Aldehydes and Ketones Introduction Nomenclature of aldehydes and







- Slides: 25

Aldehydes and Ketones

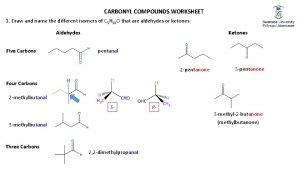
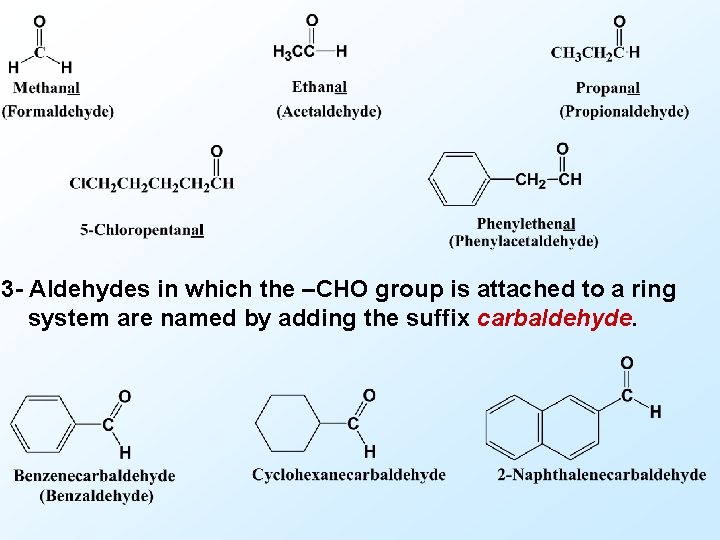
Introduction Nomenclature of aldehydes and ketones 1 - In the IUPAC system, aliphatic aldehydes are named by replacing the final -e of the name of the corresponding alkane with -al. When other substituents are present, the carbonyl group carbon is assigned position 1. 2 - Many aldehydes also have common names; these are given here in parentheses. These common names are derived from the common names for the corresponding carboxylic acids and some of them are retained by the IUPAC as acceptable names.

3 - Aldehydes in which the –CHO group is attached to a ring system are named by adding the suffix carbaldehyde.

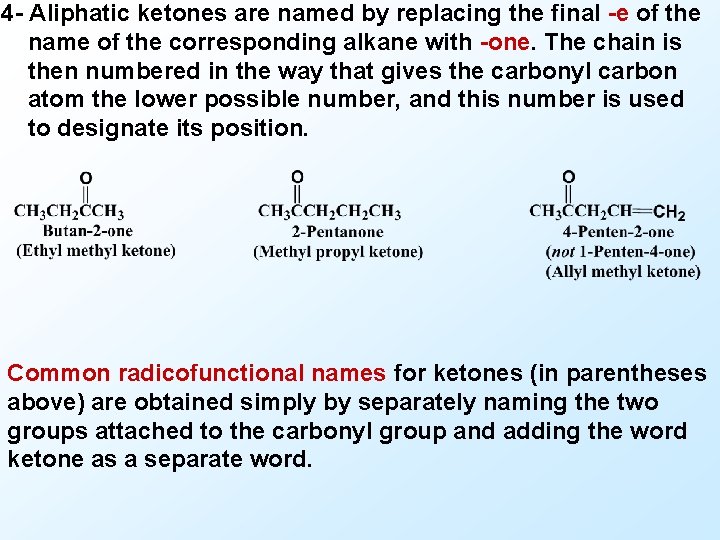
4 - Aliphatic ketones are named by replacing the final -e of the name of the corresponding alkane with -one. The chain is then numbered in the way that gives the carbonyl carbon atom the lower possible number, and this number is used to designate its position. Common radicofunctional names for ketones (in parentheses above) are obtained simply by separately naming the two groups attached to the carbonyl group and adding the word ketone as a separate word.

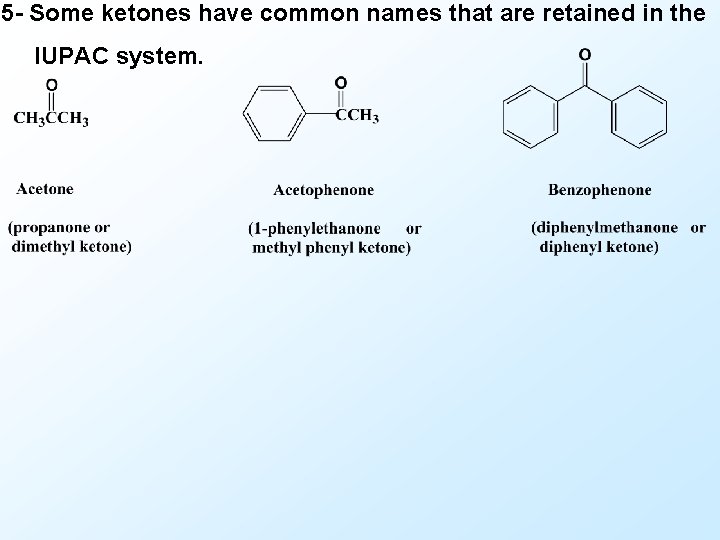
5 - Some ketones have common names that are retained in the IUPAC system.

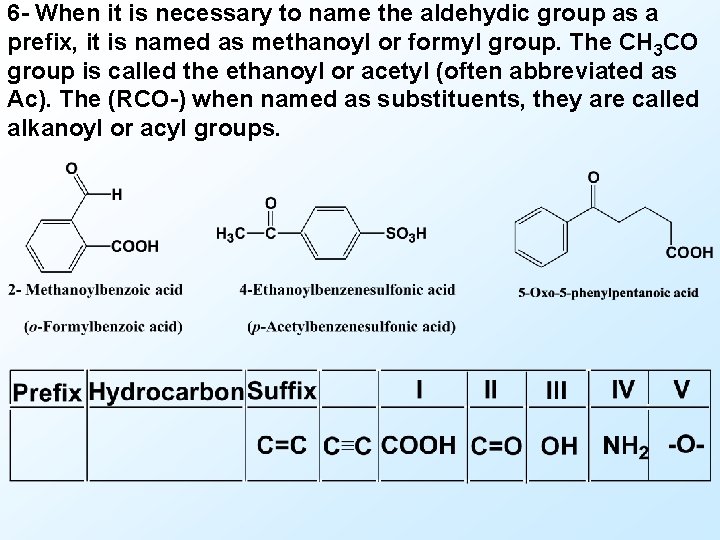
6 - When it is necessary to name the aldehydic group as a prefix, it is named as methanoyl or formyl group. The CH 3 CO group is called the ethanoyl or acetyl (often abbreviated as Ac). The (RCO-) when named as substituents, they are called alkanoyl or acyl groups.


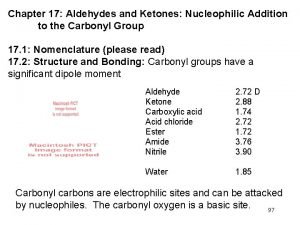
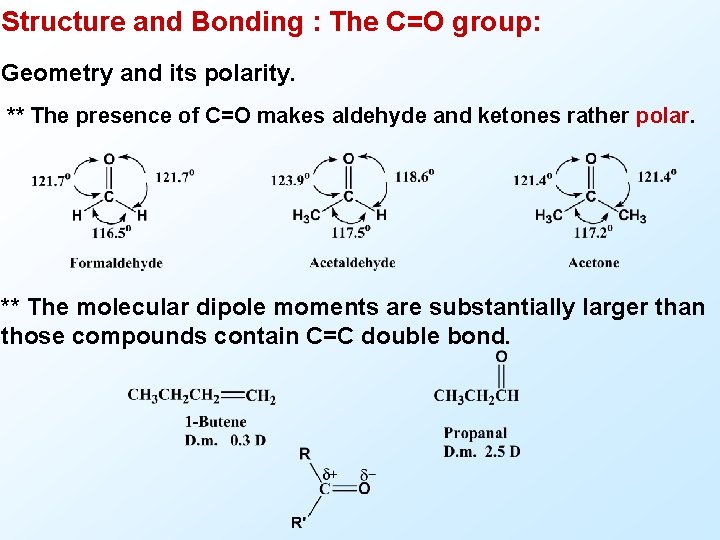
Structure and Bonding : The C=O group: Geometry and its polarity. ** The presence of C=O makes aldehyde and ketones rather polar. ** The molecular dipole moments are substantially larger than those compounds contain C=C double bond.

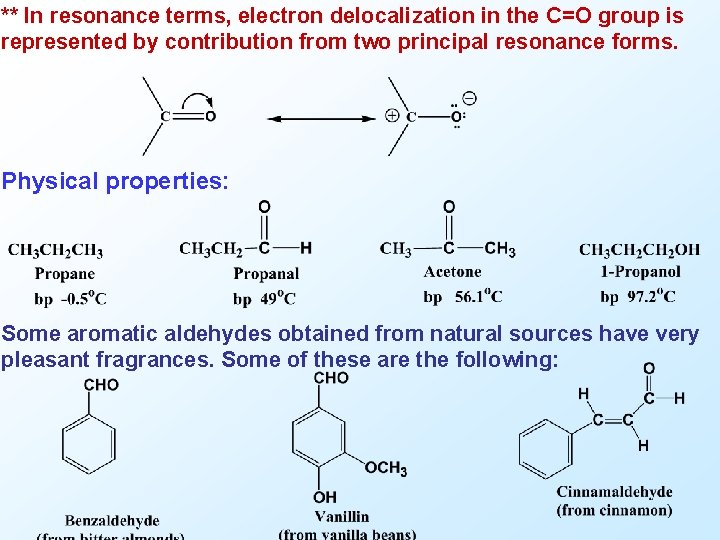
** In resonance terms, electron delocalization in the C=O group is represented by contribution from two principal resonance forms. Physical properties: Some aromatic aldehydes obtained from natural sources have very pleasant fragrances. Some of these are the following:


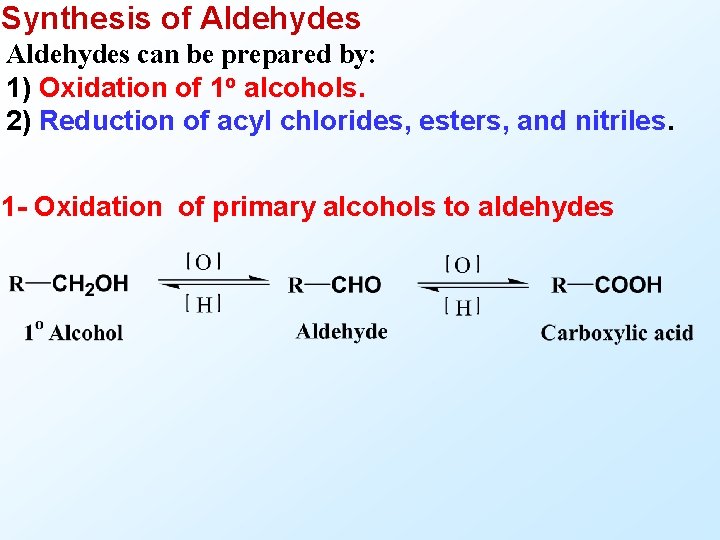
Synthesis of Aldehydes can be prepared by: 1) Oxidation of 1 o alcohols. 2) Reduction of acyl chlorides, esters, and nitriles. 1 - Oxidation of primary alcohols to aldehydes

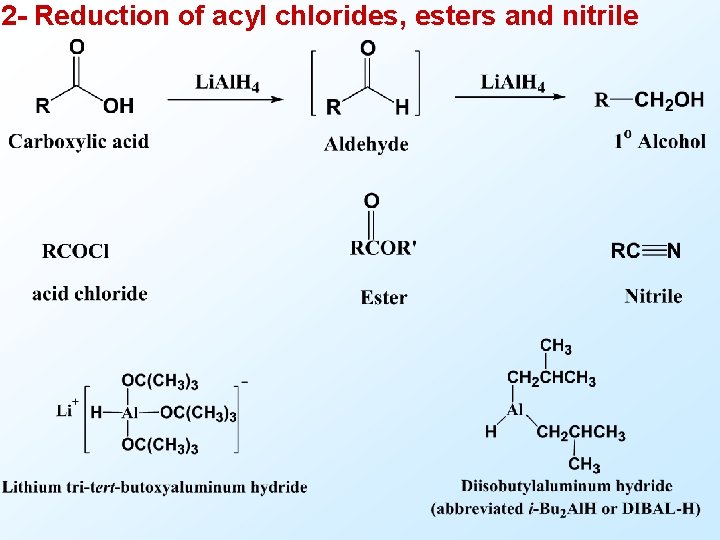
2 - Reduction of acyl chlorides, esters and nitrile


A- Reduction of acyl chlorides to aldehydes: Specific example

Mechanism for this reaction

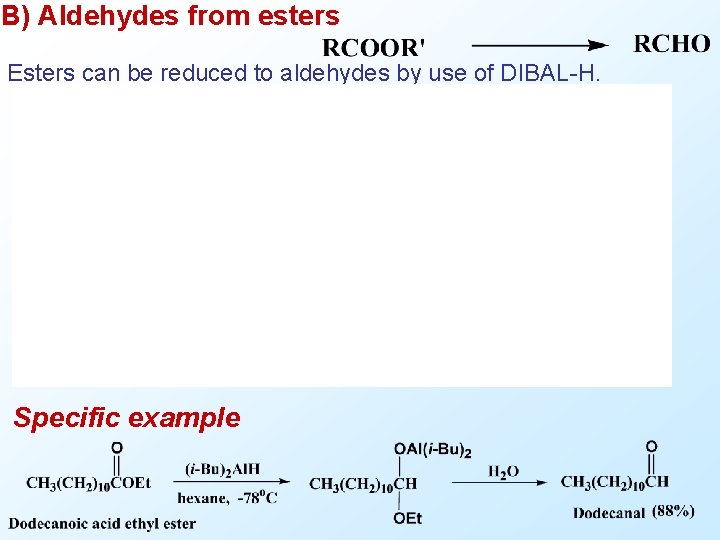
B) Aldehydes from esters Esters can be reduced to aldehydes by use of DIBAL-H. Specific example

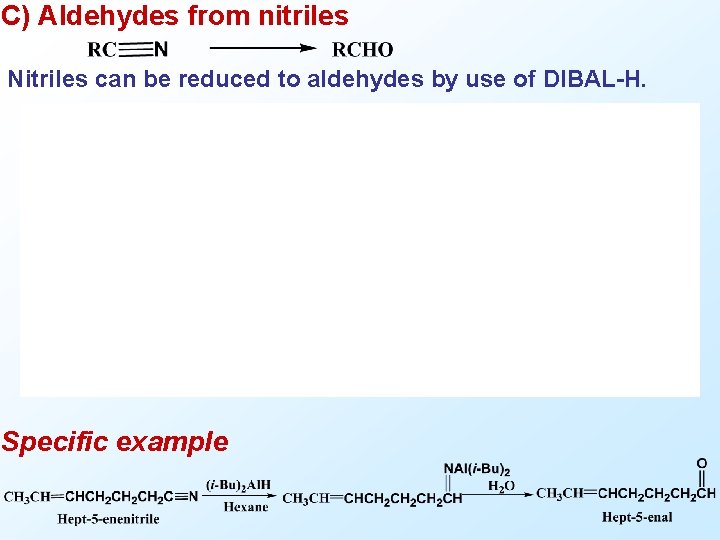
C) Aldehydes from nitriles Nitriles can be reduced to aldehydes by use of DIBAL-H. Specific example

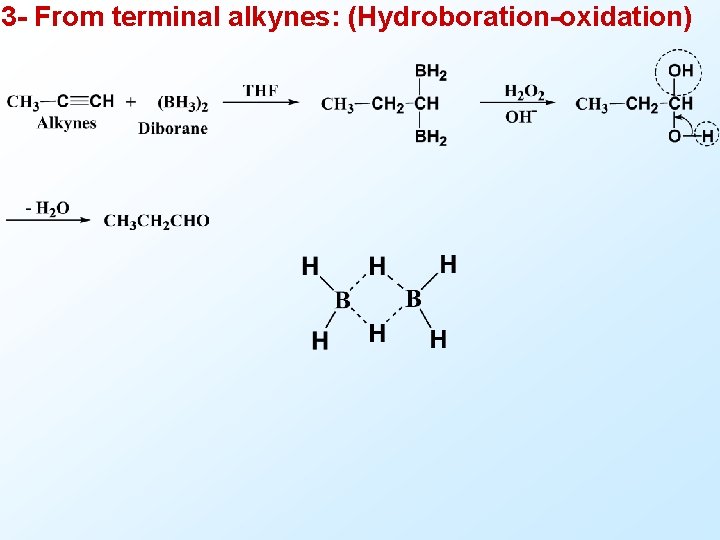
3 - From terminal alkynes: (Hydroboration-oxidation)

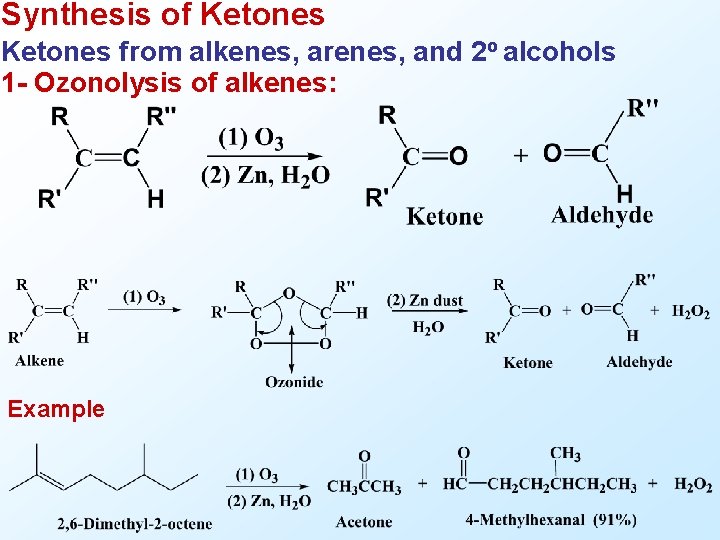
Synthesis of Ketones from alkenes, arenes, and 2 o alcohols 1 - Ozonolysis of alkenes: Example

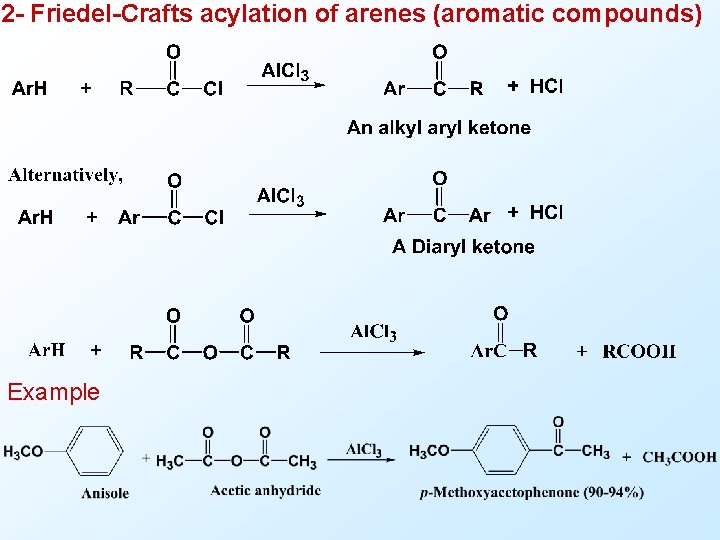
2 - Friedel-Crafts acylation of arenes (aromatic compounds) Example

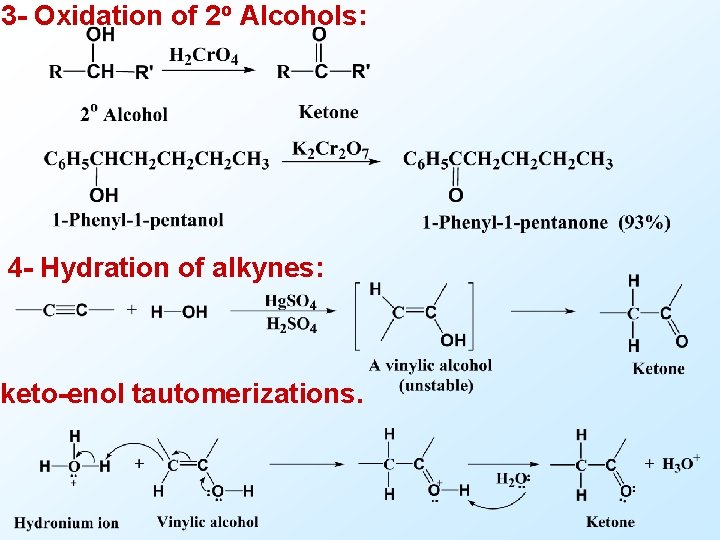
3 - Oxidation of 2 o Alcohols: 4 - Hydration of alkynes: keto-enol tautomerizations.

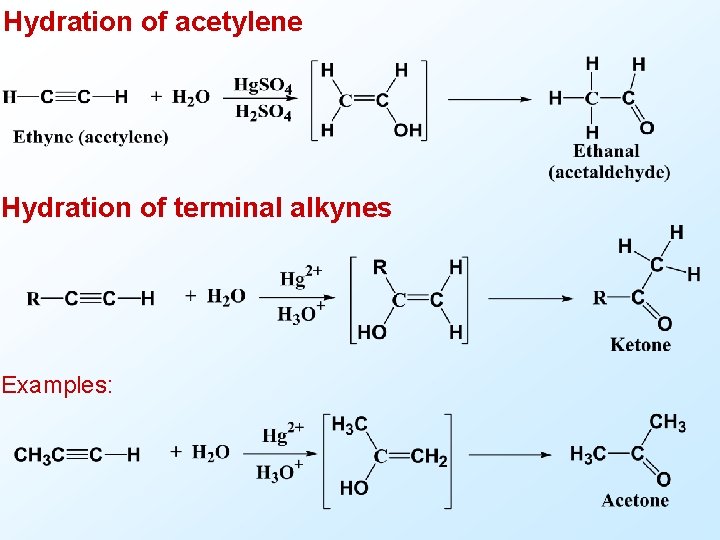
Hydration of acetylene Hydration of terminal alkynes Examples:

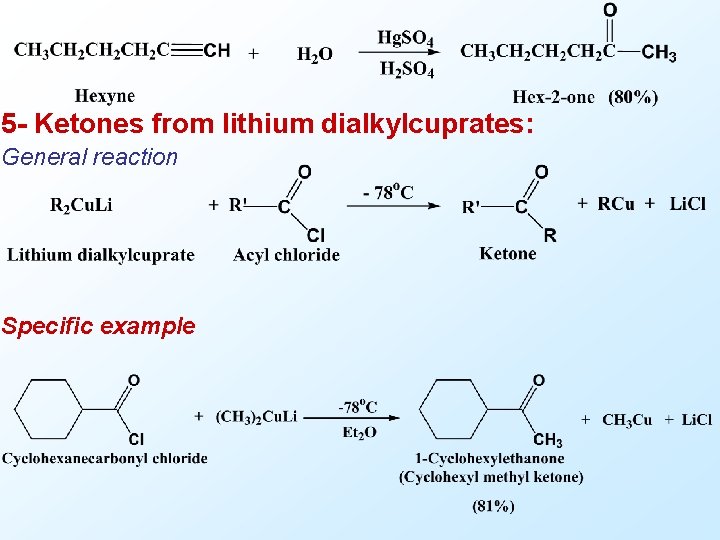
5 - Ketones from lithium dialkylcuprates: General reaction Specific example

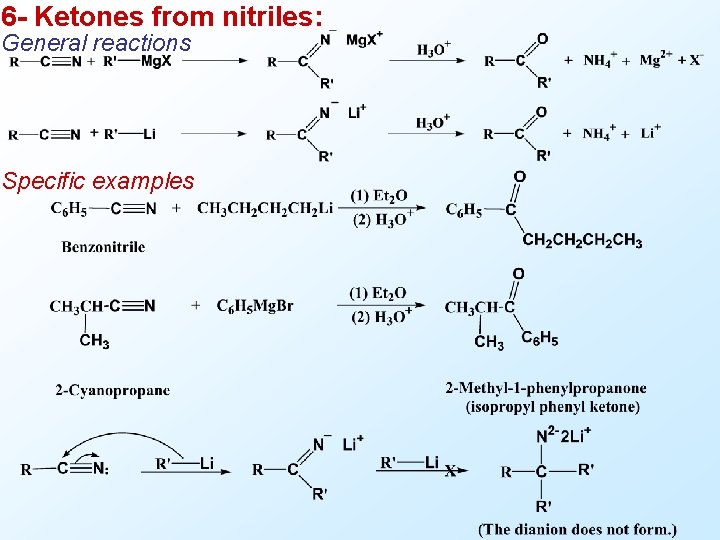
6 - Ketones from nitriles: General reactions Specific examples
 Aldehydes and ketones structure
Aldehydes and ketones structure Polyhydroxy aldehydes and ketones
Polyhydroxy aldehydes and ketones Naming of aldehydes
Naming of aldehydes Ketone iupac name
Ketone iupac name Ketone naming iupac
Ketone naming iupac Carbonyl vs ketone
Carbonyl vs ketone Ketone diagram
Ketone diagram Reactions of aldehydes and ketones chemsheets answers
Reactions of aldehydes and ketones chemsheets answers Chemical properties of ketones
Chemical properties of ketones Ketone reactivity
Ketone reactivity Aldehyde protecting group
Aldehyde protecting group Carbonyl compounds
Carbonyl compounds Aldehydes and ketones nucleophilic addition
Aldehydes and ketones nucleophilic addition Cyanohydrin formation
Cyanohydrin formation Psyhomotor
Psyhomotor Aldehyde and ketones
Aldehyde and ketones Hart's test for ketones
Hart's test for ketones Aciclosis
Aciclosis Ketones in urine moderate
Ketones in urine moderate Oxidation of ketones
Oxidation of ketones Primary aldehyde
Primary aldehyde Properties of ketones
Properties of ketones Ketone group
Ketone group Ketones
Ketones Site:slidetodoc.com
Site:slidetodoc.com Aldehydes scary
Aldehydes scary