Titrations of Weak Acids and Weak Bases Chapter

Titrations of Weak Acids and Weak Bases Chapter 17
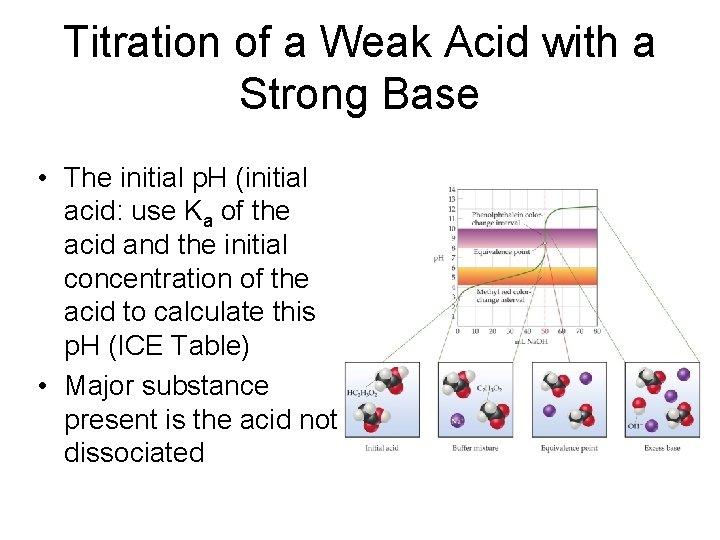
Titration of a Weak Acid with a Strong Base • The initial p. H (initial acid: use Ka of the acid and the initial concentration of the acid to calculate this p. H (ICE Table) • Major substance present is the acid not dissociated
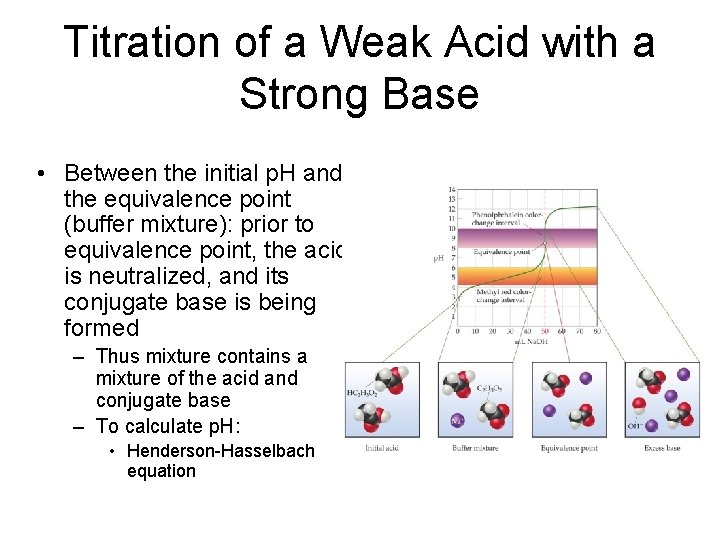
Titration of a Weak Acid with a Strong Base • Between the initial p. H and the equivalence point (buffer mixture): prior to equivalence point, the acid is neutralized, and its conjugate base is being formed – Thus mixture contains a mixture of the acid and conjugate base – To calculate p. H: • Henderson-Hasselbach equation
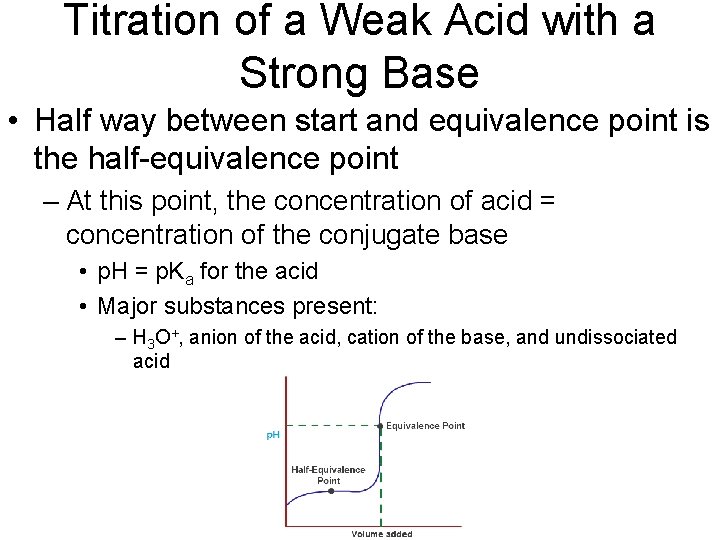
Titration of a Weak Acid with a Strong Base • Half way between start and equivalence point is the half-equivalence point – At this point, the concentration of acid = concentration of the conjugate base • p. H = p. Ka for the acid • Major substances present: – H 3 O+, anion of the acid, cation of the base, and undissociated acid
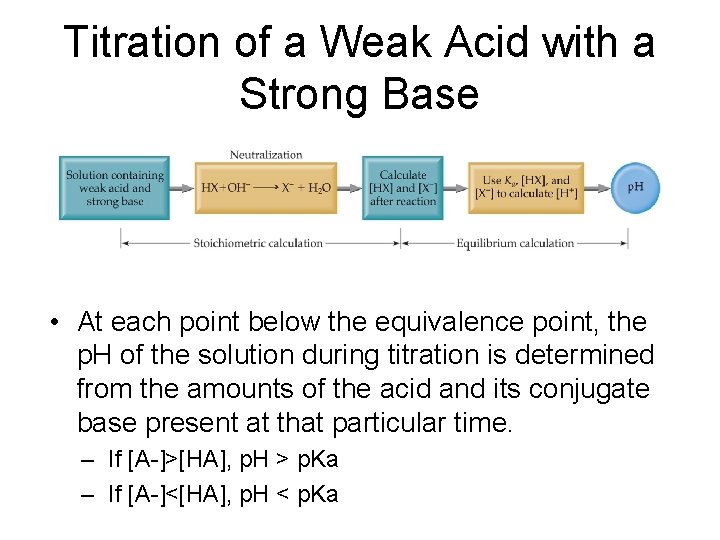
Titration of a Weak Acid with a Strong Base • At each point below the equivalence point, the p. H of the solution during titration is determined from the amounts of the acid and its conjugate base present at that particular time. – If [A-]>[HA], p. H > p. Ka – If [A-]<[HA], p. H < p. Ka
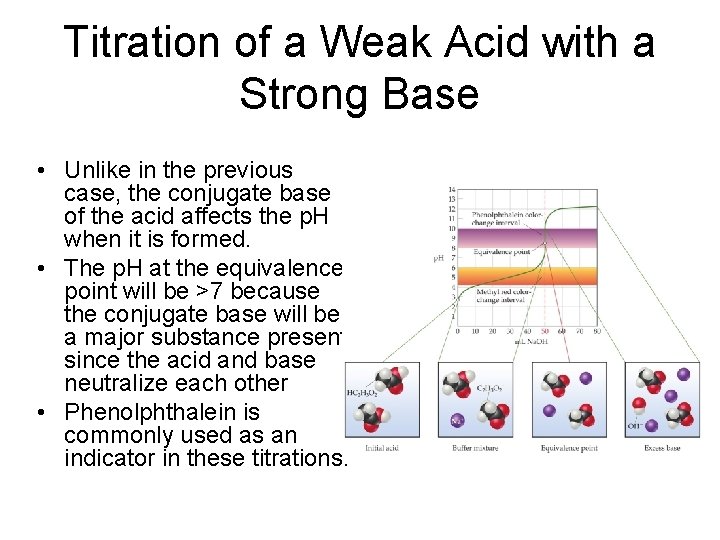
Titration of a Weak Acid with a Strong Base • Unlike in the previous case, the conjugate base of the acid affects the p. H when it is formed. • The p. H at the equivalence point will be >7 because the conjugate base will be a major substance present since the acid and base neutralize each other • Phenolphthalein is commonly used as an indicator in these titrations.
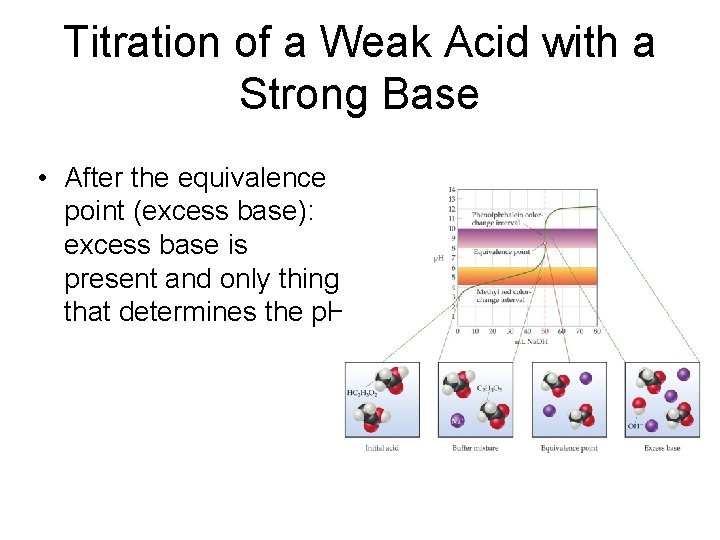
Titration of a Weak Acid with a Strong Base • After the equivalence point (excess base): excess base is present and only thing that determines the p. H
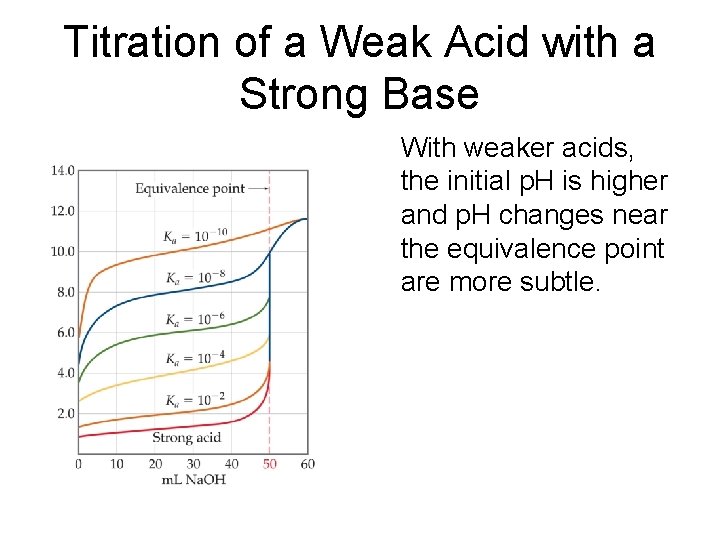
Titration of a Weak Acid with a Strong Base With weaker acids, the initial p. H is higher and p. H changes near the equivalence point are more subtle.

3 differences between strong acidstrong base & weak acid-strong base titrations • The solution of the weak acid has a higher initial p. H than a solution of a strong acid of the same concentration • The p. H change at the rapid-rise portion of the curve near the equivalence point is smaller for the weak acid than it is for the strong acid • The p. H at the equivalence point is above 7. 00 for the weak acid-strong base titration
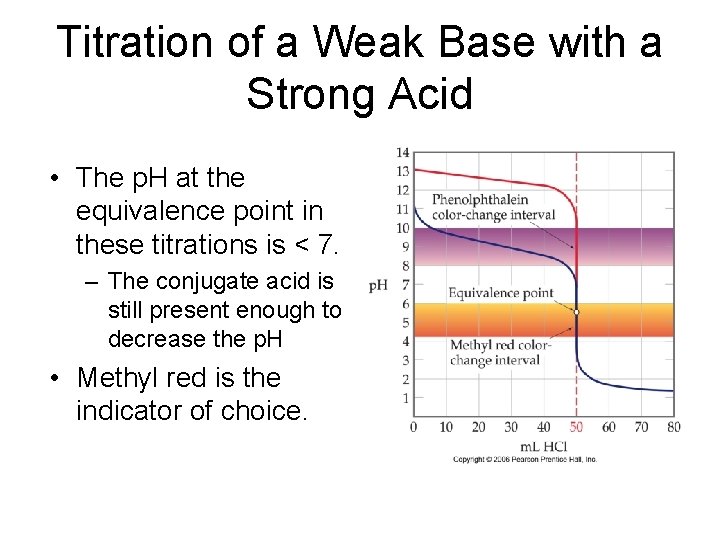
Titration of a Weak Base with a Strong Acid • The p. H at the equivalence point in these titrations is < 7. – The conjugate acid is still present enough to decrease the p. H • Methyl red is the indicator of choice.
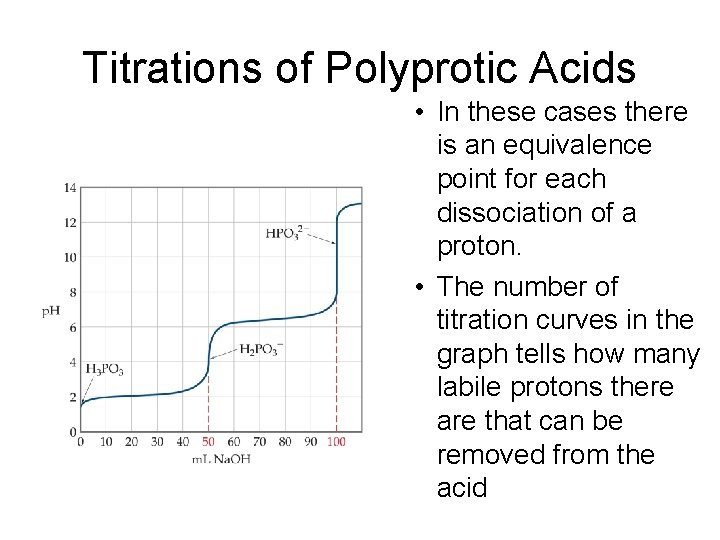
Titrations of Polyprotic Acids • In these cases there is an equivalence point for each dissociation of a proton. • The number of titration curves in the graph tells how many labile protons there are that can be removed from the acid
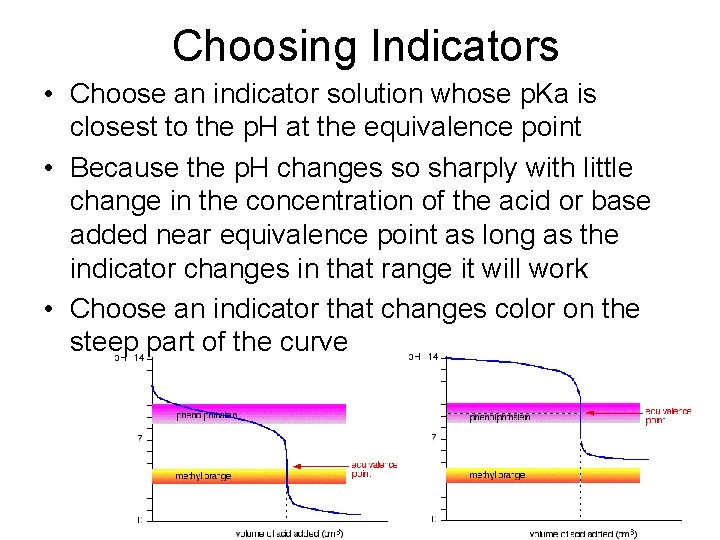
Choosing Indicators • Choose an indicator solution whose p. Ka is closest to the p. H at the equivalence point • Because the p. H changes so sharply with little change in the concentration of the acid or base added near equivalence point as long as the indicator changes in that range it will work • Choose an indicator that changes color on the steep part of the curve
- Slides: 12