CHAPTER 13 ALDEHYDES and KETONES Lecture 32 Aldehydes





- Slides: 11

CHAPTER 13 ALDEHYDES and KETONES

Lecture 32: Aldehydes and Ketones- Aldehydes in medicine, preparation of aldehydes and ketones Subject: Organic Chemistry CLO LLO By the end of this Lecture, students should be able to: K 2. 1 L 32. 1 Recognize the medicinally important aldehydes and their reactions C 2. 1 L 32. 2 Write the IUPAC nomenclature of aldehydes and ketones and their preparation Essential reading: General, Organic, and Biochemistry 9 th Edition By Katherine J. Denniston, Joseph J. Topping, Danae R. Quirk Dorr, and Robert L. Caret

Aldehydes & Ketones • Aldehydes and ketones are characterized by presence of a carbonyl group which is carbon atom bonded to oxygen atom by a double bond • Aldehydes contain a –CHO group and are named by dropping the -e and adding -al to the root name. The carbon carrying the aldehyde group is given the number 1 in naming. • Ketones contain the functional group C=O and are named by dropping the -e and adding -one. The functional group is given the lowest possible number. • Aldehydes, Ketones, Amides, Esters, Acid chloride, Acid anhydride and Carboxylic acids contain carbonyl group General, Organic, and Biochemistry 9 th Edition By Katherine J. Denniston, Joseph J. Topping and Danae R. Quirk Dorr, Robert L. Caret Page Number : 451 -454

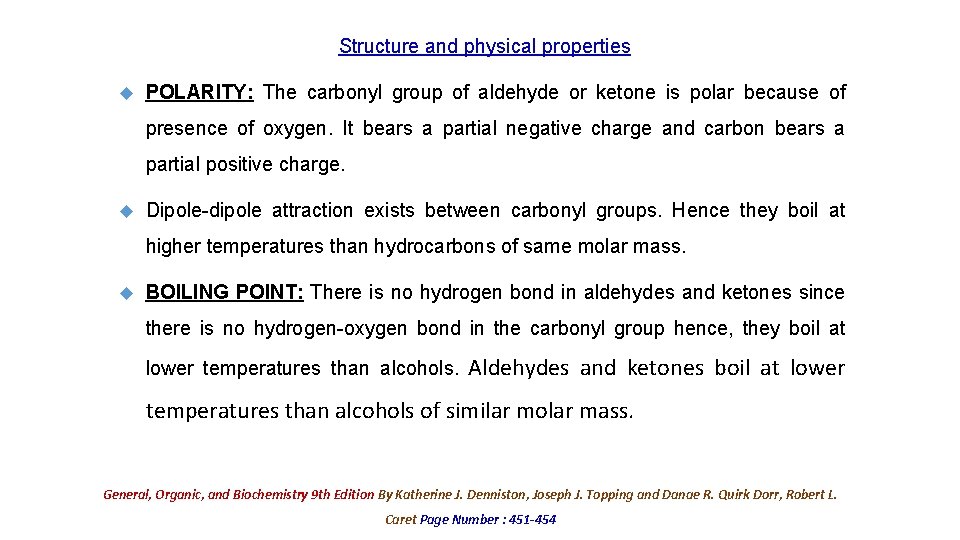
Structure and physical properties POLARITY: The carbonyl group of aldehyde or ketone is polar because of presence of oxygen. It bears a partial negative charge and carbon bears a partial positive charge. Dipole-dipole attraction exists between carbonyl groups. Hence they boil at higher temperatures than hydrocarbons of same molar mass. BOILING POINT: There is no hydrogen bond in aldehydes and ketones since there is no hydrogen-oxygen bond in the carbonyl group hence, they boil at lower temperatures than alcohols. Aldehydes and ketones boil at lower temperatures than alcohols of similar molar mass. General, Organic, and Biochemistry 9 th Edition By Katherine J. Denniston, Joseph J. Topping and Danae R. Quirk Dorr, Robert L. Caret Page Number : 451 -454

SOLUBILITY: The smaller members of the two families (five or fewer carbon atoms) are reasonably soluble in water. However, as the carbon chain length increases, the compounds become less polar and more hydrocarbon like. These larger compounds are soluble in nonpolar organic solvents. Which member in each pairs will be more soluble in water? a. CH 3 CH 2 CH 3 or CH 3(CO)CH 3 b. CH 3 COCH 2 CH 3 or CH 3 CH(OH)CH 2 CH 3 c. HOH 2 CH 2 OH or CHO-CHO Which member in each pairs will have a higher boiling point? a. CH 3 CH 2 COOH or CH 3 CH 2 CHO b. CH 3 COOH or CH 3 COCH 3 c. CH 3 CH 2 OH or CH 3 CHO General, Organic, and Biochemistry 9 th Edition By Katherine J. Denniston, Joseph J. Topping and Danae R. Quirk Dorr, Robert L. Caret Page Number : 451 -454

IUPAC NOMENCLATURE a. Provide the IUPAC names for each of the aldehydes and ketones or Draw the structures for the following a. 2 -Methylpropanal b. 2 -Methylpentanal c. 4 -Bromopentanal d. 2, 3 -Dimethylbutanal e. 6, 8 -Dimethyl-2 -nonane General, Organic, and Biochemistry 9 th Edition By Katherine J. Denniston, Joseph J. Topping and Danae R. Quirk Dorr, Robert L. Caret Page Number : 455 -459

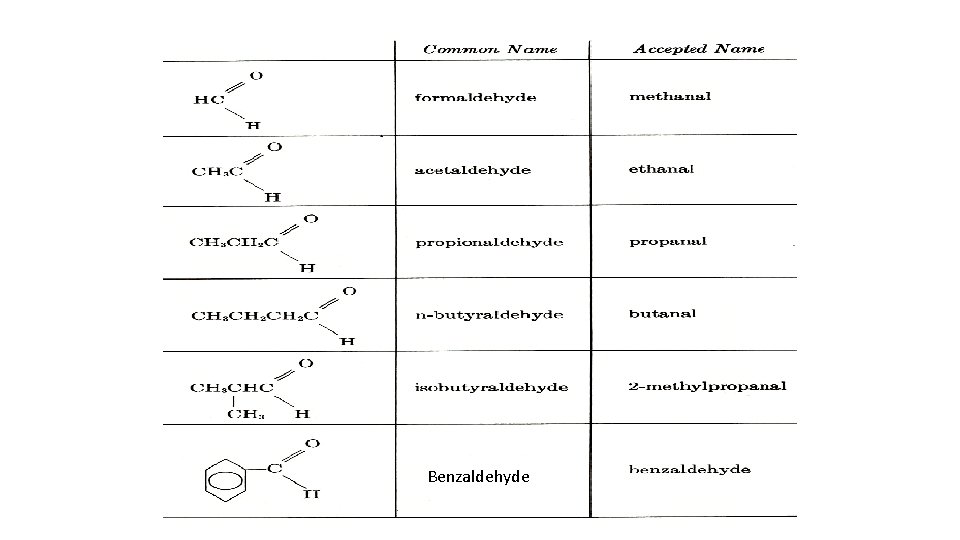
Benzaldehyde

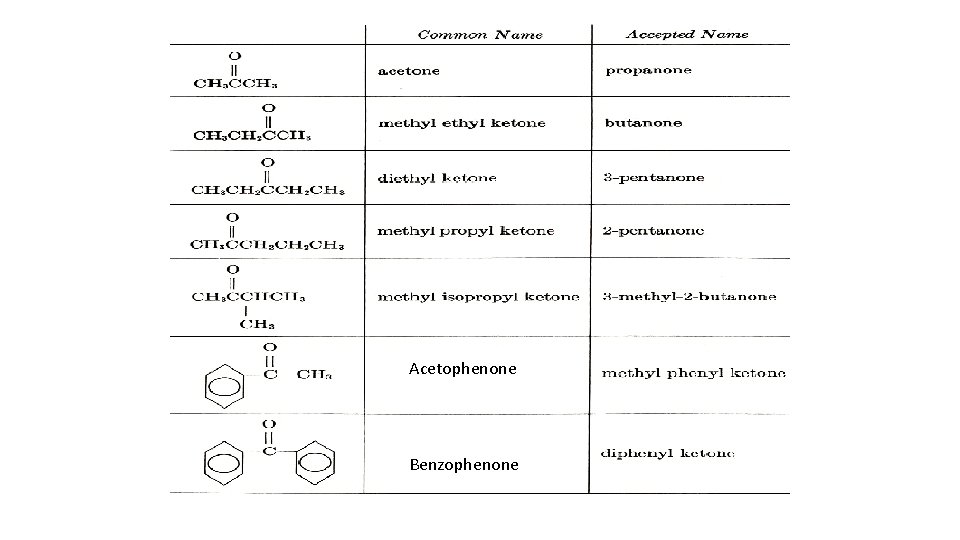
Acetophenone Benzophenone

Medicinal Importance of Aldehydes ØMethanal or formalin is a gas. Formalin is used as a preservative for tissues and as embalming fluid. ØEthanal is produced from ethanol in liver. It is responsible for symptoms of a hangover. Ø Propanone is an important solvent and has the ability to dissolve organic compounds and is nail polish remover, varnish or glue ØVanillin imparts aroma of vanilla- flavoring agent General, Organic, and Biochemistry 9 th Edition By Katherine J. Denniston, Joseph J. Topping and Danae R. Quirk Dorr, Robert L. Caret Page Number : 460

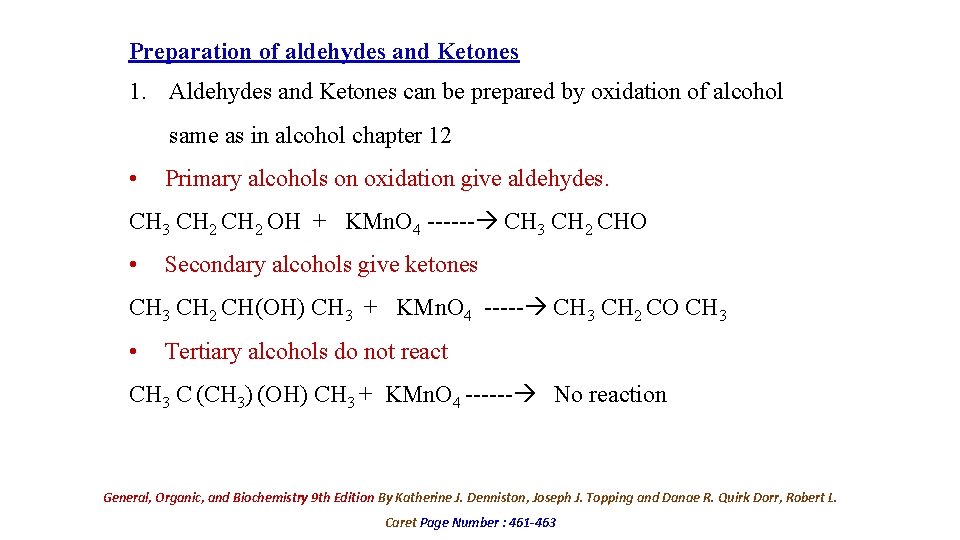
Preparation of aldehydes and Ketones 1. Aldehydes and Ketones can be prepared by oxidation of alcohol same as in alcohol chapter 12 • Primary alcohols on oxidation give aldehydes. CH 3 CH 2 OH + KMn. O 4 ------ CH 3 CH 2 CHO • Secondary alcohols give ketones CH 3 CH 2 CH(OH) CH 3 + KMn. O 4 ----- CH 3 CH 2 CO CH 3 • Tertiary alcohols do not react CH 3 C (CH 3) (OH) CH 3 + KMn. O 4 ------ No reaction General, Organic, and Biochemistry 9 th Edition By Katherine J. Denniston, Joseph J. Topping and Danae R. Quirk Dorr, Robert L. Caret Page Number : 461 -463

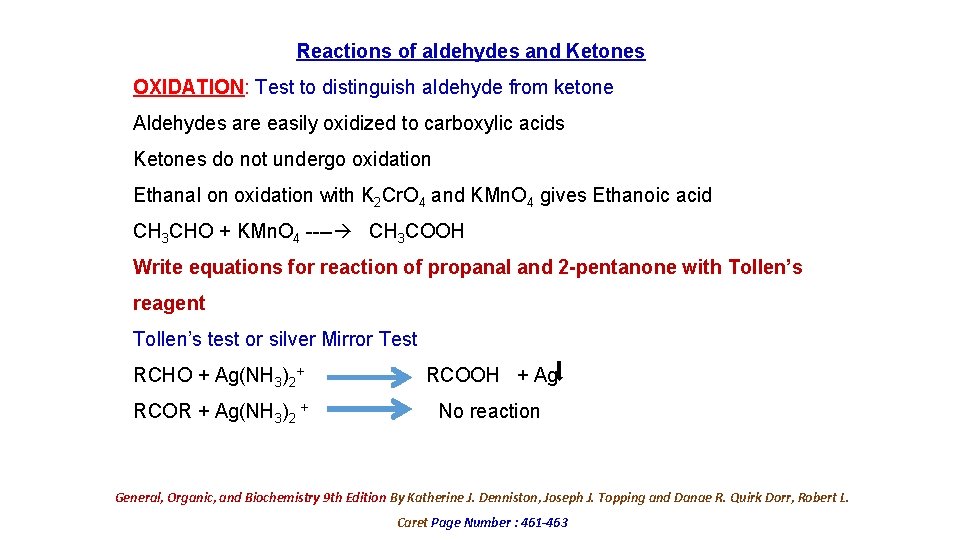
Reactions of aldehydes and Ketones OXIDATION: Test to distinguish aldehyde from ketone Aldehydes are easily oxidized to carboxylic acids Ketones do not undergo oxidation Ethanal on oxidation with K 2 Cr. O 4 and KMn. O 4 gives Ethanoic acid CH 3 CHO + KMn. O 4 ---- CH 3 COOH Write equations for reaction of propanal and 2 -pentanone with Tollen’s reagent Tollen’s test or silver Mirror Test RCHO + Ag(NH 3)2+ RCOOH + Ag RCOR + Ag(NH 3)2 + No reaction General, Organic, and Biochemistry 9 th Edition By Katherine J. Denniston, Joseph J. Topping and Danae R. Quirk Dorr, Robert L. Caret Page Number : 461 -463