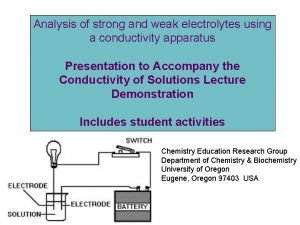
II Types of Electrolytes A Strong Electrolytes zcompletely






- Slides: 18

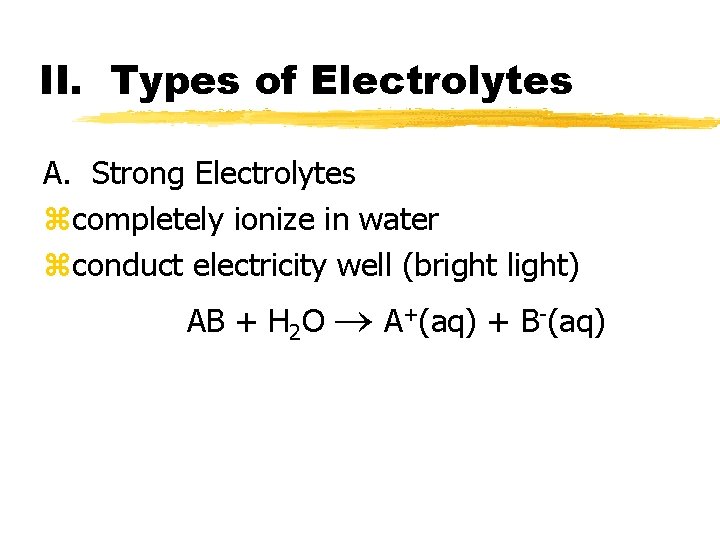
II. Types of Electrolytes A. Strong Electrolytes zcompletely ionize in water

II. Types of Electrolytes A. Strong Electrolytes zcompletely ionize in water zconduct electricity well (bright light) AB + H 2 O A+(aq) + B-(aq)

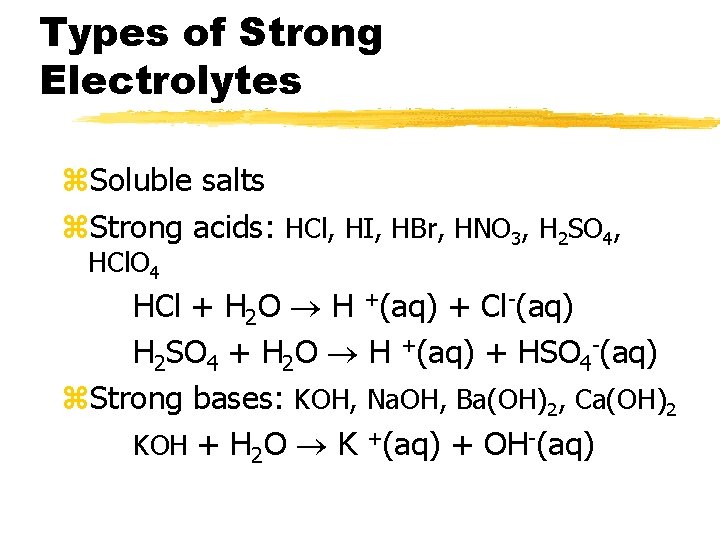
Types of Strong Electrolytes z. Soluble salts z. Strong acids: HCl, HI, HBr, HNO 3, H 2 SO 4, HCl. O 4 HCl + H 2 O H +(aq) + Cl-(aq) H 2 SO 4 + H 2 O H +(aq) + HSO 4 -(aq) z. Strong bases: KOH, Na. OH, Ba(OH)2, Ca(OH)2 KOH + H 2 O K +(aq) + OH-(aq)

II. Types of Electrolytes B. Weak Electrolytes z. Only some dissociation z. Most remains intact in water z. Don’t conduct electricity well (dim light)

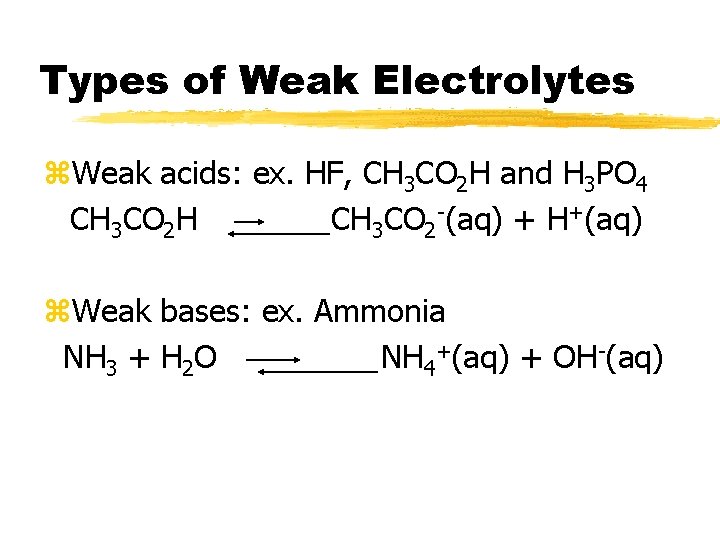
Types of Weak Electrolytes z. Weak acids: ex. HF, CH 3 CO 2 H and H 3 PO 4 CH 3 CO 2 H CH 3 CO 2 -(aq) + H+(aq) z. Weak bases: ex. Ammonia NH 3 + H 2 O NH 4+(aq) + OH-(aq)

II. Types of Electrolytes z. C. Non- Electrolytes zdissolve in water but don’t form ions zdo not conduct electricity (no light) zpolar, non-ion forming compounds zex. Sugar, ethanol

III. When Do Exchange Rx Occur? z. A water insoluble product forms (a precipitation rx) z. A stable molecule forms and removes ions from solution (ex. Neutralization rx-more details in chapt 11) z. A gas is produced

IV. Precipitation Rx z. A type of exchange reaction zsoluble reactants form insoluble product

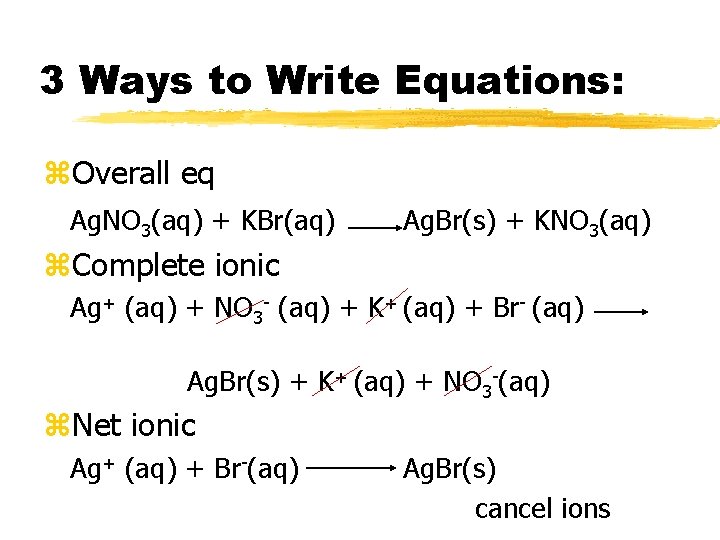
3 Ways to Write Equations: z. Overall eq Ag. NO 3(aq) + KBr(aq) Ag. Br(s) + KNO 3(aq) z. Complete ionic Ag+ (aq) + NO 3 - (aq) + K+ (aq) + Br- (aq) Ag. Br(s) + K+ (aq) + NO 3 -(aq) z. Net ionic Ag+ (aq) + Br-(aq) Ag. Br(s) cancel ions

How do you know when a solid forms? *Understand concepts and memorize rules! *Solids must have a net charge of zero-2 cations can’t form a solid.

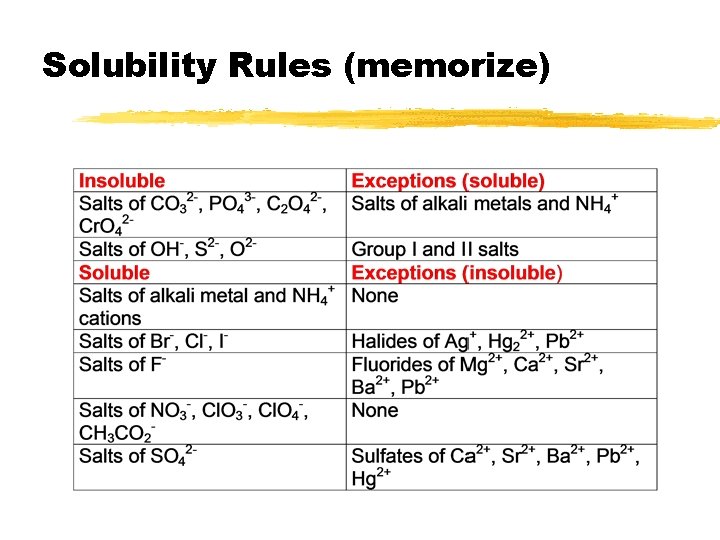
Solubility Rules (memorize)

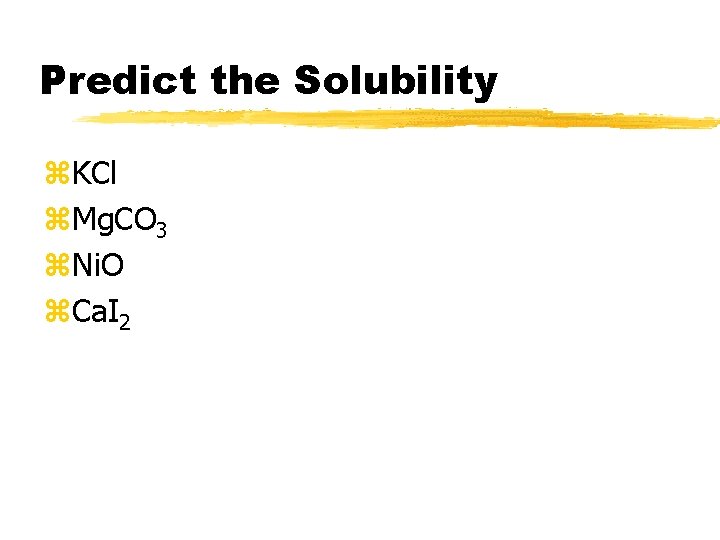
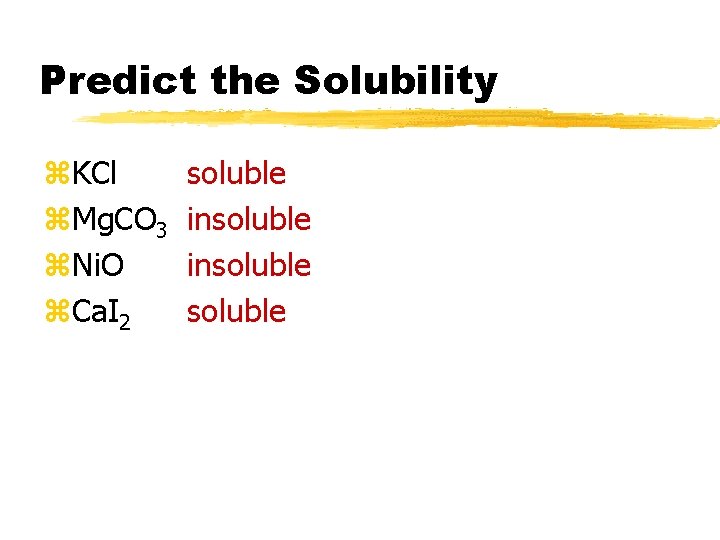
Predict the Solubility z. KCl z. Mg. CO 3 z. Ni. O z. Ca. I 2

Predict the Solubility z. KCl z. Mg. CO 3 z. Ni. O z. Ca. I 2 soluble insoluble

What Possible Solids Could Form in a Reaction? z. Exchange anions and use solubility rules to see if products are solid or aqueous. z. If both are aqueous there is no reaction.

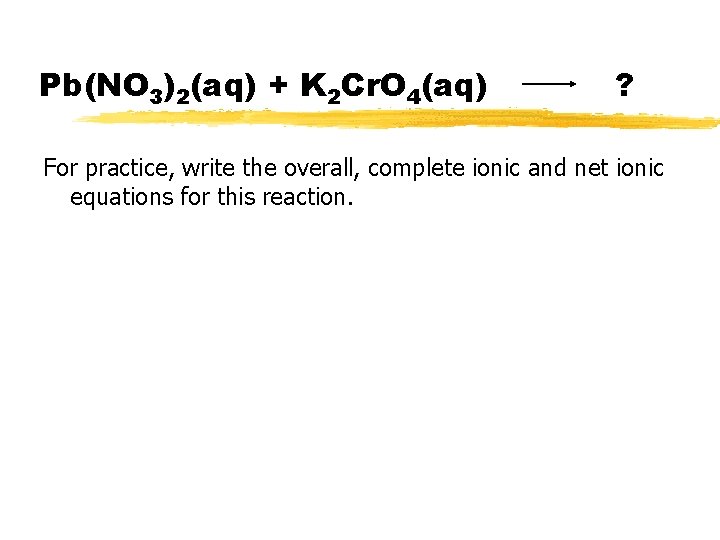
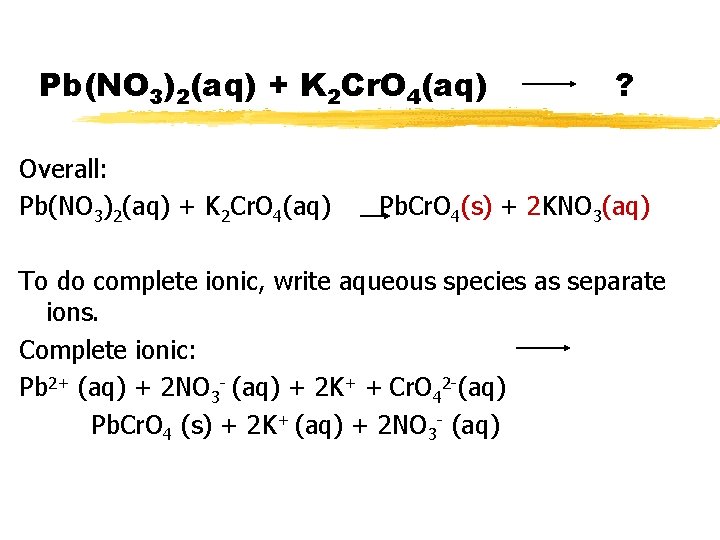
Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) ? For practice, write the overall, complete ionic and net ionic equations for this reaction.

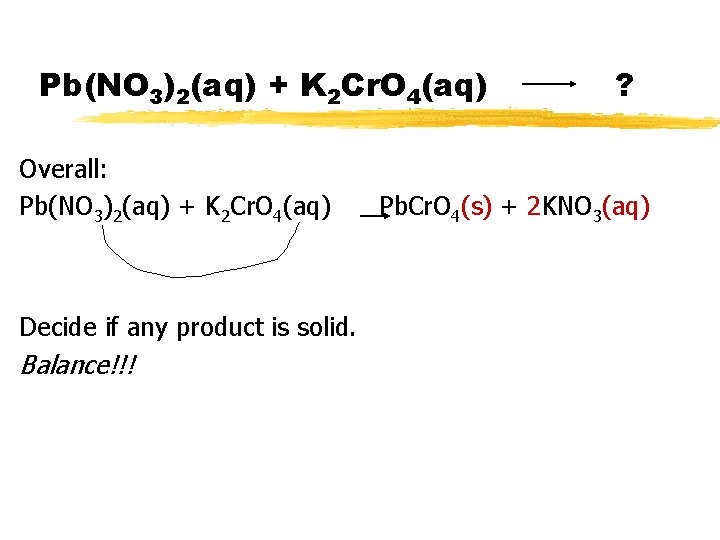
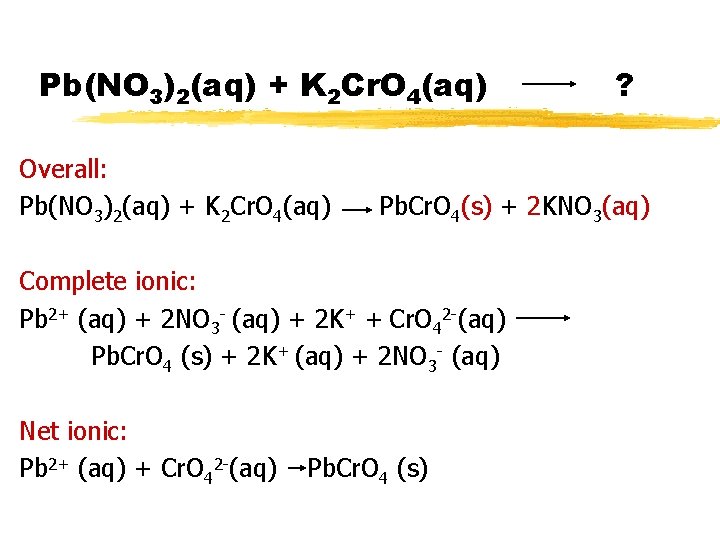
Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) Overall: Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) Decide if any product is solid. Balance!!! ? Pb. Cr. O 4(s) + 2 KNO 3(aq)

Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) Overall: Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) ? Pb. Cr. O 4(s) + 2 KNO 3(aq) To do complete ionic, write aqueous species as separate ions. Complete ionic: Pb 2+ (aq) + 2 NO 3 - (aq) + 2 K+ + Cr. O 42 -(aq) Pb. Cr. O 4 (s) + 2 K+ (aq) + 2 NO 3 - (aq)

Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) Overall: Pb(NO 3)2(aq) + K 2 Cr. O 4(aq) Pb. Cr. O 4(s) + 2 KNO 3(aq) Complete ionic: Pb 2+ (aq) + 2 NO 3 - (aq) + 2 K+ + Cr. O 42 -(aq) Pb. Cr. O 4 (s) + 2 K+ (aq) + 2 NO 3 - (aq) Net ionic: Pb 2+ (aq) + Cr. O 42 -(aq) ? Pb. Cr. O 4 (s)
 Be strong be strong be strong in the lord
Be strong be strong be strong in the lord Strong vs weak electrolytes
Strong vs weak electrolytes Strong vs weak electrolytes
Strong vs weak electrolytes Seven strong acids
Seven strong acids Strong acid strong base titration
Strong acid strong base titration Strong acid and strong base titration curve
Strong acid and strong base titration curve How to remember strong acids and strong bases
How to remember strong acids and strong bases Weak base strong acid titration curve
Weak base strong acid titration curve Strong base
Strong base Neutralization equation
Neutralization equation Weak electrolytes examples
Weak electrolytes examples Pabrinex 1 and 2
Pabrinex 1 and 2 Pn
Pn Non classical adrenal hyperplasia
Non classical adrenal hyperplasia Normal electrolytes values
Normal electrolytes values Normal electrolytes values
Normal electrolytes values Dka and electrolytes
Dka and electrolytes How does electrolytes affects the chemical equilibria
How does electrolytes affects the chemical equilibria Vacid meaning
Vacid meaning