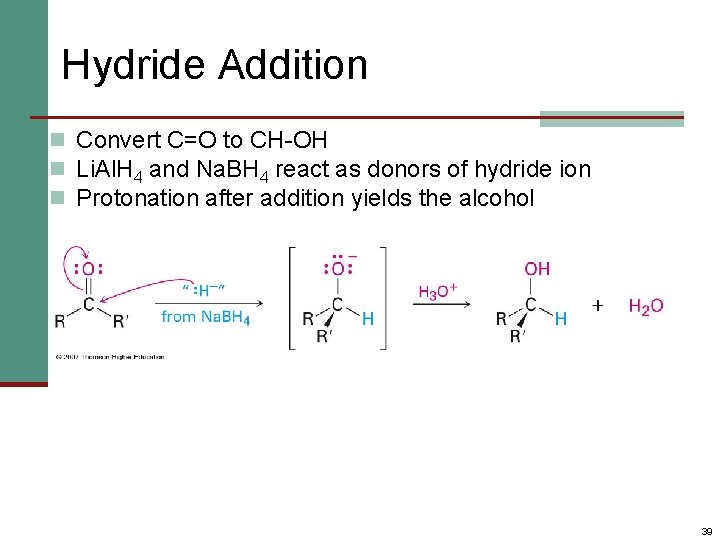
Hydride Addition n Convert C=O to CH-OH n Li. Al. H 4 and Na. BH 4 react as donors of hydride ion n Protonation after addition yields the alcohol 39

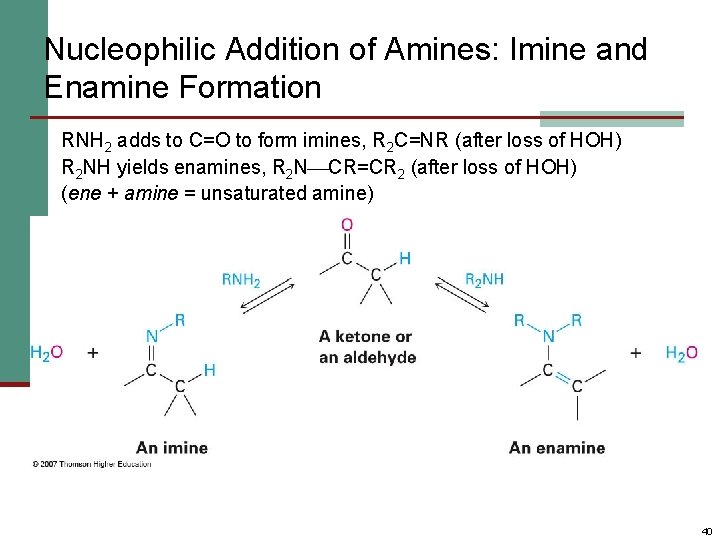
Nucleophilic Addition of Amines: Imine and Enamine Formation RNH 2 adds to C=O to form imines, R 2 C=NR (after loss of HOH) R 2 NH yields enamines, R 2 N CR=CR 2 (after loss of HOH) (ene + amine = unsaturated amine) 40

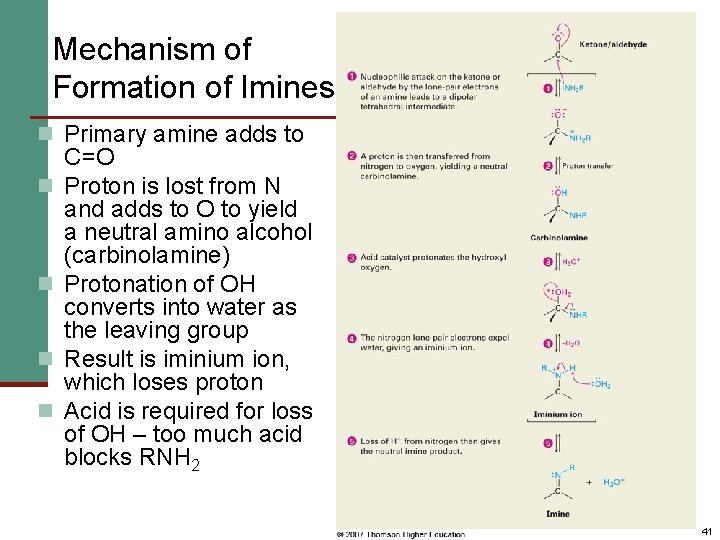
Mechanism of Formation of Imines n Primary amine adds to n n C=O Proton is lost from N and adds to O to yield a neutral amino alcohol (carbinolamine) Protonation of OH converts into water as the leaving group Result is iminium ion, which loses proton Acid is required for loss of OH – too much acid blocks RNH 2 41

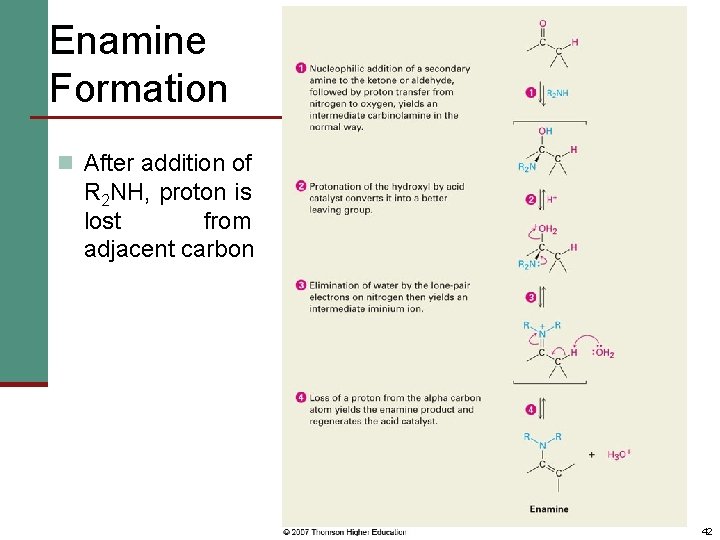
Enamine Formation n After addition of R 2 NH, proton is lost from adjacent carbon 42

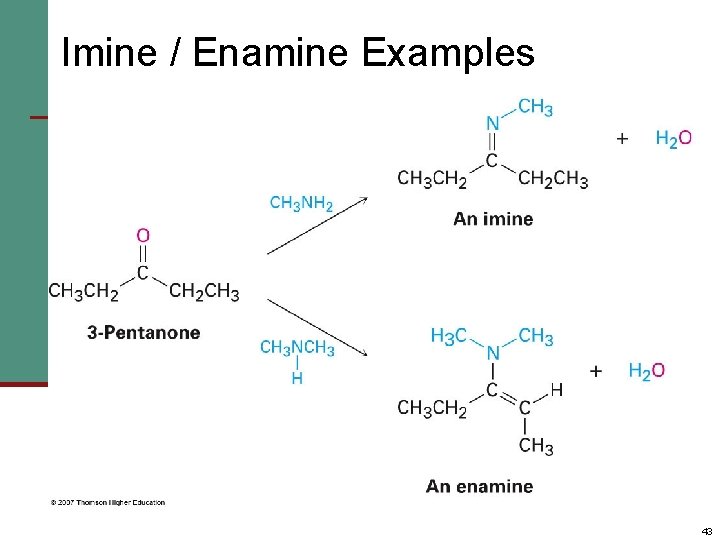
Imine / Enamine Examples 43

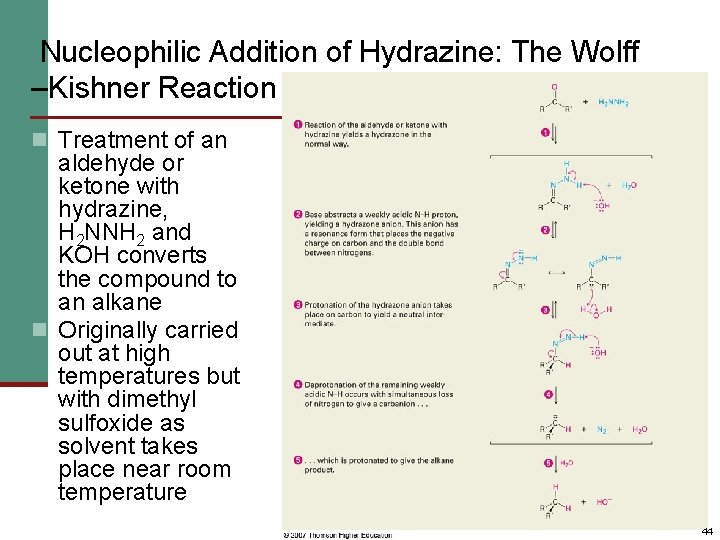
Nucleophilic Addition of Hydrazine: The Wolff –Kishner Reaction n Treatment of an aldehyde or ketone with hydrazine, H 2 NNH 2 and KOH converts the compound to an alkane n Originally carried out at high temperatures but with dimethyl sulfoxide as solvent takes place near room temperature 44

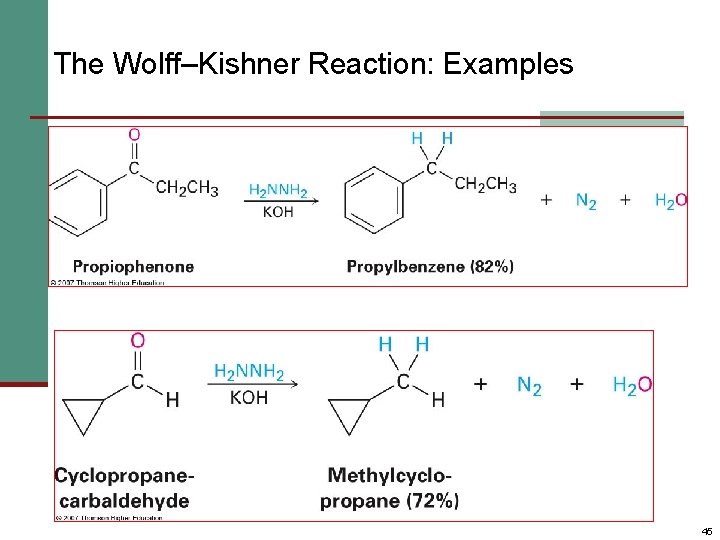
The Wolff–Kishner Reaction: Examples 45

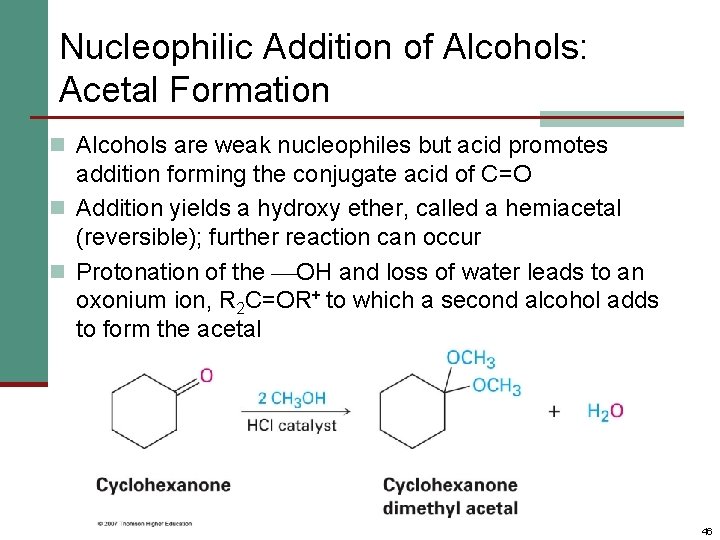
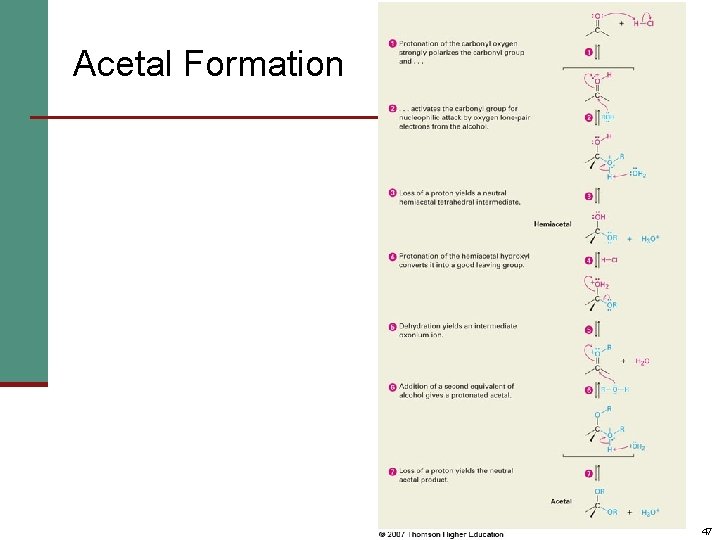
Nucleophilic Addition of Alcohols: Acetal Formation n Alcohols are weak nucleophiles but acid promotes addition forming the conjugate acid of C=O n Addition yields a hydroxy ether, called a hemiacetal (reversible); further reaction can occur n Protonation of the OH and loss of water leads to an oxonium ion, R 2 C=OR+ to which a second alcohol adds to form the acetal 46

Acetal Formation 47

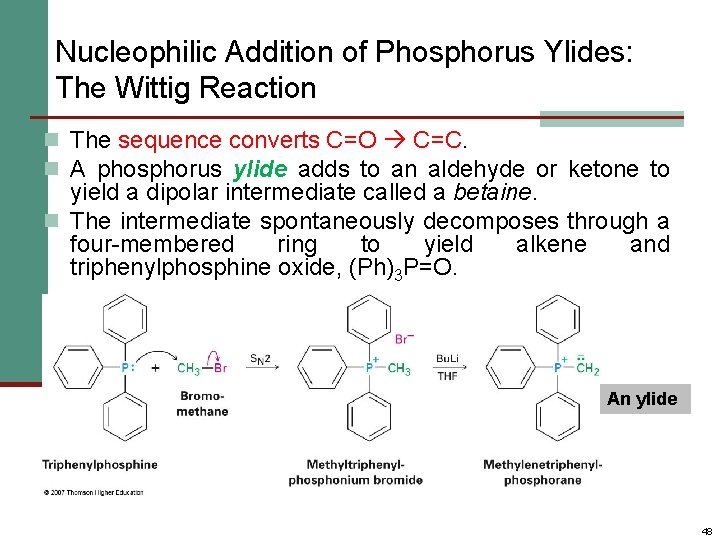
Nucleophilic Addition of Phosphorus Ylides: The Wittig Reaction n The sequence converts C=O C=C. n A phosphorus ylide adds to an aldehyde or ketone to yield a dipolar intermediate called a betaine. n The intermediate spontaneously decomposes through a four-membered ring to yield alkene and triphenylphosphine oxide, (Ph)3 P=O. An ylide 48

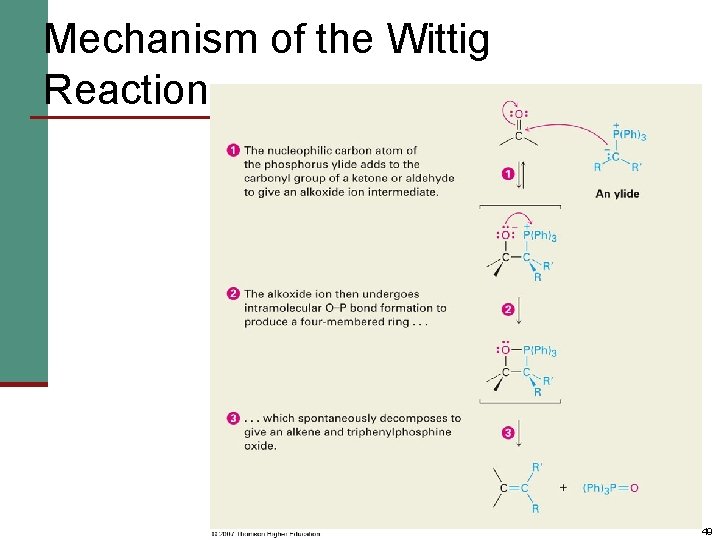
Mechanism of the Wittig Reaction 49
 Aldehydes and ketones structure
Aldehydes and ketones structure Polyhydroxy aldehyde examples
Polyhydroxy aldehyde examples Aldehydes to carboxylic acids
Aldehydes to carboxylic acids Difference between alkanal and alkanone
Difference between alkanal and alkanone How to name ketones and aldehydes
How to name ketones and aldehydes Carbonyl vs ketone
Carbonyl vs ketone Aldehyde and ketone structure
Aldehyde and ketone structure Chemsheets
Chemsheets Chemical properties of aldehyde and ketone
Chemical properties of aldehyde and ketone Ketone reactivity
Ketone reactivity Aldehyde protecting group
Aldehyde protecting group Naming aldehydes and ketones worksheet with answers doc
Naming aldehydes and ketones worksheet with answers doc Aldehydes and ketones nucleophilic addition
Aldehydes and ketones nucleophilic addition Hydration of aldehydes and ketones
Hydration of aldehydes and ketones Rabi mohtar
Rabi mohtar What is galactosemia
What is galactosemia Aldehyde and ketones
Aldehyde and ketones Aldehyde functional group
Aldehyde functional group Uses of ketones
Uses of ketones Naming ether
Naming ether Examples of aldehydes
Examples of aldehydes Polyhydroxy aldehyde structure
Polyhydroxy aldehyde structure Aldol condensation introduction
Aldol condensation introduction Principle of fouchet's test
Principle of fouchet's test Aciclosis
Aciclosis Ketones in urine moderate
Ketones in urine moderate Self oxidation reduction reaction of aldehyde
Self oxidation reduction reaction of aldehyde Properties of ketones
Properties of ketones Keton group
Keton group Ketones
Ketones Nosotros salir
Nosotros salir Normal pt and ptt
Normal pt and ptt Wac 296-155-305
Wac 296-155-305 Monarc gun
Monarc gun Stanford cs 155
Stanford cs 155 Tcrp report 155
Tcrp report 155 Cs 155
Cs 155 Convenio 155
Convenio 155 155 as a fraction in simplest form
155 as a fraction in simplest form The diagram below shows a toy cart possessing 16 joules
The diagram below shows a toy cart possessing 16 joules Cs 155
Cs 155 Cs 155
Cs 155 Cs 155
Cs 155 Aapm tg 155
Aapm tg 155 Cs 155
Cs 155 Convenio 155
Convenio 155 Bentuk sederhana dari 2 cos 80 sin 50 adalah
Bentuk sederhana dari 2 cos 80 sin 50 adalah 3 doğal sayının toplamı 155 tir
3 doğal sayının toplamı 155 tir Jika 67 - (-28) + 12a = 155 nilai a adalah
Jika 67 - (-28) + 12a = 155 nilai a adalah Ley general del ambiente resumen
Ley general del ambiente resumen