SOLUTIONS PART II Electrolytes vs Nonelectrolytes Electrolytes vs





- Slides: 17

SOLUTIONS PART II Electrolytes vs Nonelectrolytes

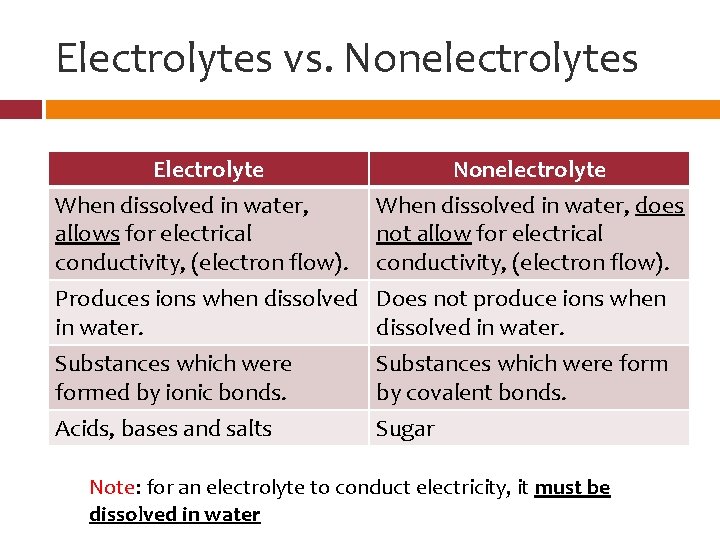
Electrolytes vs. Nonelectrolytes Electrolyte When dissolved in water, allows for electrical conductivity, (electron flow). Nonelectrolyte When dissolved in water, does not allow for electrical conductivity, (electron flow). Produces ions when dissolved in water. Substances which were formed by ionic bonds. Acids, bases and salts Does not produce ions when dissolved in water. Substances which were form by covalent bonds. Sugar Note: for an electrolyte to conduct electricity, it must be dissolved in water

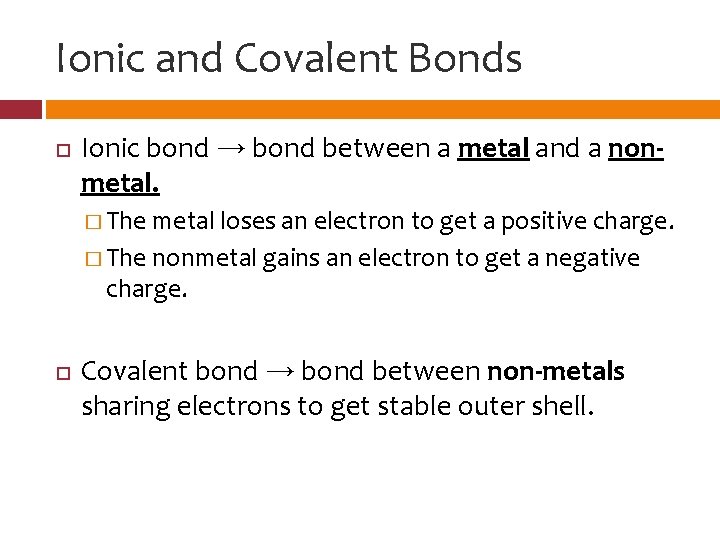
Ionic and Covalent Bonds Ionic bond → bond between a metal and a nonmetal. � The metal loses an electron to get a positive charge. � The nonmetal gains an electron to get a negative charge. Covalent bond → bond between non-metals sharing electrons to get stable outer shell.

Electrolytic Dissociation Definition: electrolytes are dissolved in water, dissociating from one another to their respective positively and negatively charged ions, allowing for electrical conductivity. Key thing → the production of ions during dissociation. � No ions, no electrical conductivity.

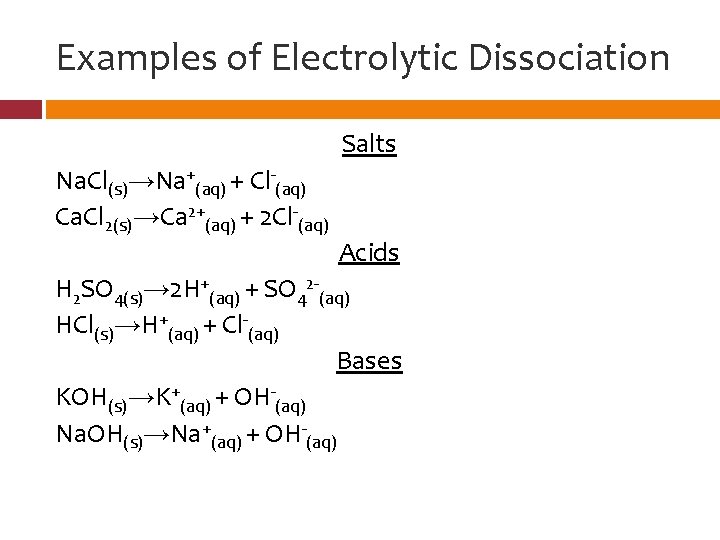
Examples of Electrolytic Dissociation Salts Na. Cl(s)→Na+(aq) + Cl-(aq) Ca. Cl 2(s)→Ca 2+(aq) + 2 Cl-(aq) Acids H 2 SO 4(s)→ 2 H+(aq) + SO 42 -(aq) HCl(s)→H+(aq) + Cl-(aq) Bases KOH(s)→K+(aq) + OH-(aq) Na. OH(s)→Na+(aq) + OH-(aq)

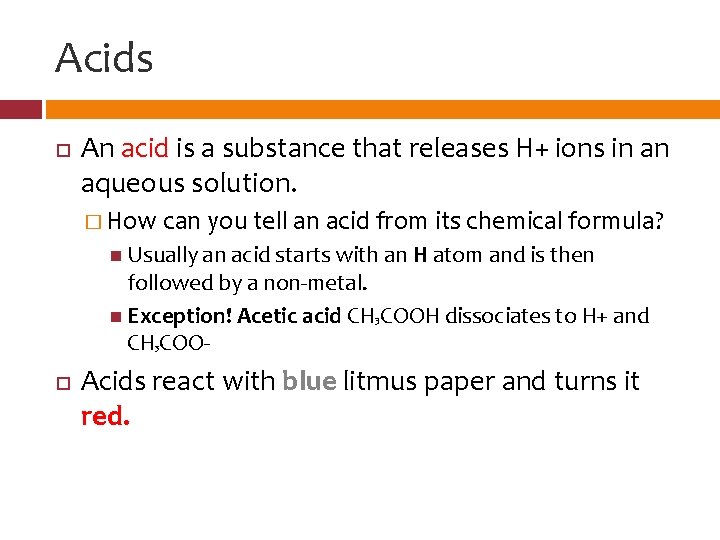
Acids An acid is a substance that releases H+ ions in an aqueous solution. � How can you tell an acid from its chemical formula? Usually an acid starts with an H atom and is then followed by a non-metal. Exception! Acetic acid CH 3 COOH dissociates to H+ and CH COO 3 Acids react with blue litmus paper and turns it red.

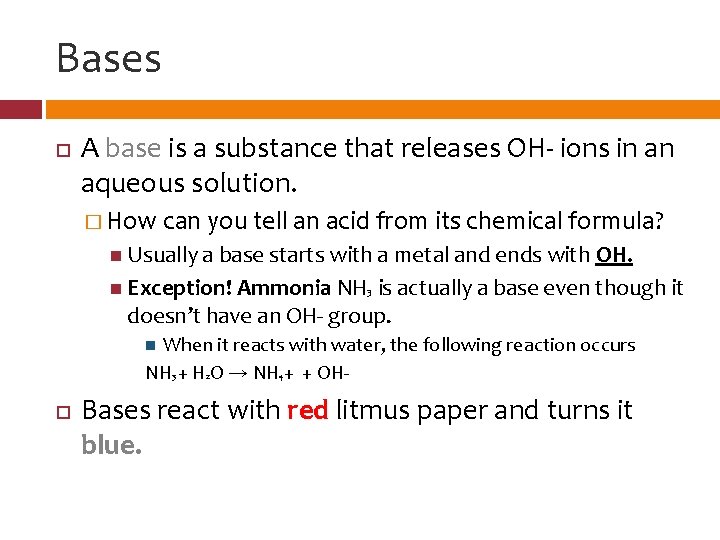
Bases A base is a substance that releases OH- ions in an aqueous solution. � How can you tell an acid from its chemical formula? Usually a base starts with a metal and ends with OH. Exception! Ammonia NH 3 is actually a base even though it doesn’t have an OH- group. When it reacts with water, the following reaction occurs NH 3 + H 2 O → NH + + OH 4 Bases react with red litmus paper and turns it blue.

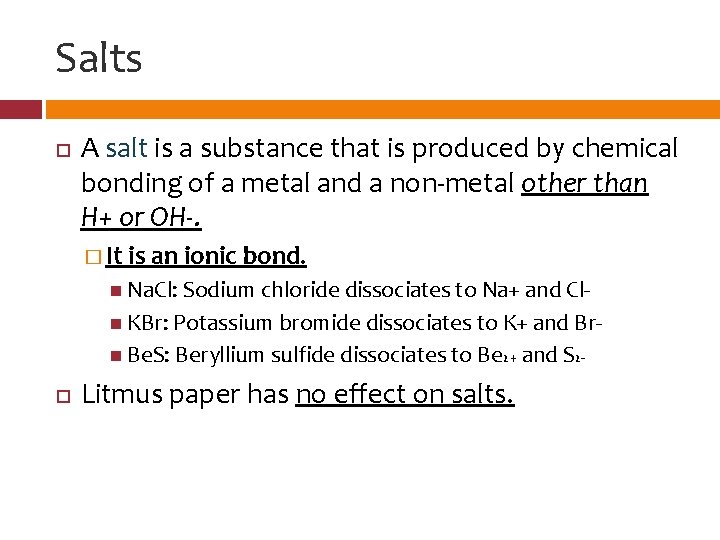
Salts A salt is a substance that is produced by chemical bonding of a metal and a non-metal other than H+ or OH-. � It is an ionic bond. Na. Cl: Sodium chloride dissociates to Na+ and Cl KBr: Potassium bromide dissociates to K+ and Br Be. S: Beryllium sulfide dissociates to Be 2+ and S 2 - Litmus paper has no effect on salts.

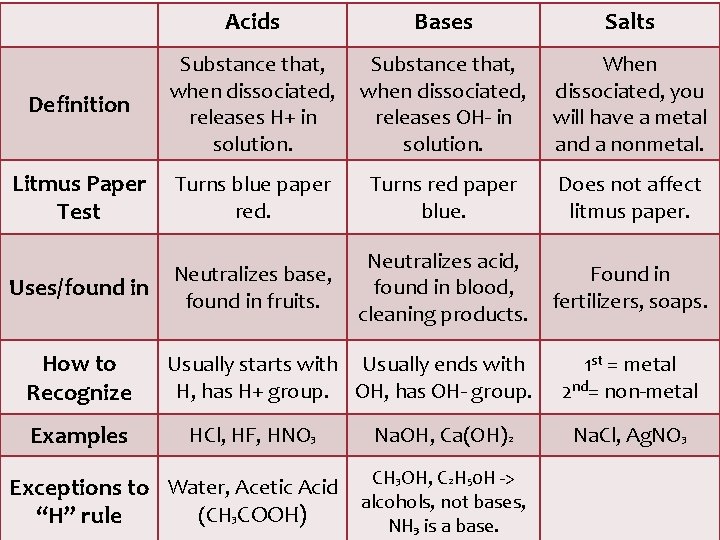
Acids Bases Salts Definition Substance that, when dissociated, releases H+ in solution. Substance that, when dissociated, releases OH- in solution. When dissociated, you will have a metal and a nonmetal. Litmus Paper Test Turns blue paper red. Turns red paper blue. Does not affect litmus paper. Uses/found in Neutralizes base, found in fruits. Neutralizes acid, found in blood, cleaning products. Found in fertilizers, soaps. How to Recognize Examples Usually starts with Usually ends with H, has H+ group. OH, has OH- group. HCl, HF, HNO 3 Na. OH, Ca(OH)2 Exceptions to Water, Acetic Acid CH 3 OH, C 2 H 50 H -> alcohols, not bases, (CH 3 COOH) “H” rule NH₃ is a base. 1 st = metal 2 nd= non-metal Na. Cl, Ag. NO 3

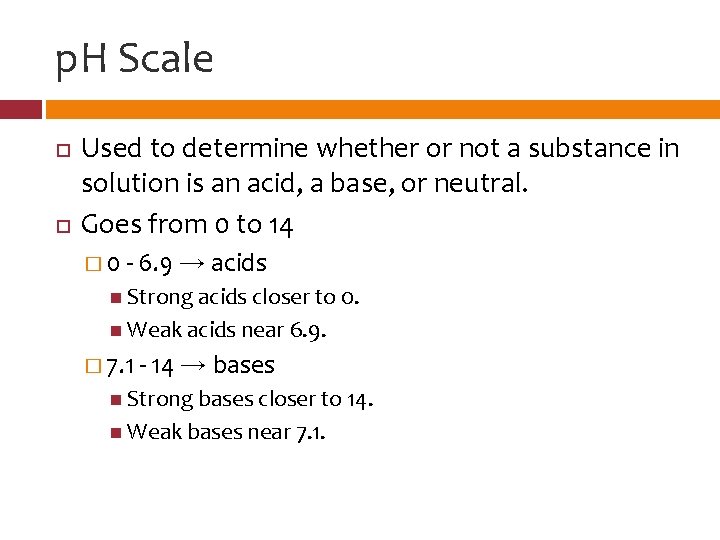
p. H Scale Used to determine whether or not a substance in solution is an acid, a base, or neutral. Goes from 0 to 14 � 0 - 6. 9 → acids Strong acids closer to 0. Weak acids near 6. 9. � 7. 1 - 14 → bases Strong bases closer to 14. Weak bases near 7. 1.

p. H Scale The scale goes up by a 10 -fold factor. � Meaning if you are comparing an acid with a p. H 2 and p. H of 3, the acid which has a p. H 2 is 10 times stronger. � If you are comparing an acid with p. H 2 and p. H 6, the acid which has p. H 2 is 104 times stronger.

How to Identify Acids, Bases and Neutral Substances Litmus Paper Test 1. • Tells you if your substance is an acid, base or neutral. Buffer Solution + Indicators 2. • A buffer solution is a solution composed of a weak acid and its associated base. • Key: its p. H changes very little when strong acid/base added to it, meaning it has specific p. H levels. An indicator is a chemical which undergoes a colour change at specific p. H’s.

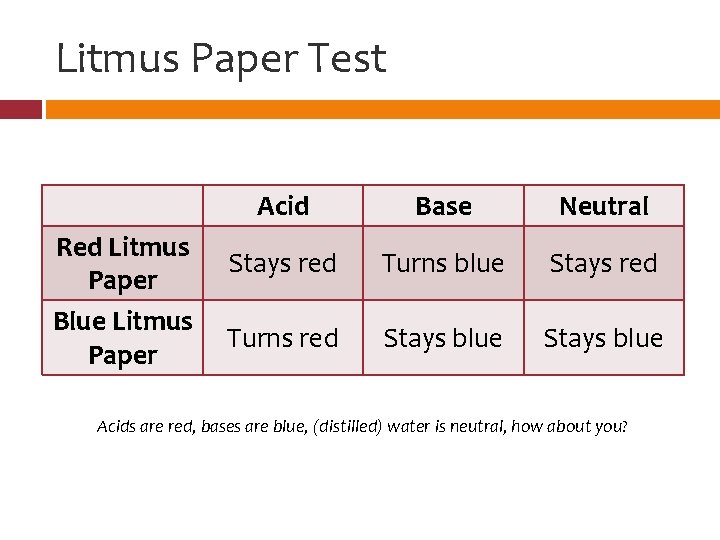
Litmus Paper Test Red Litmus Paper Blue Litmus Paper Acid Base Neutral Stays red Turns blue Stays red Turns red Stays blue Acids are red, bases are blue, (distilled) water is neutral, how about you?

Buffer Solution + Indicator This procedure plays on the specificity of buffer solutions. The first step you do is introduce your solution to the indicator and record that colour. Then you introduce your indicator to buffer solutions ranging from p. H 1 to p. H 14 and match the colour. � You may have to use more than one indicator, for their ranges overlap, thus allowing to get a more precise p. H value.

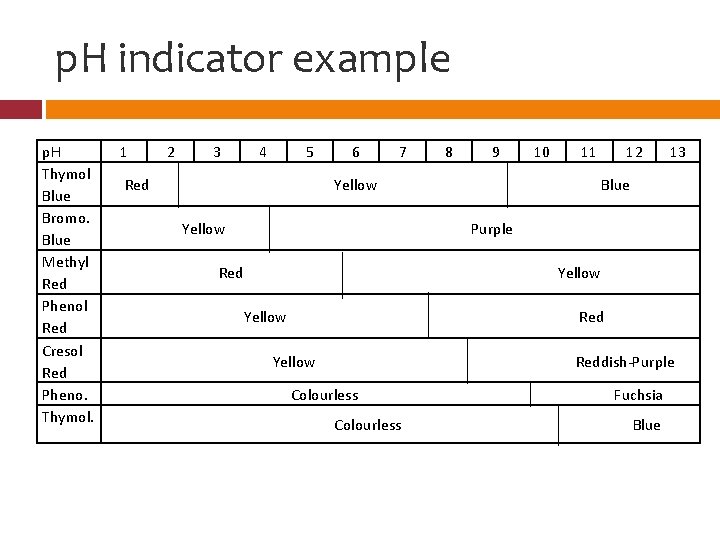
p. H indicator example p. H Thymol Blue Bromo. Blue Methyl Red Phenol Red Cresol Red Pheno. Thymol. 1 2 3 4 5 Red 6 7 8 9 10 11 Yellow 12 13 Blue Purple Red Yellow Reddish-Purple Colourless Fuchsia Blue

p. H Indicator Example 1. 2. 3. Which indicator(s) would you use to find a strong acid? Strong base? Neutral substance? My solution turned red using Methyl Red and purple using Bromophenol Blue. What’s the p. H of my solution (range)? My solution turned blue using Thymol Blue and fuchsia using Phenolphthalein. What’s the p. H of my solution (range)?

Mnemonic to remember for acids and bases Acids are red, bases are blue, water is neutral, what about you? Acid → turn blue litmus paper red Bases → turn red litmus paper blue Water → as a neutral substance, does not rnx with litmus paper (same as salts)