Pathophysiology Fluid and electrolytes Terms Intracellular fluid fluid



![Starling law of the capillaries � Example EF=[18 mm+3 mm]-[25 mm+0 mm]= -3 mm. Starling law of the capillaries � Example EF=[18 mm+3 mm]-[25 mm+0 mm]= -3 mm.](https://slidetodoc.com/presentation_image_h2/0706a684d7542acb1177a784949edf3e/image-12.jpg)




























- Slides: 62

Pathophysiology � Fluid and electrolytes � Terms ◦ Intracellular fluid- fluid in the cells ◦ Extracellular fluid- fluid outside the cells �Interstitial fluid- fluid between the cells �Plasma- fluid in the blood

Terms �Oncotic pressure �-the pressure exerted by plasma proteins on the capillary wall �Osmotic pressure �: the pressure produced by or associated with osmosis and dependent on solute concentration and temperature � Oncotic pressure in the circulatory system is a form of osmotic pressure exerted by proteins in blood plasma that normally tends to pull water into the circulatory system. Colloid osmotic pressure is another name for oncotic pressure. �Colloid osmotic pressure is used in calculations related to Starling’s law of the capillaries

Fluid intake and output � Fluid intake is from water in foods, liquids ingested and water of catabolism ◦ The total is around 2, 400 to 2, 800 ml a day � Fluid output is loss from the skin by diffusion and sweat, by the kidneys (urine) about 1500 ml/day, and by the intestines (feces) ◦ Output is about 2, 400 -2, 800 ml per day ◦ Note 2, 800 ml =2. 8 Liters

Memory check m. Eq/L � m. Eq/L= mg of ion per liter x number of charges of one ion (valence)/ atomic weight of ion(s) � Note: If mg of ion is per 100 ml X by 10 to bring to liter � Example Patient blood work shows the following: � laboratory results indicate that the plasma sodium = 110 m. Eq/L, look at normal levels � Too low

Memory check 2 � An acid is a substance/chemical that donates a hydrogen ion to the solution ◦ Examples of acids are HCL (hydrochloric acid and H 2 CO 3 (carbonic acid � Acid/ base balance in body fluids is affected by the formation of carbonic acid � CO 2 + H 2 O H 2 CO 3 H+ + HCO 3

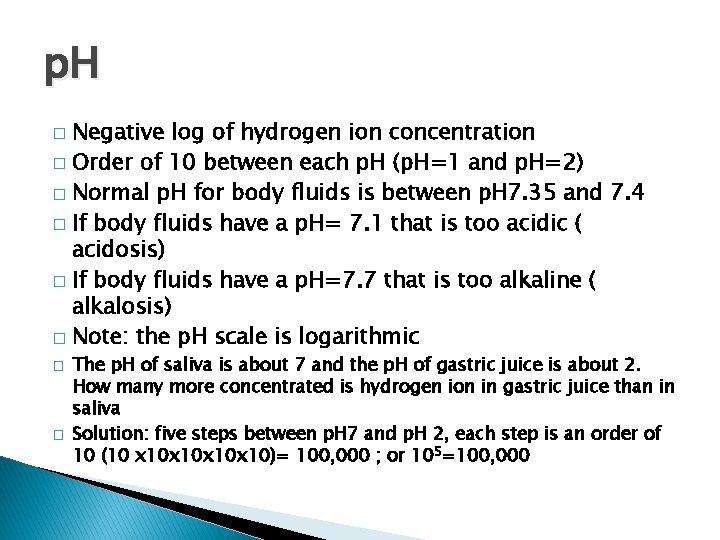
p. H Negative log of hydrogen ion concentration � Order of 10 between each p. H (p. H=1 and p. H=2) � Normal p. H for body fluids is between p. H 7. 35 and 7. 4 � If body fluids have a p. H= 7. 1 that is too acidic ( acidosis) � If body fluids have a p. H=7. 7 that is too alkaline ( alkalosis) � Note: the p. H scale is logarithmic � � � The p. H of saliva is about 7 and the p. H of gastric juice is about 2. How many more concentrated is hydrogen ion in gastric juice than in saliva Solution: five steps between p. H 7 and p. H 2, each step is an order of 10 (10 x 10 x 10)= 100, 000 ; or 105=100, 000

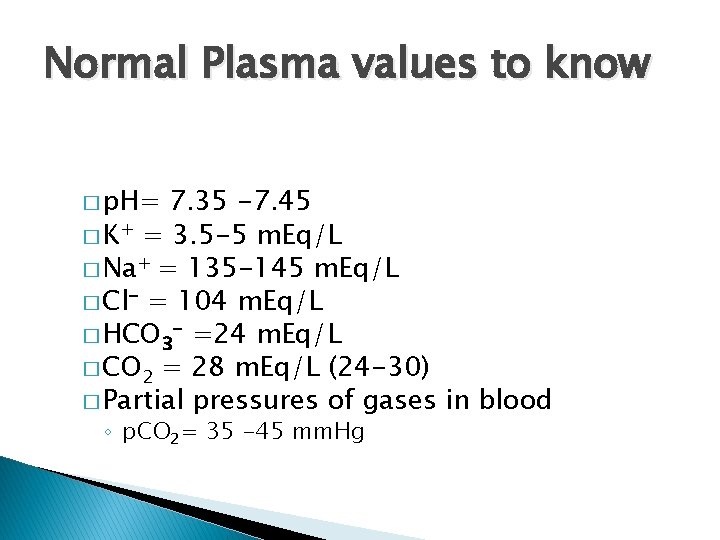
Normal Plasma values to know � p. H= 7. 35 -7. 45 � K+ = 3. 5 -5 m. Eq/L � Na+ = 135 -145 m. Eq/L � Cl- = 104 m. Eq/L � HCO 3 - =24 m. Eq/L � CO 2 = 28 m. Eq/L (24 -30) � Partial pressures of gases in blood ◦ p. CO 2= 35 -45 mm. Hg

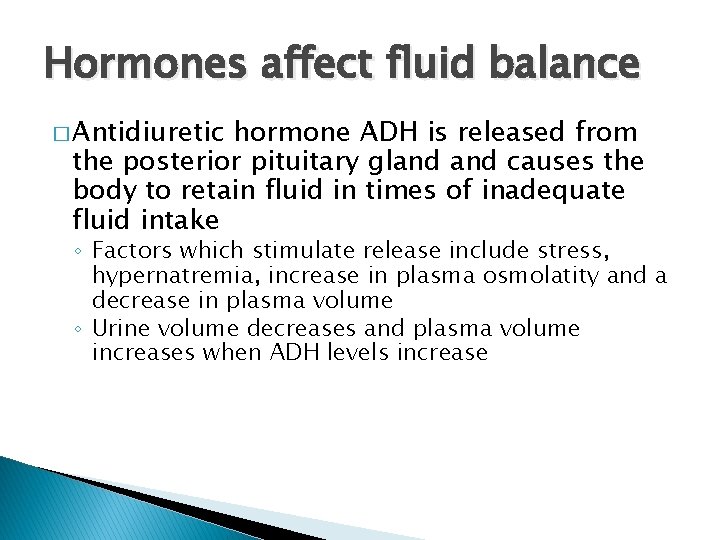
Hormones affect fluid balance � Antidiuretic hormone ADH is released from the posterior pituitary gland causes the body to retain fluid in times of inadequate fluid intake ◦ Factors which stimulate release include stress, hypernatremia, increase in plasma osmolatity and a decrease in plasma volume ◦ Urine volume decreases and plasma volume increases when ADH levels increase

High Aldosterone � Aldosterone is secreted by the adrenal cortex � Causes retention (reabsorption) of sodium ions and the excretion of potassium ions which results in the retention of body fluids ◦ Urine volume decreases and plasma volume increases ◦ Blood sodium increases and potassium levels in the blood decrease

Alterations in water movement � Edema ◦ Forces pushing out are greater than normal, fluid excess is not returned to venous and or lymphatic vessels

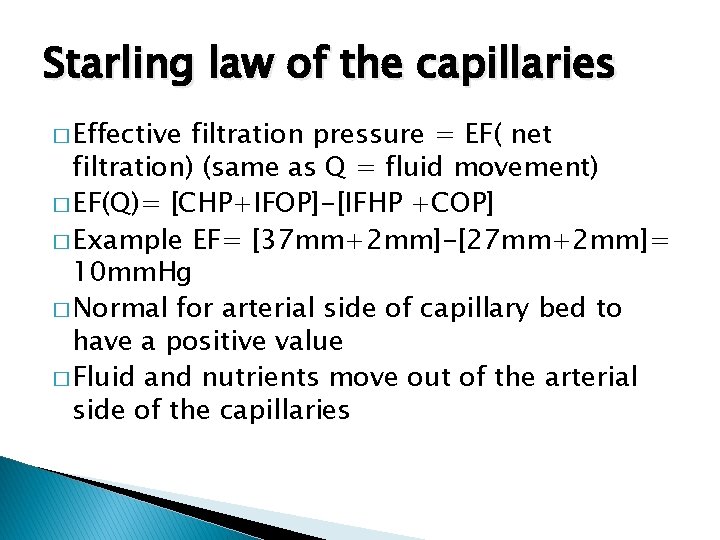
Starling law of the capillaries � Effective filtration pressure = EF( net filtration) (same as Q = fluid movement) � EF(Q)= [CHP+IFOP]-[IFHP +COP] � Example EF= [37 mm+2 mm]-[27 mm+2 mm]= 10 mm. Hg � Normal for arterial side of capillary bed to have a positive value � Fluid and nutrients move out of the arterial side of the capillaries
![Starling law of the capillaries Example EF18 mm3 mm25 mm0 mm 3 mm Starling law of the capillaries � Example EF=[18 mm+3 mm]-[25 mm+0 mm]= -3 mm.](https://slidetodoc.com/presentation_image_h2/0706a684d7542acb1177a784949edf3e/image-12.jpg)
Starling law of the capillaries � Example EF=[18 mm+3 mm]-[25 mm+0 mm]= -3 mm. Hg � Normal for venous side of capillary bed to have a negative value � Fluid and wastes move into the venous side of the capillaries

Causes of edema- increased HP � Hydrostatic pressure increases because of venous obstruction � Thrombophlebitis � Hepatic obstruction � Tight clothing around extremities � Prolonged standing (without movement)

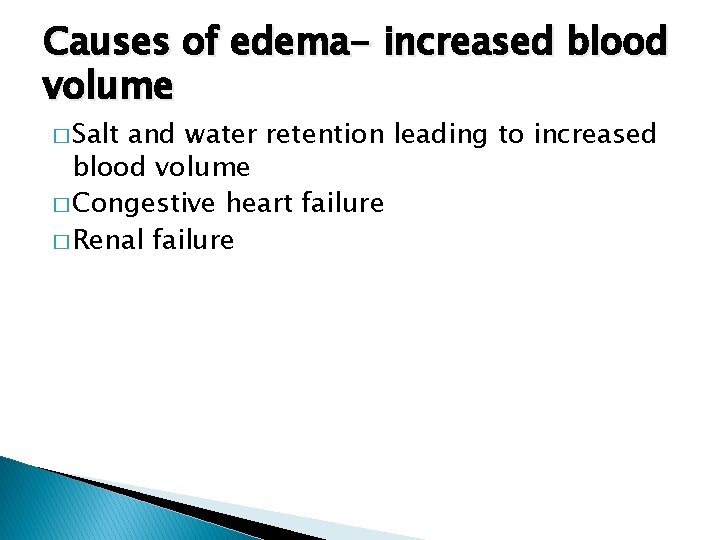
Causes of edema- increased blood volume � Salt and water retention leading to increased blood volume � Congestive heart failure � Renal failure

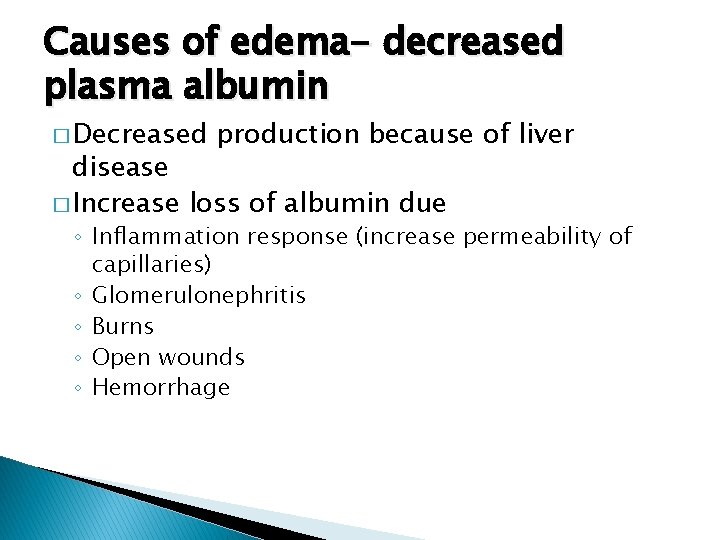
Causes of edema- decreased plasma albumin � Decreased production because of liver disease � Increase loss of albumin due ◦ Inflammation response (increase permeability of capillaries) ◦ Glomerulonephritis ◦ Burns ◦ Open wounds ◦ Hemorrhage

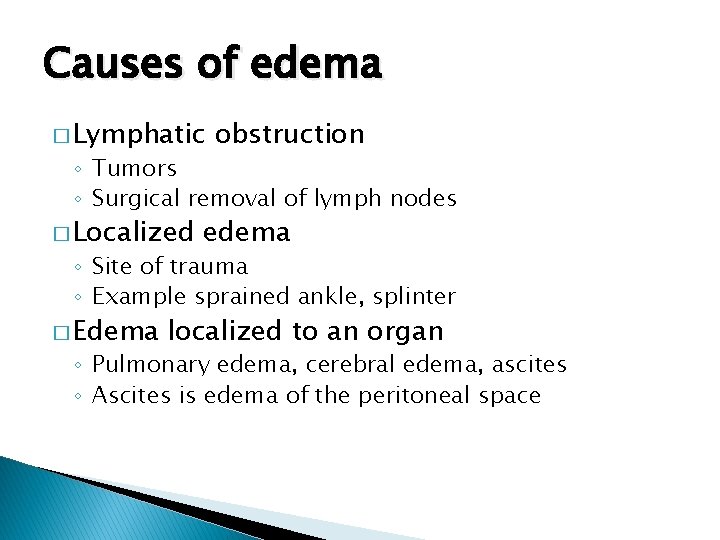
Causes of edema � Lymphatic obstruction ◦ Tumors ◦ Surgical removal of lymph nodes � Localized edema ◦ Site of trauma ◦ Example sprained ankle, splinter � Edema localized to an organ ◦ Pulmonary edema, cerebral edema, ascites ◦ Ascites is edema of the peritoneal space

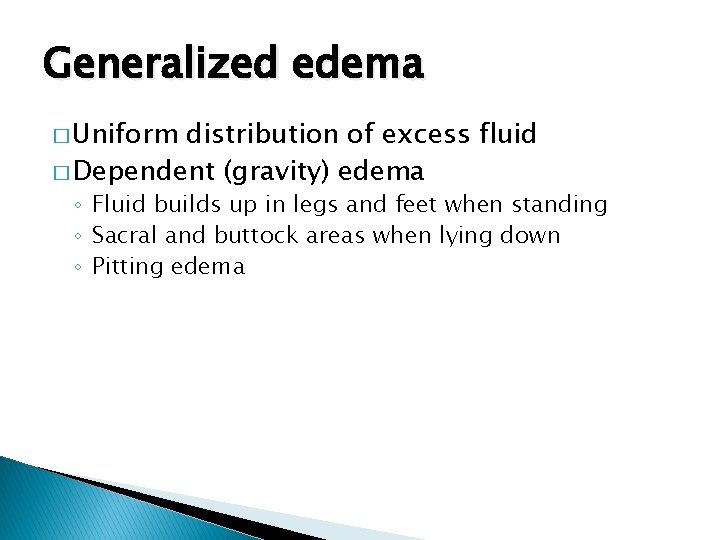
Generalized edema � Uniform distribution of excess fluid � Dependent (gravity) edema ◦ Fluid builds up in legs and feet when standing ◦ Sacral and buttock areas when lying down ◦ Pitting edema

Complications � Because of trapping of fluid causes sequestering of fluid � Dehydration occurs � Timely fluid and electrolyte replacement is critical and treating the cause of edema is essential to prevent complications

Alterations in Sodium, Chloride, and water balance � Hormones that help regulate fluid balance include- antidiuretic hormone (ADH), aldosterone, (atrial) natriuretic hormone (ANH) � Types- isotonic alterations, hypertonic alterations, hypotonic alterations

Isotonic alterations (fluid volume deficits) � Occurs when fluid loss (or gain) and electrolyte loss (or gain) are proportional

Isotonic fluid loss (isotonic dehydration) � Causes- hemorrhage, severe wound drainage, excessive diaphoresis � Signs and symptoms include- loss of weight, dry skin, dry mucous membranes, decreased urine output � Hypovolemia symptoms include rapid heart rate, decreased blood pressure, shock may occur. Sometimes because of flattened veins it is difficult to start IV

Isotonic fluid excesses � Causes- excessive IV, hypersecretion of aldosterone, side effects of cortisone � Signs and symptoms include- hypervolemia, weight gain, decreased hematocrit, increase blood pressure, increased BHP � Complications- pulmonary edema, heart failure

Hypertonic alterations � Osmolality of ECF increases � Most common cause hypernatremia or deficit of ECF fluid

hypernatremia � Serum sodium levels greater than 147 m. Eq/L � Loss of water, gain of sodium � When water loss occurs in hypernatremia ICF and ECF dehydration occurs � Causes hyperosmolaity

Hypernatremia � Causes does not include excess dietary sodium � Causes include inappropriate administration of hypertonic saline � (Sodium bicarbonate is often given to treat acidosis during cardiac arrest) � Over secretion of aldosterone as in Cushing syndrome

Increase retention of sodium � Can also be a result of complications associated with � Respiratory infections � Diabetes mellitus � Diabetes insipidus � Polyuria � Profuse sweating � Diarrhea in children

Signs and symptoms of hypernatremia � Signs and symptoms- thirst, fever, dry mucous membranes, restlessness � Complications- convulsions, pulmonary edema

Water deficit � Dehydration � Causes- lack of intake, excess of loss of water vapor as in hyperventilation, increased renal clearance, diabetes insipidus

Signs and symptoms of water deficit � Thirst, dry skin, dry mucous membranes, concentrated urine, tachycardia, weak pulses, postural hypotension

Hyperchloremia � Too much chloride � Occurs with hypernatremia or with too little bicarbonate � Can occur with metabolic acidosis � Ingestion of ammonium chloride diuretic is a infrequent cause (excess loss of chloride occurs)

Hyponatremia � Serum levels below 135 m. Eq/L � Pure sodium deficits- extrarenal lossesvomiting, diarrhea, burns, GI suction � Inadequate intake is rare but possible in person on very low sodium diets accompanied by use of diuretics

Hyponatremia, cont. � IV of 5% dextrose can cause dilution hyponatremia � Excess sweating and intake of sodium free water � Renal dysfunction can lead to hypoosmolar hyponatremia

Hypertonic hyponatremia � Complications of hyperlipidemia, hyperproteinemia and or hyperglycemia � Increases in plasma lipids, proteins, displace water volume and decrease sodium concentrations

Signs and symptoms of hyponatremia � Behavioral and neurologic function includelethargy, confusion, apprehension, seizures, coma � if loss of ECF also occurs symptoms of hypotension also occur

Water excess � Causes- compulsive water drinking � IV of 5% dextrose in patients with acute renal failure, congestive heart failure, cirrhosis of liver can cause water excess � Decreased urine formation ◦ Associated with renal disease or decreased renal blood flow

SIADH � Syndrome of inappropriate secretion of ADH � Causes of SIADH include fear, pain, acute infections, brain trauma, surgery, side effects of some medications (analgesics and anesthetics) and bronchogenic cancer � Decrease renal excretion of water occurs

Signs and symptoms of water excess � Acute excesses- confusion and convulsions � Long term excesses- weakness, nausea, muscle twitching, headache, weight gain

Hypochloremia � Loss of chloride � Result of hyponatremia � Elevated bicarbonate as in metabolic alkalosis � Vomiting with loss of HCl � Salt restricted diets and or use of diuretics � Cystic fibrosis is characterized by hypochloremia

Potassium and health � Potassium intake associated with reduction in BP � Stroke risk is increased with increase loss of potassium � Foods high in potassium are cranberries and bananas

Hypokalemia � Serum plasma levels below 3. 5 m. Eq/L � Loss of potassium- diarrhea, laxative abuse, vomiting, eating disorders, some diuretics, kidney disorders

Signs and symptoms of hypokalemia � Dysrhythmia, EKG depressed T wave, irregular pulse, lethargy, fatigue, confusion, nausea, water loss, thirst, muscle weakness

Hyperkalemia � Above 5. 5 m. Eq/L � Causes- shift from intracellular to extracellular space as in massive trauma or burns, decreased renal excretion, excess intake (rare as use of KCl by mistake) and in metabolic acidosis

Example �A patient suffers from weakness, dizziness, irritability, and intestinal cramps: The EKG shows tall peaked T wave but otherwise normal. � The lab results show Plasma sodium=138 m. Eq/L � Blood potassium levels =6. 8 m. Eq/L � � � This individual is suffering from a. hypernatremia b. hyponatremia c. hypercalcemia d. hyperkalemia e. hypokalemia

Signs and symptoms of hyperkalemia � Dysrhythmia, bradycardia, anxiety, tingling, numbness, nausea, vomiting, earlyhyperactive muscles, late- weakness of muscle

Acid Base balance � Buffers include bicarbonate, phosphate (renal), hemoglobin (protein)

Carbonic acid-bicarbonate buffering � Normal blood p. H=7. 4 which is a 20: 1 ratio of BB/CA � For example if the bicarbonate (BB)level is 24 m. Eq/L and the carbonic acid(CA) level is 1. 2 m. Eq/L the ratio is 20: 1

Compensation mechanisms to control p. H � Acidification of urine- slowest (kidneys) � Respiration rate – medium (lungs) � Buffer systems of blood- fastest (blood buffers)

The four types of Acid-Base imbalances � Metabolic acidosis � Metabolic alkalosis � Respiratory acidosis � Respiratory alkalosis

Metabolic acidosis � Increased H+ load ◦ Ketoacidosis-diabetes mellitus, starvation ◦ Lactic acidosis- shock ◦ Ingestion- ammonium chloride, ethylene glycol, methanol

Decreased H+ excretion � Uremia � Distal renal tubule acidosis

Bicarbonate loss � Renal failure � Diarrhea � Proximal renal tubule acidosis

Signs and symptoms of metabolic acidosis � Headache, lethargy, rapid and deep respirations, anorexia, nausea, vomiting, diarrhea, abdominal discomfort

Metabolic acidosis � Ratio BB/CA is 10: 1 p. H=7. 1 � Body compensation- respiratory- increased breathing rate blows off excess CO 2 ; kidney conserves HCO 3 - and excretes H+, urine is acidic � Therapy- use of lactate IV- increases HCO 3 in liver

Metabolic alkalosis � Cause- excess ingestion of bicarbonate � Excess bicarbonate due to loss of chloride ions

Signs and symptoms of metabolic alkalosis � Weakness, muscle cramps, hyperactive reflexes, tetany, shallow slow respirations, confusion, convulsion, atrial tachycardia

Metabolic alkalosis � BB/CC ratio is 40: 1 p. H=7. 7 � Body compensation- breathing is suppressed � Alkaline urine is produced � Therapy- IV containing chloride

Respiratory acidosis � Causes pneumonia, emphysema, asthma, bronchitis, pulmonary edema, depression of respiratory center, dyphoscoliosis

Signs and symptoms of respiratory acidosis � Apprehension, lethargy, muscle twitching, tremors, convulsions,

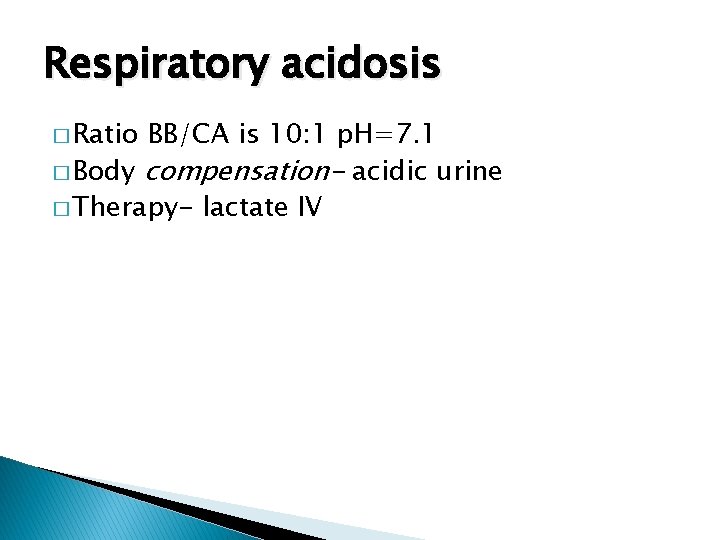
Respiratory acidosis � Ratio BB/CA is 10: 1 p. H=7. 1 � Body compensation- acidic urine � Therapy- lactate IV

Respiratory alkalosis � Alveolar hyperventilation � Hypoxemia caused by pulmonary disease, congestive heard failure, high altitudes, hypermetabolic states, hysteria, cirrhosis of gram negative sepsis which cause hyperventilation

Signs and symptoms of respiratory alkalosis � Confusion, dizziness, tingling of extremities, convulsions, coma

Respiratory alkalosis � BB/CA ratio of 40: 1 p. H=7. 7 � Body compensation- alkaline urine � Therapy- IV of chloride solution