Aldehydes and Ketones Miss Sineenard Songsri Introduction Aldehydes




![[2] Reaction at the α carbon. A second general reaction of aldehydes and ketones [2] Reaction at the α carbon. A second general reaction of aldehydes and ketones](https://slidetodoc.com/presentation_image/3989475c9c910293a3cfde9918c28c39/image-18.jpg)
![[1] Nucleophilic addition reaction. Two general mechanisms are usually drawn for nucleophilic addition, depending [1] Nucleophilic addition reaction. Two general mechanisms are usually drawn for nucleophilic addition, depending](https://slidetodoc.com/presentation_image/3989475c9c910293a3cfde9918c28c39/image-19.jpg)
![Ø Absence of an acid catalyzed nucleophilic addition Step [1]: The nucleophile attacks the Ø Absence of an acid catalyzed nucleophilic addition Step [1]: The nucleophile attacks the](https://slidetodoc.com/presentation_image/3989475c9c910293a3cfde9918c28c39/image-20.jpg)
![In Step [2], the nucleophile attacks, and then deprotonation forms the neutral addition product In Step [2], the nucleophile attacks, and then deprotonation forms the neutral addition product](https://slidetodoc.com/presentation_image/3989475c9c910293a3cfde9918c28c39/image-22.jpg)
- Slides: 24

Aldehydes and Ketones Miss Sineenard Songsri

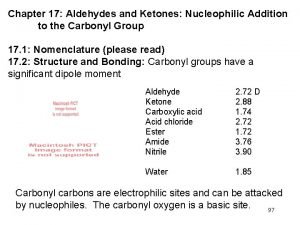
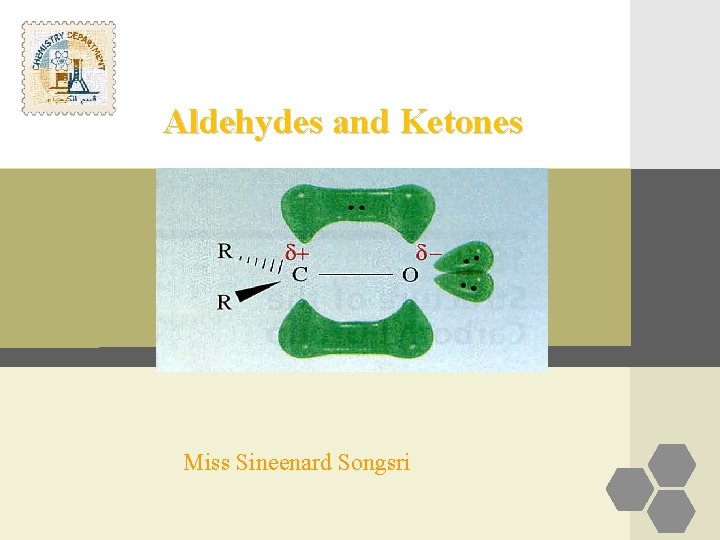
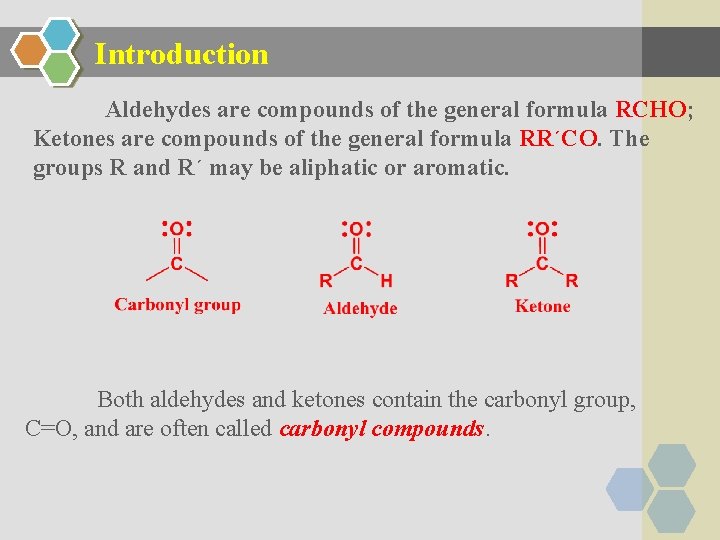
Introduction Aldehydes are compounds of the general formula RCHO; Ketones are compounds of the general formula RR´CO. The groups R and R´ may be aliphatic or aromatic. Both aldehydes and ketones contain the carbonyl group, C=O, and are often called carbonyl compounds.

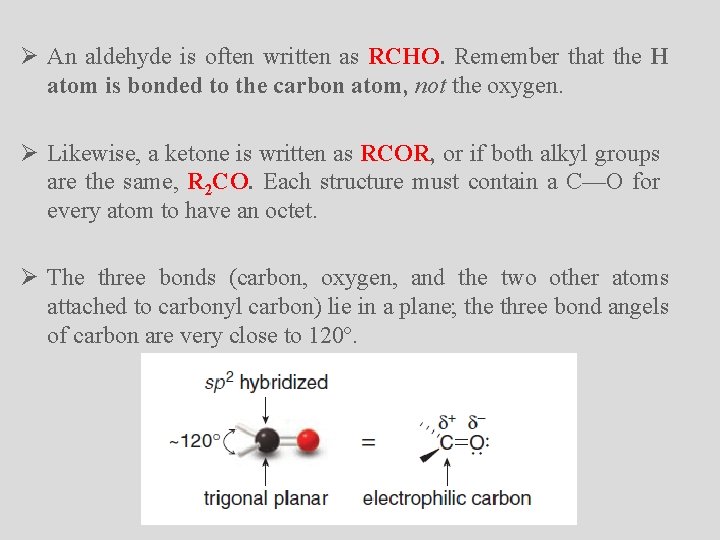
Ø An aldehyde is often written as RCHO. Remember that the H atom is bonded to the carbon atom, not the oxygen. Ø Likewise, a ketone is written as RCOR, or if both alkyl groups are the same, R 2 CO. Each structure must contain a C––O for every atom to have an octet. Ø The three bonds (carbon, oxygen, and the two other atoms attached to carbonyl carbon) lie in a plane; the three bond angels of carbon are very close to 120º.

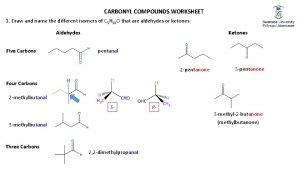
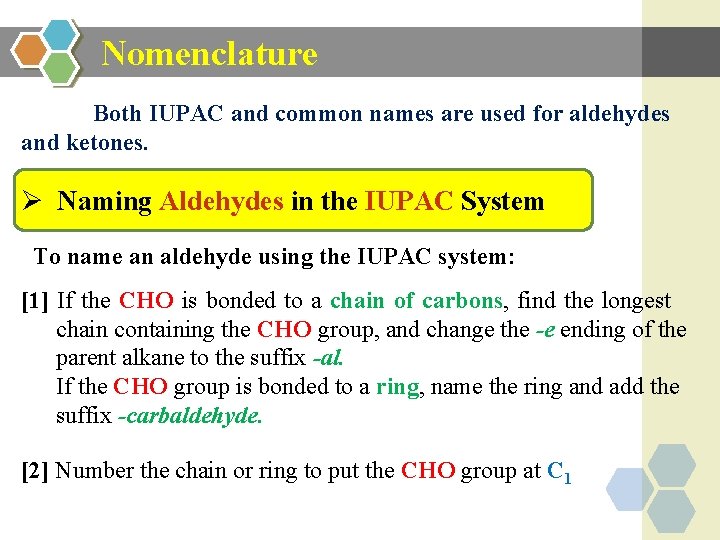
Nomenclature Both IUPAC and common names are used for aldehydes and ketones. Ø Naming Aldehydes in the IUPAC System To name an aldehyde using the IUPAC system: [1] If the CHO is bonded to a chain of carbons, find the longest chain containing the CHO group, and change the -e ending of the parent alkane to the suffix -al. If the CHO group is bonded to a ring, name the ring and add the suffix -carbaldehyde. [2] Number the chain or ring to put the CHO group at C 1

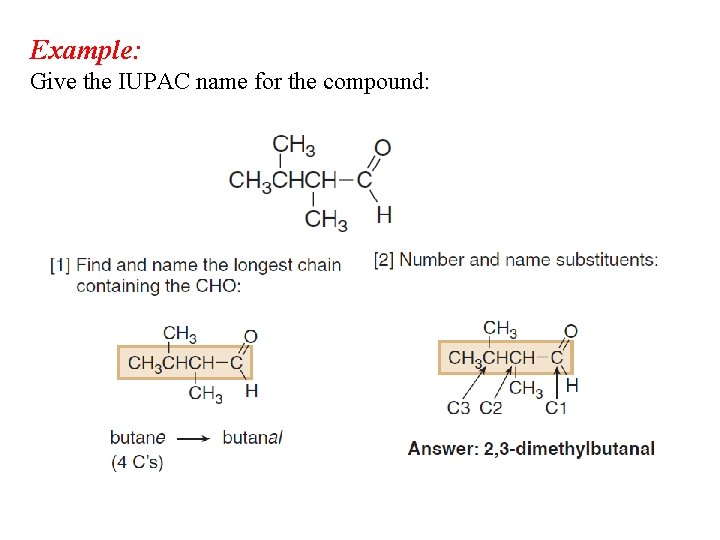
Example: Give the IUPAC name for the compound:


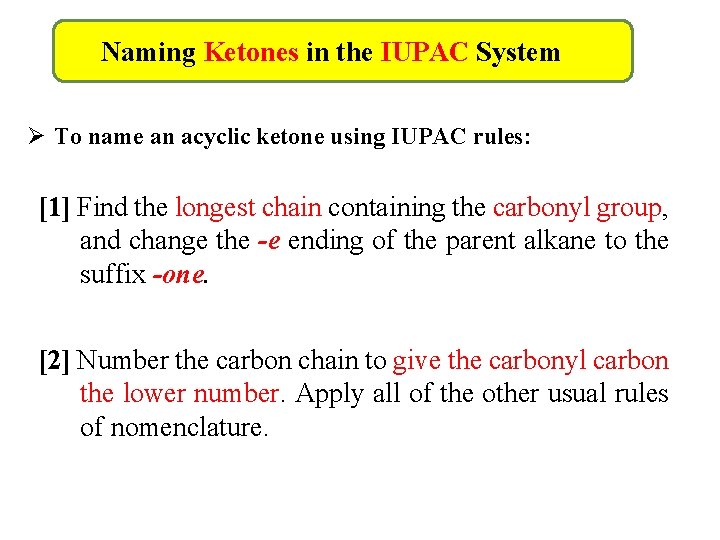
Naming Ketones in the IUPAC System Ø To name an acyclic ketone using IUPAC rules: [1] Find the longest chain containing the carbonyl group, and change the -e ending of the parent alkane to the suffix -one. [2] Number the carbon chain to give the carbonyl carbon the lower number. Apply all of the other usual rules of nomenclature.




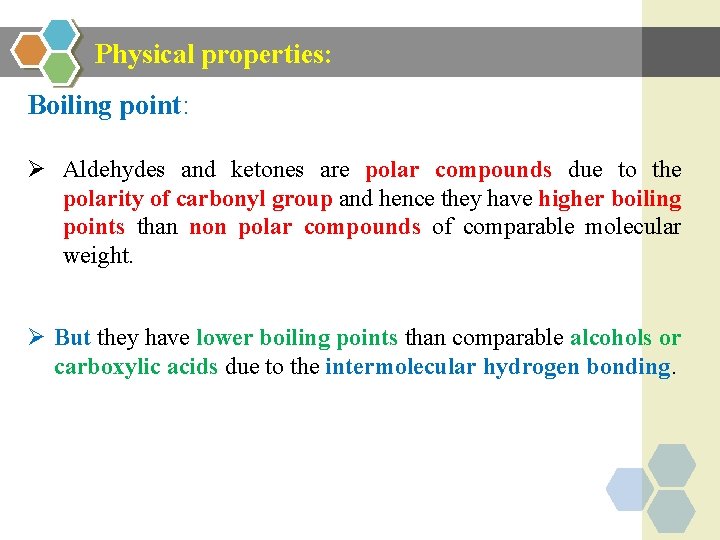
Physical properties: Boiling point: Ø Aldehydes and ketones are polar compounds due to the polarity of carbonyl group and hence they have higher boiling points than non polar compounds of comparable molecular weight. Ø But they have lower boiling points than comparable alcohols or carboxylic acids due to the intermolecular hydrogen bonding.

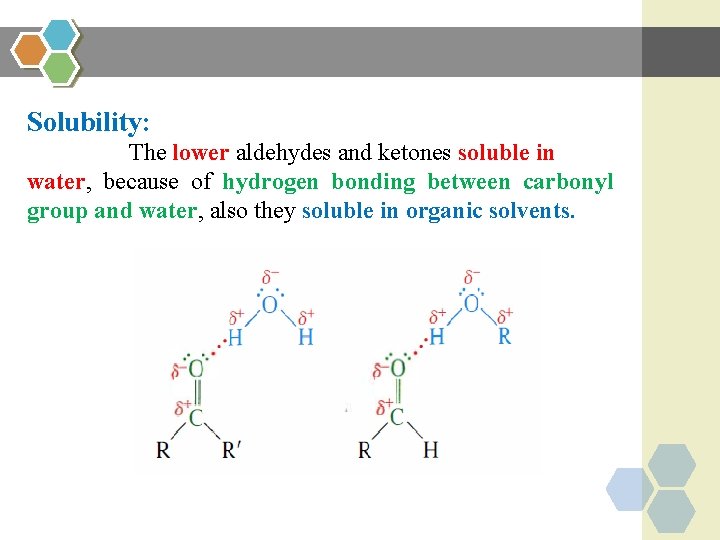
Solubility: The lower aldehydes and ketones soluble in water, because of hydrogen bonding between carbonyl group and water, also they soluble in organic solvents.

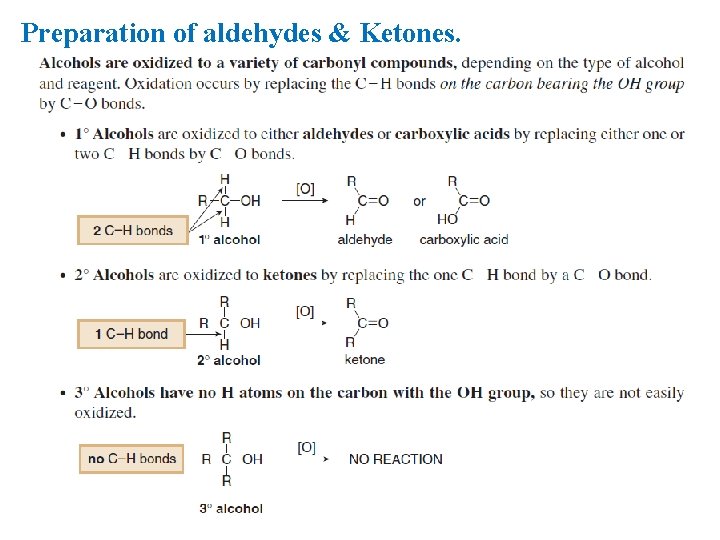
Preparation of aldehydes & Ketones.

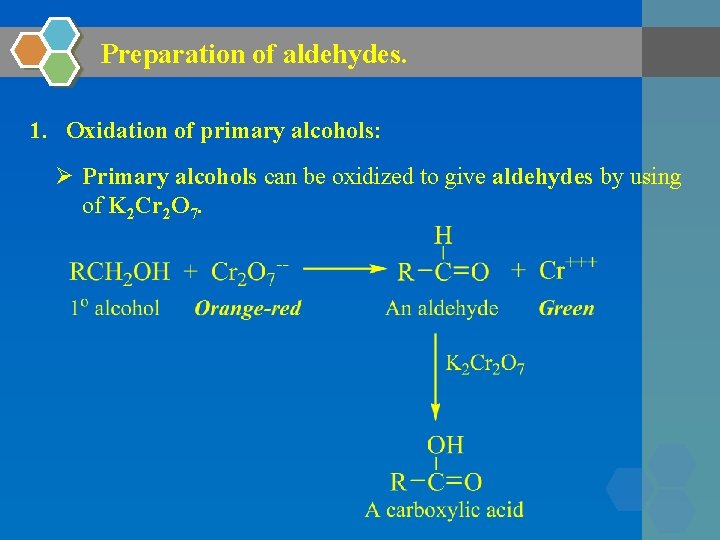
Preparation of aldehydes. 1. Oxidation of primary alcohols: Ø Primary alcohols can be oxidized to give aldehydes by using of K 2 Cr 2 O 7.

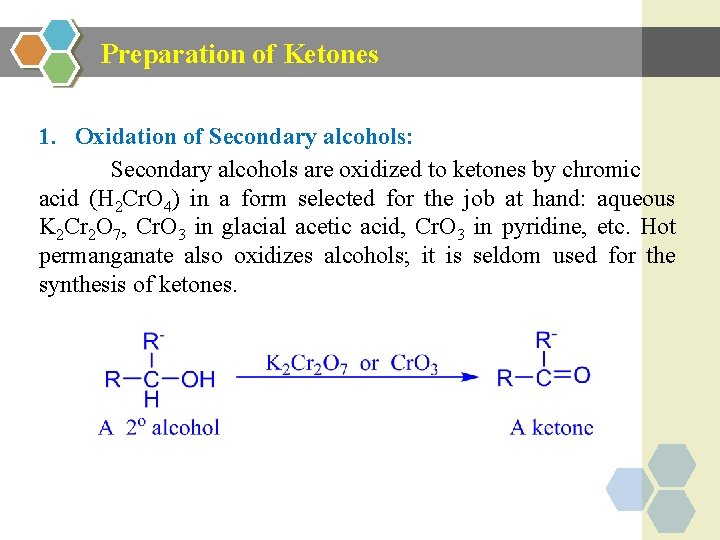
Preparation of Ketones 1. Oxidation of Secondary alcohols: Secondary alcohols are oxidized to ketones by chromic acid (H 2 Cr. O 4) in a form selected for the job at hand: aqueous K 2 Cr 2 O 7, Cr. O 3 in glacial acetic acid, Cr. O 3 in pyridine, etc. Hot permanganate also oxidizes alcohols; it is seldom used for the synthesis of ketones.

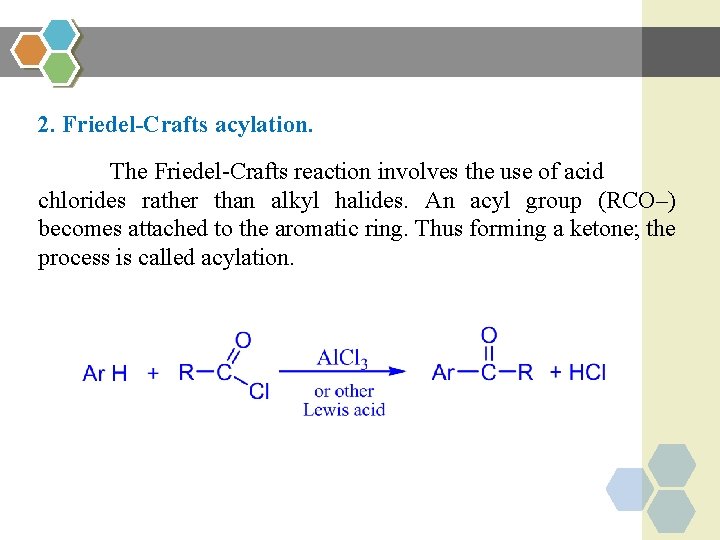
2. Friedel-Crafts acylation. The Friedel-Crafts reaction involves the use of acid chlorides rather than alkyl halides. An acyl group (RCO–) becomes attached to the aromatic ring. Thus forming a ketone; the process is called acylation.

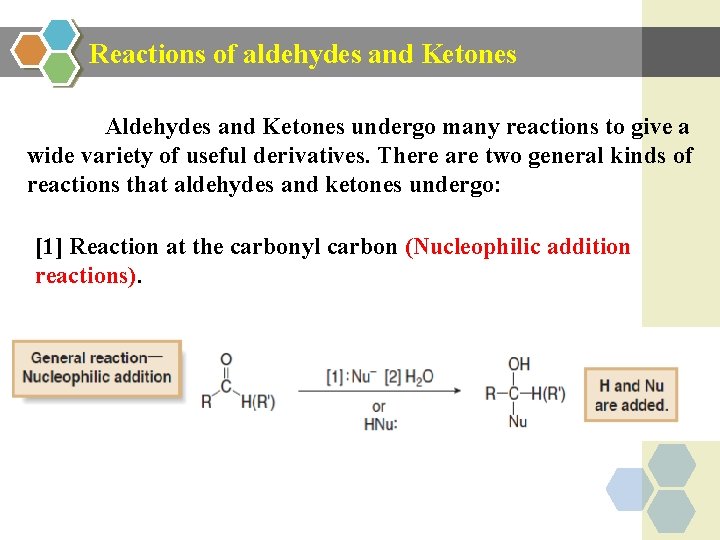
Reactions of aldehydes and Ketones Aldehydes and Ketones undergo many reactions to give a wide variety of useful derivatives. There are two general kinds of reactions that aldehydes and ketones undergo: [1] Reaction at the carbonyl carbon (Nucleophilic addition reactions).
![2 Reaction at the α carbon A second general reaction of aldehydes and ketones [2] Reaction at the α carbon. A second general reaction of aldehydes and ketones](https://slidetodoc.com/presentation_image/3989475c9c910293a3cfde9918c28c39/image-18.jpg)
[2] Reaction at the α carbon. A second general reaction of aldehydes and ketones involves reaction at the α carbon. A C–H bond on the α carbon to a carbonyl group is more acidic than many other C–H bonds, because reaction with base forms a resonance-stabilized enolate anion.
![1 Nucleophilic addition reaction Two general mechanisms are usually drawn for nucleophilic addition depending [1] Nucleophilic addition reaction. Two general mechanisms are usually drawn for nucleophilic addition, depending](https://slidetodoc.com/presentation_image/3989475c9c910293a3cfde9918c28c39/image-19.jpg)
[1] Nucleophilic addition reaction. Two general mechanisms are usually drawn for nucleophilic addition, depending on the nucleophile (negatively charged versus neutral) and the presence or absence of an acid catalyst. With negatively charged nucleophiles, nucleophilic addition follows the two-step process first (nucleophilic attack) followed by protonation.
![Ø Absence of an acid catalyzed nucleophilic addition Step 1 The nucleophile attacks the Ø Absence of an acid catalyzed nucleophilic addition Step [1]: The nucleophile attacks the](https://slidetodoc.com/presentation_image/3989475c9c910293a3cfde9918c28c39/image-20.jpg)
Ø Absence of an acid catalyzed nucleophilic addition Step [1]: The nucleophile attacks the carbonyl group, cleaving the π bond and moving an electron pair onto oxygen. This forms a sp 3 hybridized intermediate with a new C–Nu bond. Step [2]: protonation of the negatively charged O atom by H 2 O forms the addition product.

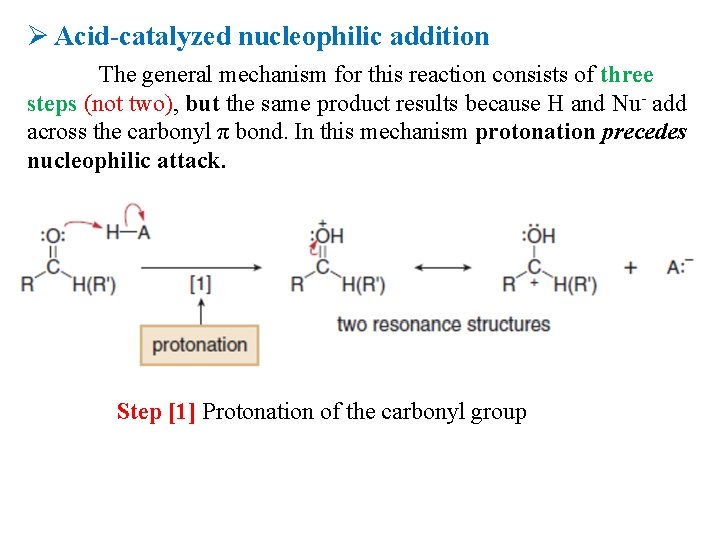
Ø Acid-catalyzed nucleophilic addition The general mechanism for this reaction consists of three steps (not two), but the same product results because H and Nu- add across the carbonyl π bond. In this mechanism protonation precedes nucleophilic attack. Step [1] Protonation of the carbonyl group
![In Step 2 the nucleophile attacks and then deprotonation forms the neutral addition product In Step [2], the nucleophile attacks, and then deprotonation forms the neutral addition product](https://slidetodoc.com/presentation_image/3989475c9c910293a3cfde9918c28c39/image-22.jpg)
In Step [2], the nucleophile attacks, and then deprotonation forms the neutral addition product in Step [3]. Steps [2]–[3] Nucleophilic attack and deprotonation

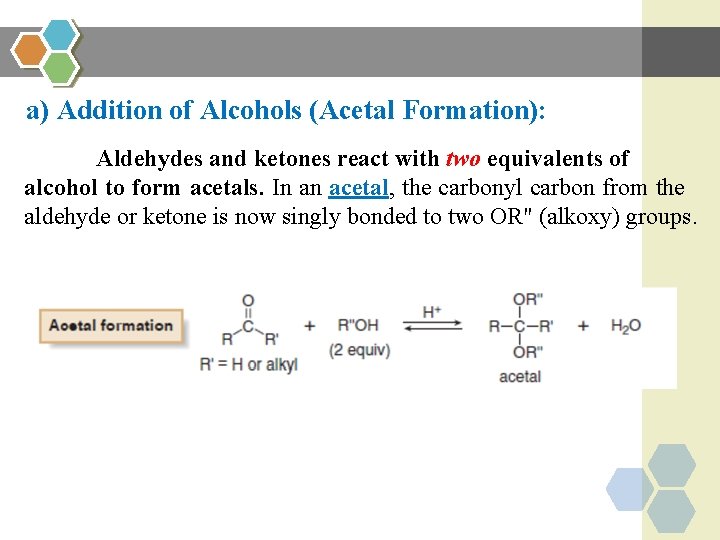
a) Addition of Alcohols (Acetal Formation): Aldehydes and ketones react with two equivalents of alcohol to form acetals. In an acetal, the carbonyl carbon from the aldehyde or ketone is now singly bonded to two OR" (alkoxy) groups.

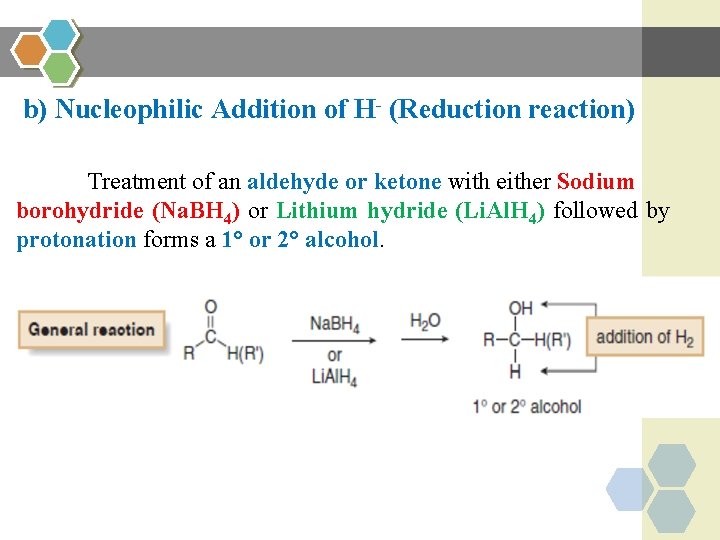
b) Nucleophilic Addition of H- (Reduction reaction) Treatment of an aldehyde or ketone with either Sodium borohydride (Na. BH 4) or Lithium hydride (Li. Al. H 4) followed by protonation forms a 1° or 2° alcohol.
 Aldehydes and ketones structure
Aldehydes and ketones structure Polyhydroxy aldehydes and ketones
Polyhydroxy aldehydes and ketones Aldehydes to carboxylic acids
Aldehydes to carboxylic acids Example of alkanone
Example of alkanone Test for carbonyl group
Test for carbonyl group Aldehydes and ketones
Aldehydes and ketones Aldehydes and ketones structure
Aldehydes and ketones structure Reactions of alcohols 1 chemsheets answers
Reactions of alcohols 1 chemsheets answers Chemical properties of ketones
Chemical properties of ketones Ketone reactivity
Ketone reactivity Reactions of aldehydes and ketones summary
Reactions of aldehydes and ketones summary Naming aldehydes and ketones worksheet with answers doc
Naming aldehydes and ketones worksheet with answers doc Aldehydes and ketones nucleophilic addition
Aldehydes and ketones nucleophilic addition Cyanohydrin formation
Cyanohydrin formation Bilateral cataract ketones and raised liver enzymes
Bilateral cataract ketones and raised liver enzymes Aldehyde and ketones
Aldehyde and ketones Urine bile salt bile pigment test procedure
Urine bile salt bile pigment test procedure Aciclosis
Aciclosis Ketones in urine moderate
Ketones in urine moderate Oxidation of ketones
Oxidation of ketones Ch3li reaction with ketone
Ch3li reaction with ketone Properties of ketones
Properties of ketones Carbonyl group
Carbonyl group Ketones
Ketones Nhr group name
Nhr group name