Aldehydes and Ketones I Nucleophilic Addition to the

- Slides: 33

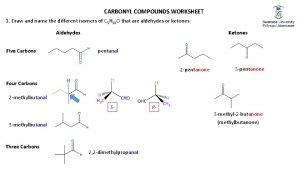
Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group

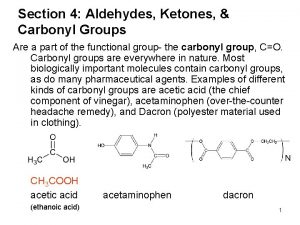
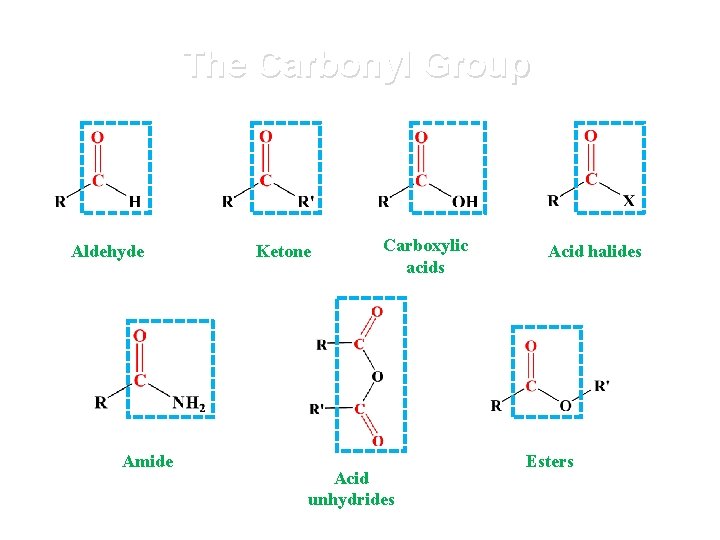
The Carbonyl Group Aldehyde Amide Ketone Carboxylic acids Acid unhydrides Acid halides Esters

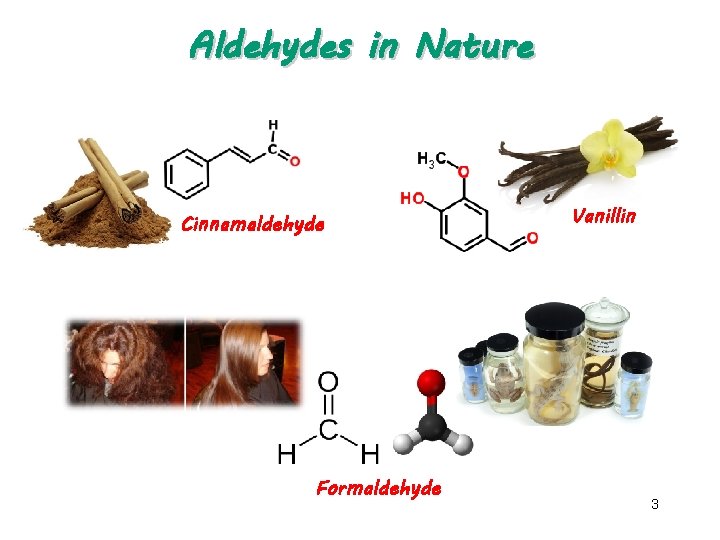
Aldehydes in Nature Cinnamaldehyde Formaldehyde Vanillin 3

Ketones in Nature Acetone 4

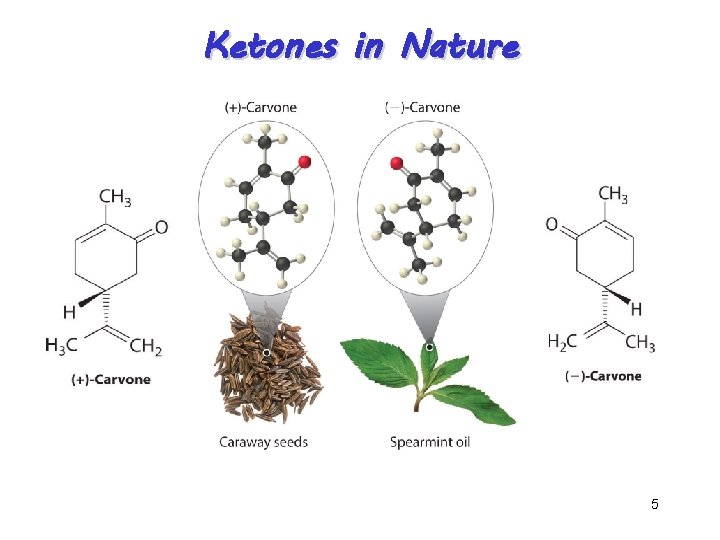
Ketones in Nature 5

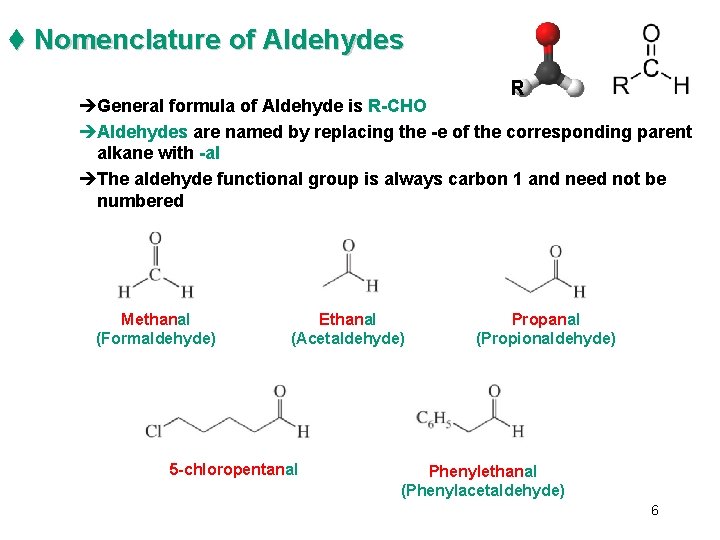
t Nomenclature of Aldehydes R èGeneral formula of Aldehyde is R-CHO èAldehydes are named by replacing the -e of the corresponding parent alkane with -al èThe aldehyde functional group is always carbon 1 and need not be numbered Methanal (Formaldehyde) Ethanal (Acetaldehyde) 5 -chloropentanal Propanal (Propionaldehyde) Phenylethanal (Phenylacetaldehyde) 6

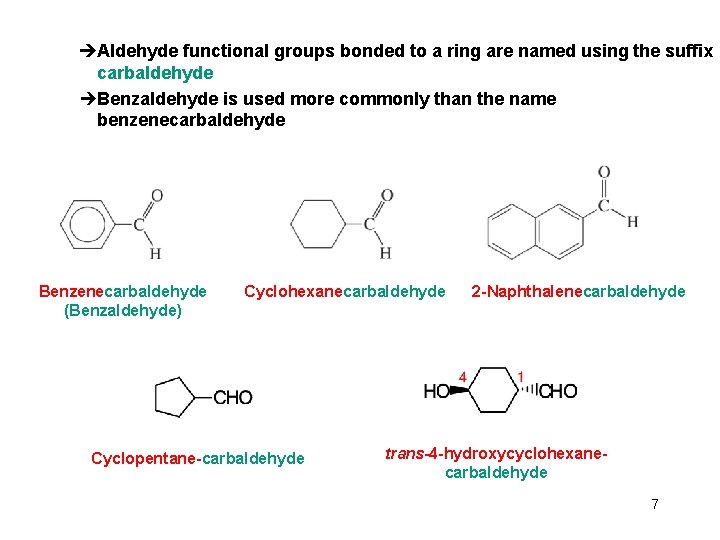
èAldehyde functional groups bonded to a ring are named using the suffix carbaldehyde èBenzaldehyde is used more commonly than the name benzenecarbaldehyde Benzenecarbaldehyde (Benzaldehyde) Cyclohexanecarbaldehyde Cyclopentane-carbaldehyde 2 -Naphthalenecarbaldehyde trans-4 -hydroxycyclohexanecarbaldehyde 7

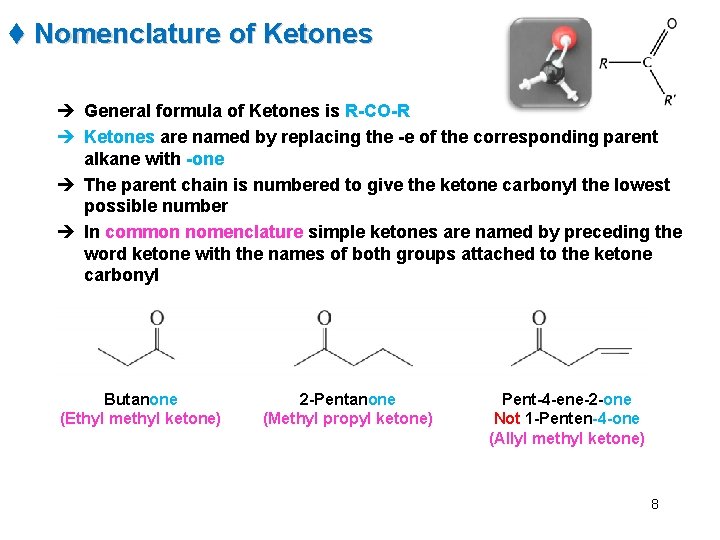
t Nomenclature of Ketones è General formula of Ketones is R-CO-R è Ketones are named by replacing the -e of the corresponding parent alkane with -one è The parent chain is numbered to give the ketone carbonyl the lowest possible number è In common nomenclature simple ketones are named by preceding the word ketone with the names of both groups attached to the ketone carbonyl Butanone (Ethyl methyl ketone) 2 -Pentanone (Methyl propyl ketone) Pent-4 -ene-2 -one Not 1 -Penten-4 -one (Allyl methyl ketone) 8

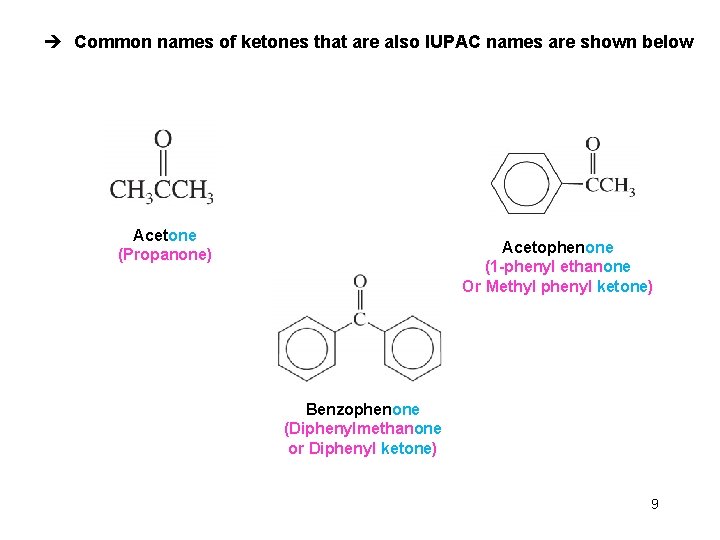
è Common names of ketones that are also IUPAC names are shown below Acetone (Propanone) Acetophenone (1 -phenyl ethanone Or Methyl phenyl ketone) Benzophenone (Diphenylmethanone or Diphenyl ketone) 9

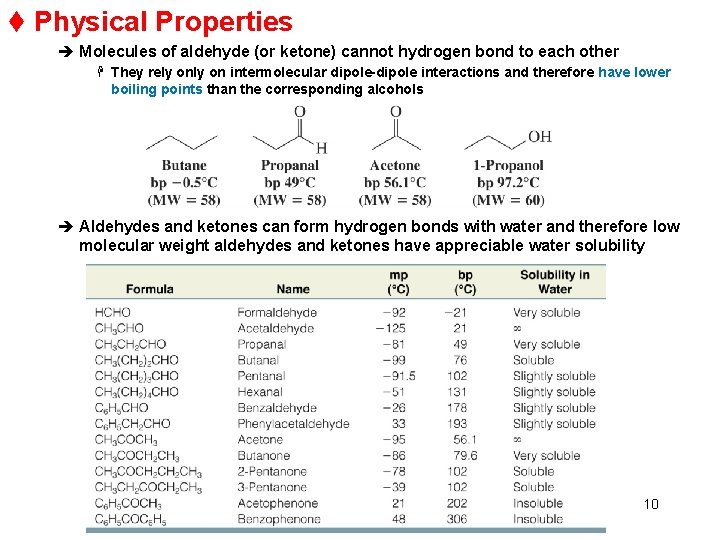
t Physical Properties è Molecules of aldehyde (or ketone) cannot hydrogen bond to each other H They rely on intermolecular dipole-dipole interactions and therefore have lower boiling points than the corresponding alcohols è Aldehydes and ketones can form hydrogen bonds with water and therefore low molecular weight aldehydes and ketones have appreciable water solubility 10

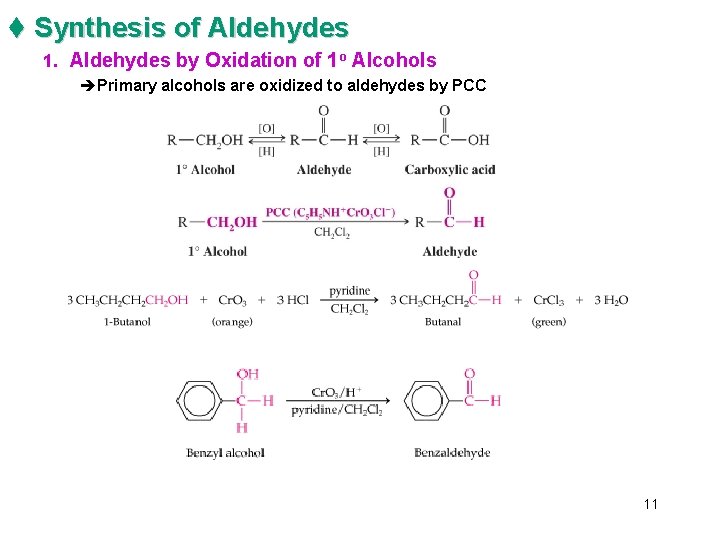
t Synthesis of Aldehydes 1. Aldehydes by Oxidation of 1 o Alcohols èPrimary alcohols are oxidized to aldehydes by PCC 11

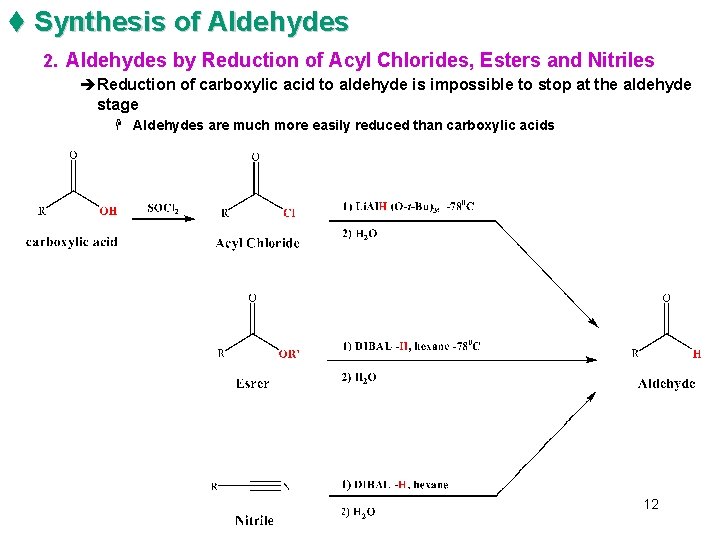
t Synthesis of Aldehydes 2. Aldehydes by Reduction of Acyl Chlorides, Esters and Nitriles èReduction of carboxylic acid to aldehyde is impossible to stop at the aldehyde stage H Aldehydes are much more easily reduced than carboxylic acids 12

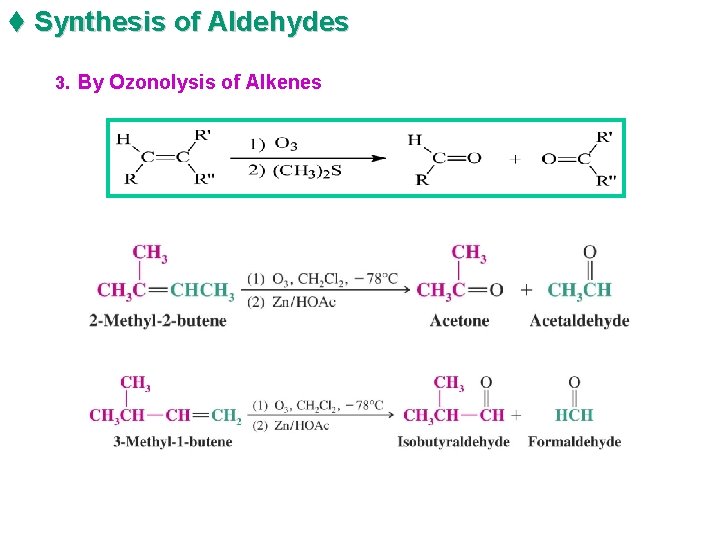
t Synthesis of Aldehydes 3. By Ozonolysis of Alkenes

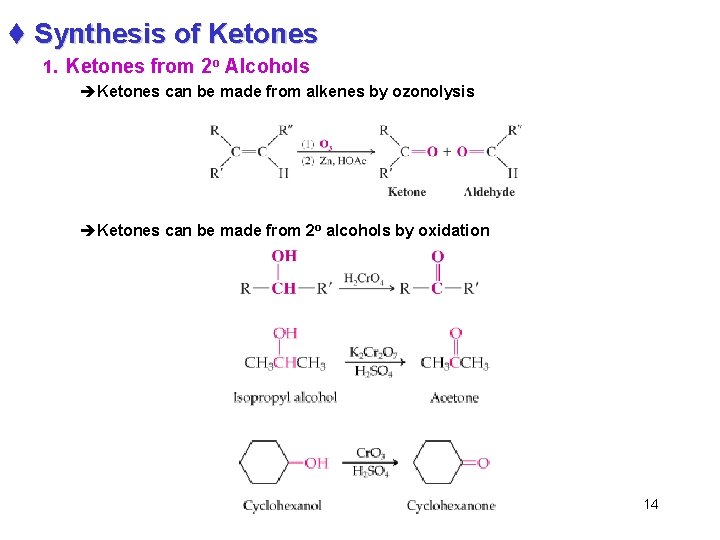
t Synthesis of Ketones 1. Ketones from 2 o Alcohols èKetones can be made from alkenes by ozonolysis èKetones can be made from 2 o alcohols by oxidation 14

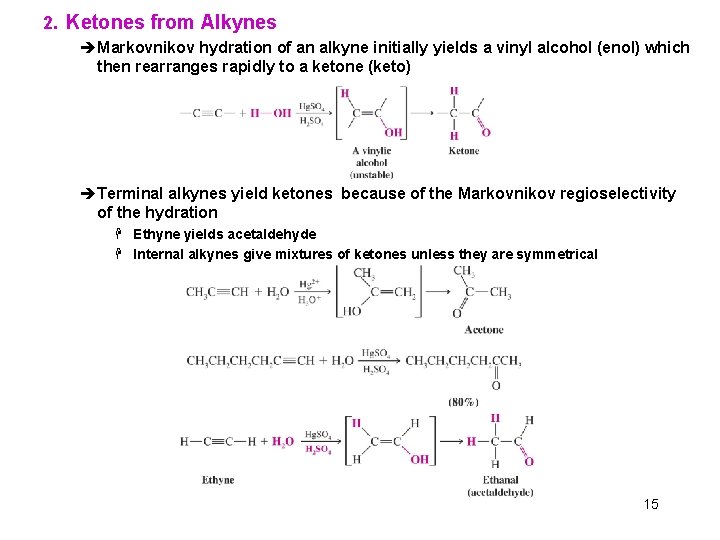
2. Ketones from Alkynes èMarkovnikov hydration of an alkyne initially yields a vinyl alcohol (enol) which then rearranges rapidly to a ketone (keto) èTerminal alkynes yield ketones because of the Markovnikov regioselectivity of the hydration H Ethyne yields acetaldehyde H Internal alkynes give mixtures of ketones unless they are symmetrical 15

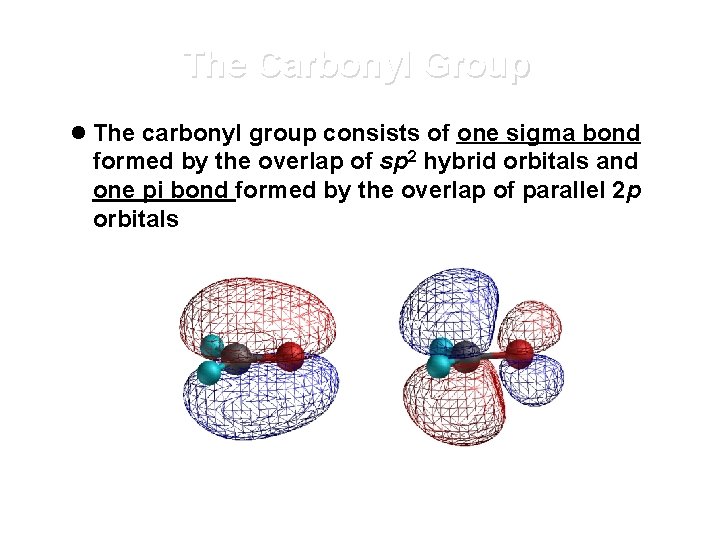
The Carbonyl Group l The carbonyl group consists of one sigma bond formed by the overlap of sp 2 hybrid orbitals and one pi bond formed by the overlap of parallel 2 p orbitals

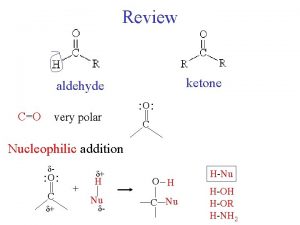
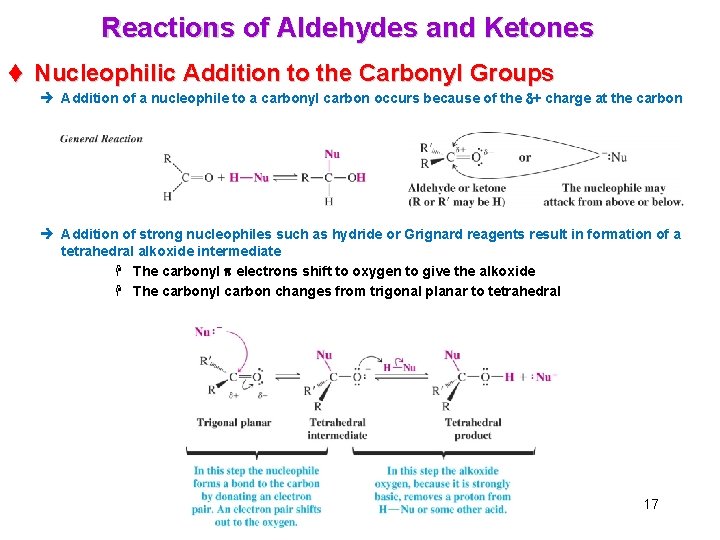
Reactions of Aldehydes and Ketones t Nucleophilic Addition to the Carbonyl Groups è Addition of a nucleophile to a carbonyl carbon occurs because of the d+ charge at the carbon è Addition of strong nucleophiles such as hydride or Grignard reagents result in formation of a tetrahedral alkoxide intermediate H The carbonyl p electrons shift to oxygen to give the alkoxide H The carbonyl carbon changes from trigonal planar to tetrahedral 17

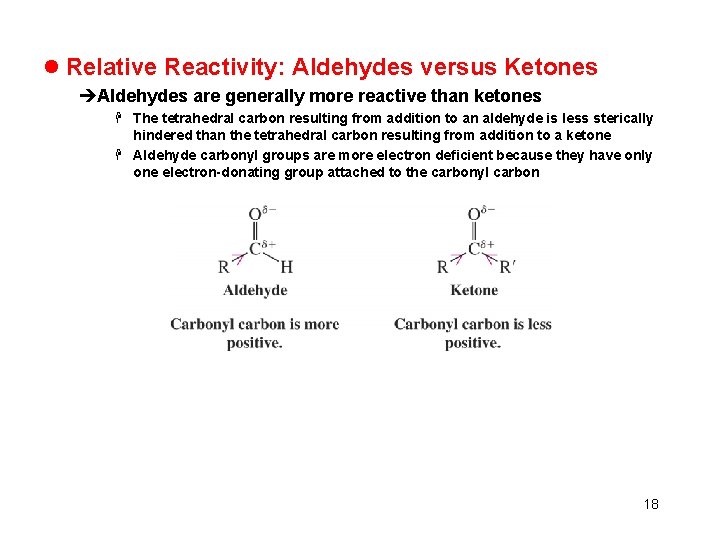
l Relative Reactivity: Aldehydes versus Ketones èAldehydes are generally more reactive than ketones H The tetrahedral carbon resulting from addition to an aldehyde is less sterically hindered than the tetrahedral carbon resulting from addition to a ketone H Aldehyde carbonyl groups are more electron deficient because they have only one electron-donating group attached to the carbonyl carbon 18

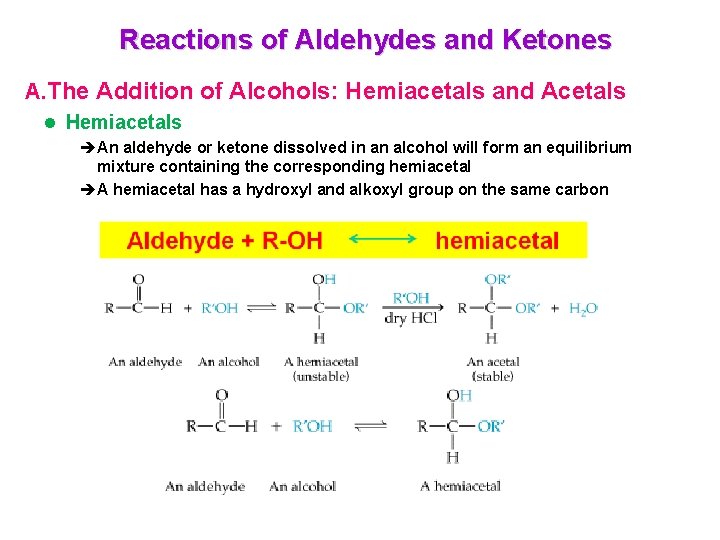
Reactions of Aldehydes and Ketones A. The Addition of Alcohols: Hemiacetals and Acetals l Hemiacetals èAn aldehyde or ketone dissolved in an alcohol will form an equilibrium mixture containing the corresponding hemiacetal èA hemiacetal has a hydroxyl and alkoxyl group on the same carbon

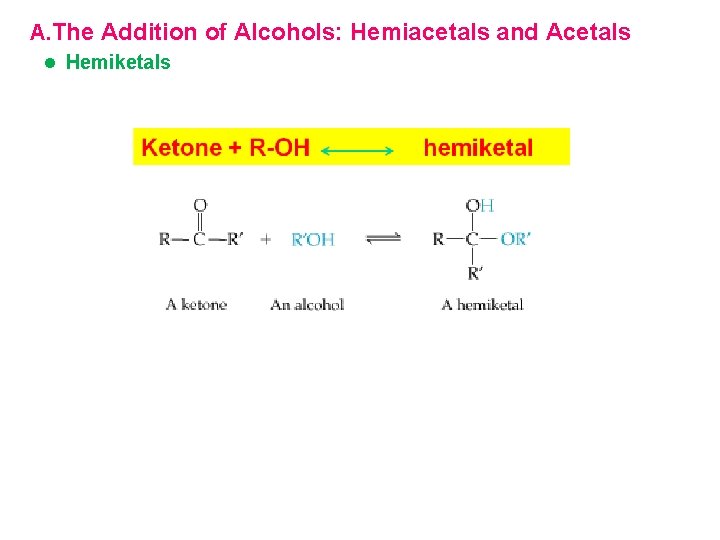
A. The Addition of Alcohols: Hemiacetals and Acetals l Hemiketals

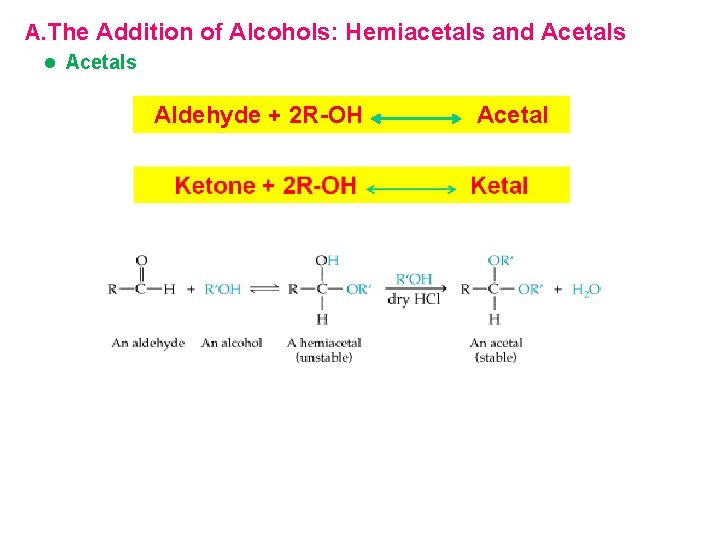
A. The Addition of Alcohols: Hemiacetals and Acetals l Acetals Aldehyde + 2 R-OH Acetal

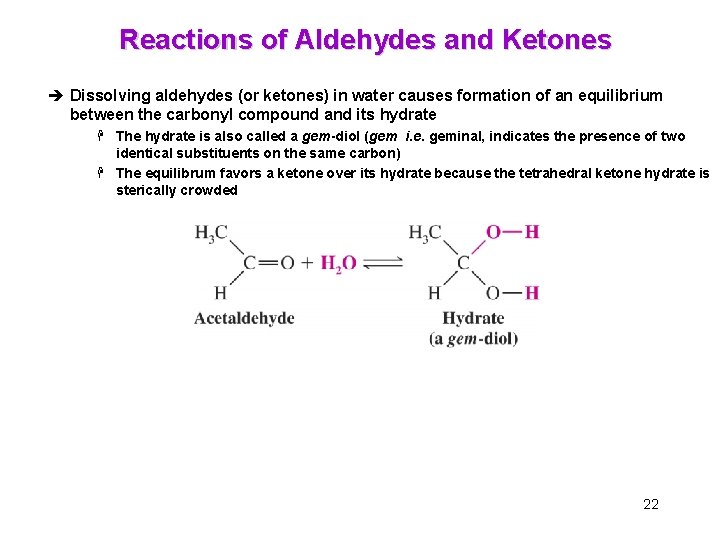
Reactions of Aldehydes and Ketones è Dissolving aldehydes (or ketones) in water causes formation of an equilibrium between the carbonyl compound and its hydrate H The hydrate is also called a gem-diol (gem i. e. geminal, indicates the presence of two identical substituents on the same carbon) H The equilibrum favors a ketone over its hydrate because the tetrahedral ketone hydrate is sterically crowded 22

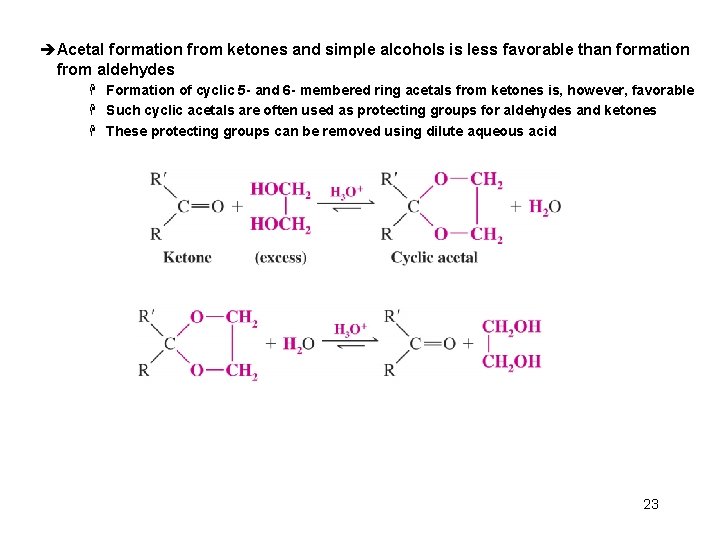
èAcetal formation from ketones and simple alcohols is less favorable than formation from aldehydes H Formation of cyclic 5 - and 6 - membered ring acetals from ketones is, however, favorable H Such cyclic acetals are often used as protecting groups for aldehydes and ketones H These protecting groups can be removed using dilute aqueous acid 23

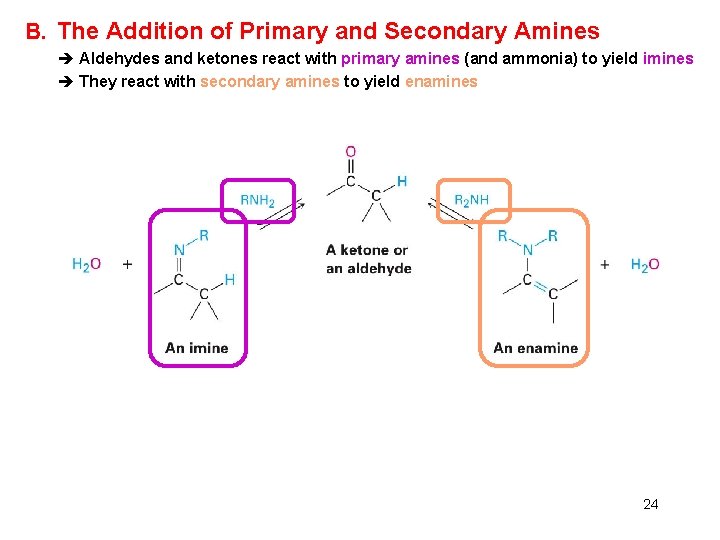
B. The Addition of Primary and Secondary Amines è Aldehydes and ketones react with primary amines (and ammonia) to yield imines è They react with secondary amines to yield enamines 24

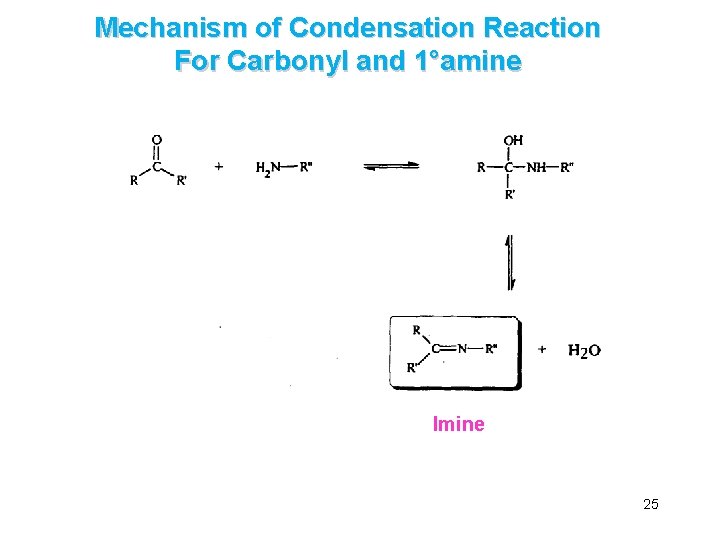
Mechanism of Condensation Reaction For Carbonyl and 1°amine Imine 25

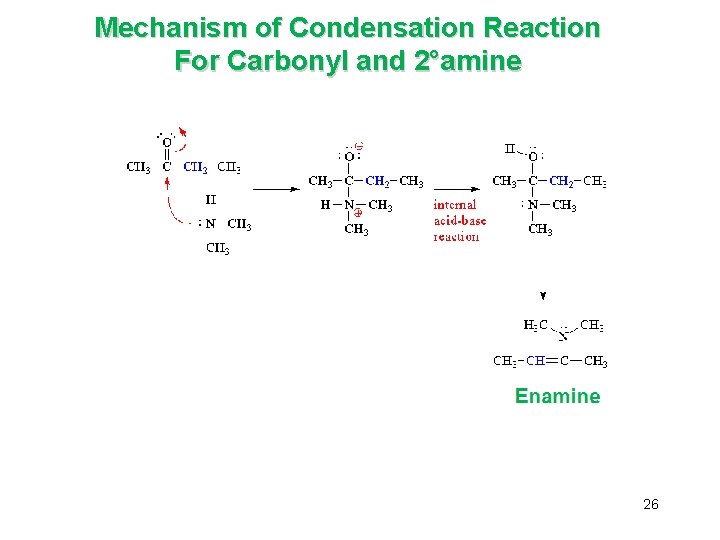
Mechanism of Condensation Reaction For Carbonyl and 2°amine 26

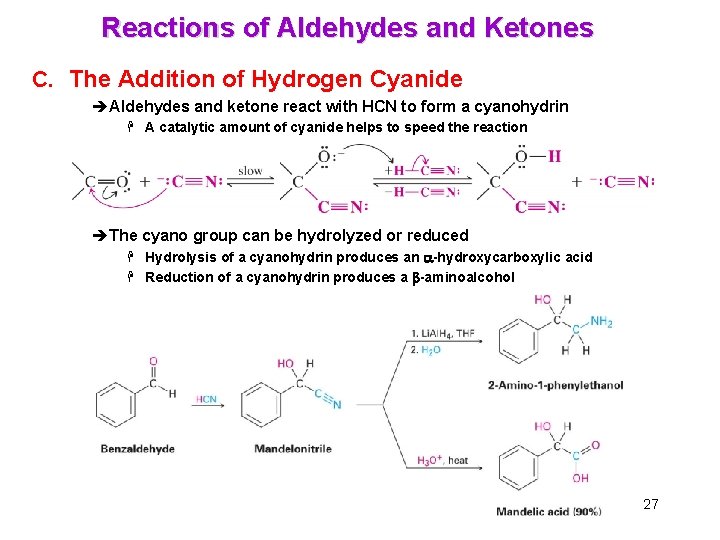
Reactions of Aldehydes and Ketones C. The Addition of Hydrogen Cyanide èAldehydes and ketone react with HCN to form a cyanohydrin H A catalytic amount of cyanide helps to speed the reaction èThe cyano group can be hydrolyzed or reduced H Hydrolysis of a cyanohydrin produces an a-hydroxycarboxylic acid H Reduction of a cyanohydrin produces a b-aminoalcohol 27

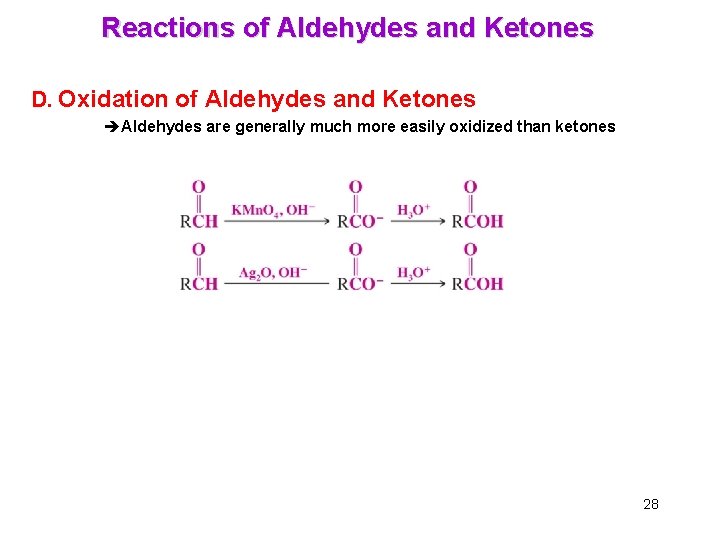
Reactions of Aldehydes and Ketones D. Oxidation of Aldehydes and Ketones èAldehydes are generally much more easily oxidized than ketones 28

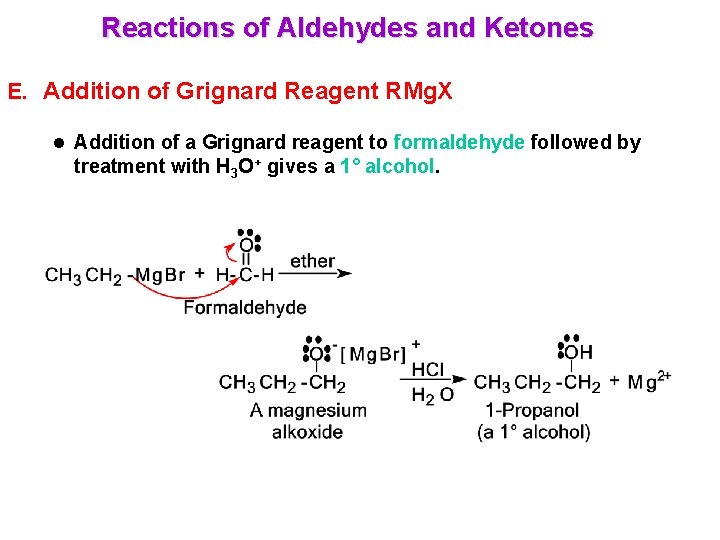
Reactions of Aldehydes and Ketones E. Addition of Grignard Reagent RMg. X l Addition of a Grignard reagent to formaldehyde followed by treatment with H 3 O+ gives a 1° alcohol.

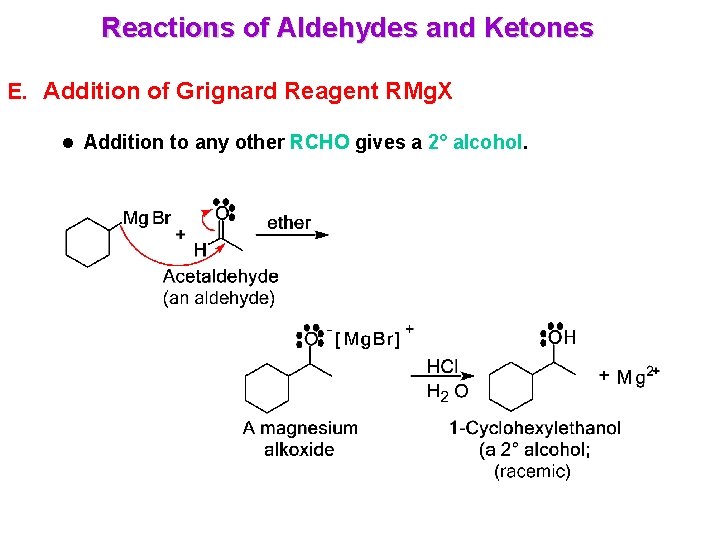
Reactions of Aldehydes and Ketones E. Addition of Grignard Reagent RMg. X l Addition to any other RCHO gives a 2° alcohol.

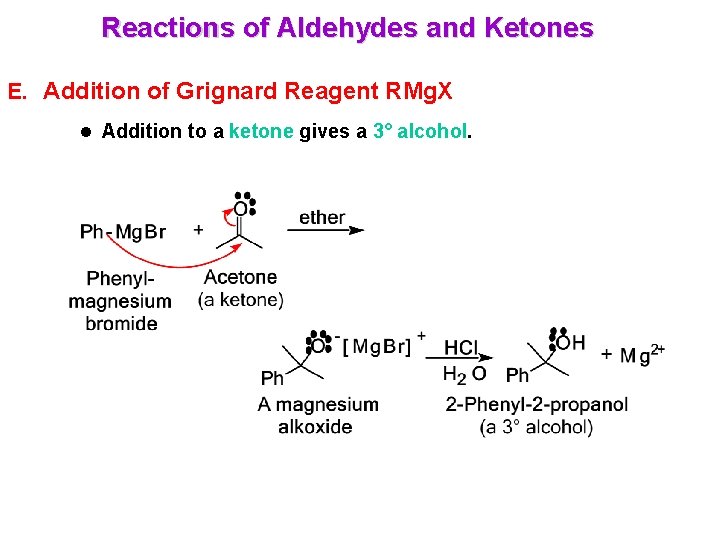
Reactions of Aldehydes and Ketones E. Addition of Grignard Reagent RMg. X l Addition to a ketone gives a 3° alcohol.

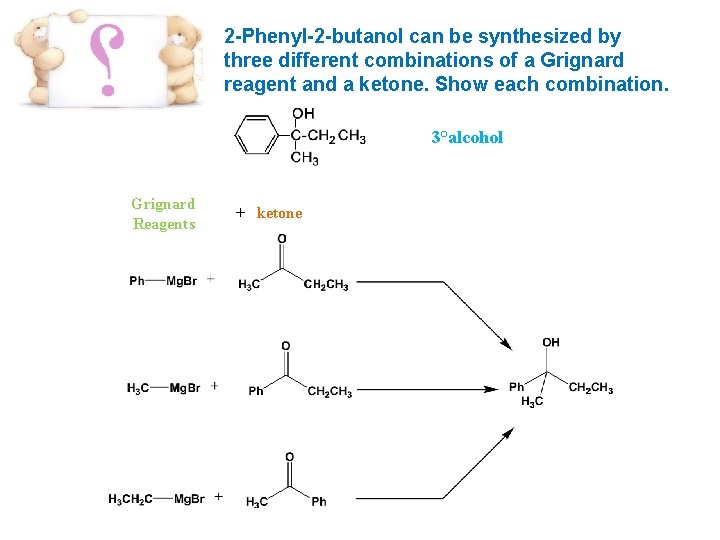
2 -Phenyl-2 -butanol can be synthesized by three different combinations of a Grignard reagent and a ketone. Show each combination. 3°alcohol Grignard Reagents + ketone

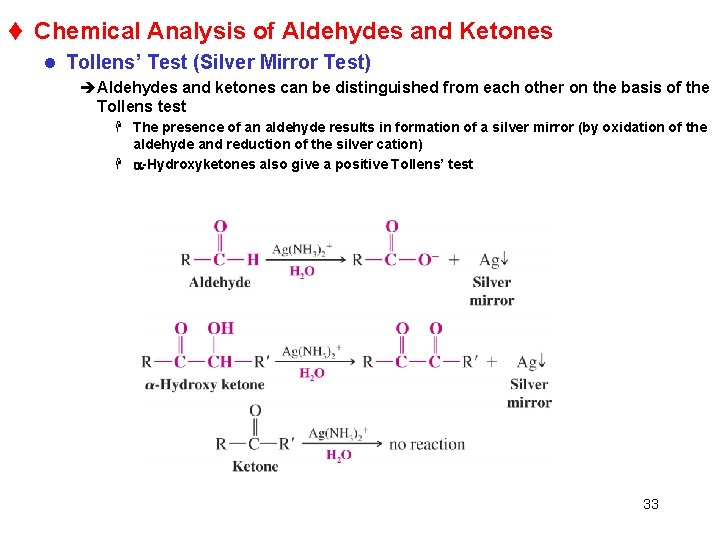
t Chemical Analysis of Aldehydes and Ketones l Tollens’ Test (Silver Mirror Test) èAldehydes and ketones can be distinguished from each other on the basis of the Tollens test H The presence of an aldehyde results in formation of a silver mirror (by oxidation of the aldehyde and reduction of the silver cation) H a-Hydroxyketones also give a positive Tollens’ test 33
 Aldehydes and ketones nucleophilic addition
Aldehydes and ketones nucleophilic addition Aldehydes and ketones structure
Aldehydes and ketones structure Polyhydroxy aldehyde examples
Polyhydroxy aldehyde examples Naming of aldehydes
Naming of aldehydes Preparation of ketones from acyl chlorides
Preparation of ketones from acyl chlorides Test for alkanal
Test for alkanal Carbonyl group aldehyde and ketone
Carbonyl group aldehyde and ketone Ketones structure
Ketones structure Chemsheets reactions of alcohols 1 answers
Chemsheets reactions of alcohols 1 answers Chemical properties of aldehyde and ketone
Chemical properties of aldehyde and ketone Ketone reactivity
Ketone reactivity Aldehyde protecting group
Aldehyde protecting group Carbonyl compounds
Carbonyl compounds Cyanohydrin formation
Cyanohydrin formation Addition reaction of carbonyl compounds
Addition reaction of carbonyl compounds Oc
Oc What is galactosemia
What is galactosemia Aldehyde and ketones
Aldehyde and ketones Identify nucleophile and electrophile
Identify nucleophile and electrophile Nhr group name
Nhr group name Uses of ketones
Uses of ketones Naming ether
Naming ether Site:slidetodoc.com
Site:slidetodoc.com Polyhydroxylated aldehydes
Polyhydroxylated aldehydes Aldol reaction
Aldol reaction Hart's test for ketones
Hart's test for ketones Sodium nitroprusside test for ketones
Sodium nitroprusside test for ketones Ketones in urine moderate
Ketones in urine moderate Oxidation of ketones
Oxidation of ketones Properties of ketones
Properties of ketones Ketone group
Ketone group Ketones
Ketones Antiperiplanar
Antiperiplanar Nucleophilicity trend
Nucleophilicity trend