PATHOLOGY WOUND HEALINGREGENERATION AND REPAIR Repair Regeneration Repair





































- Slides: 64

PATHOLOGY: WOUND HEALINGREGENERATION AND REPAIR

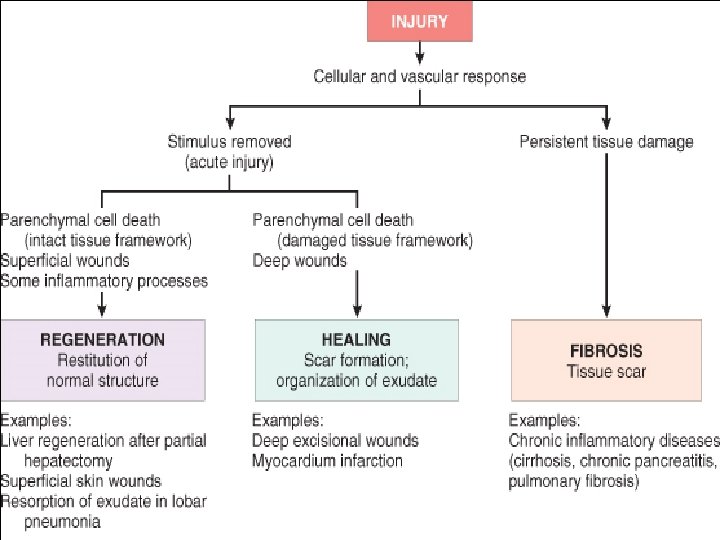
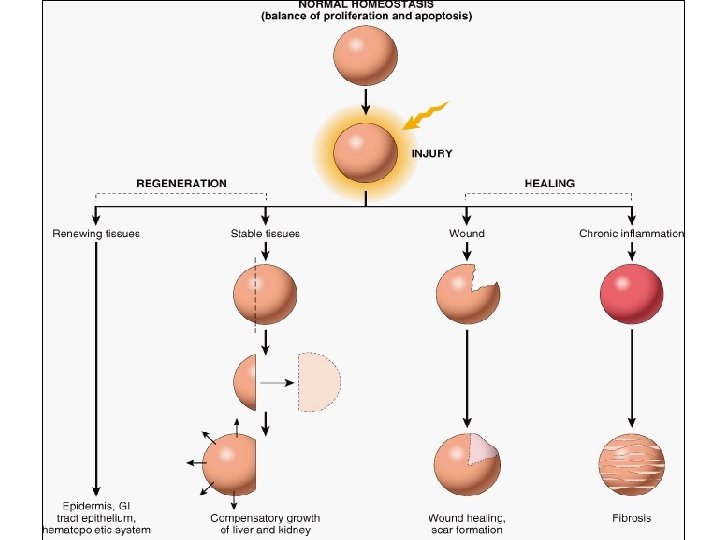
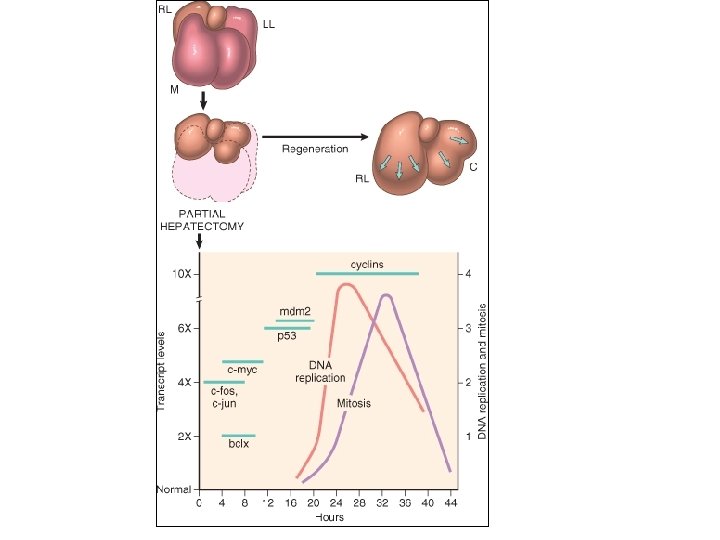
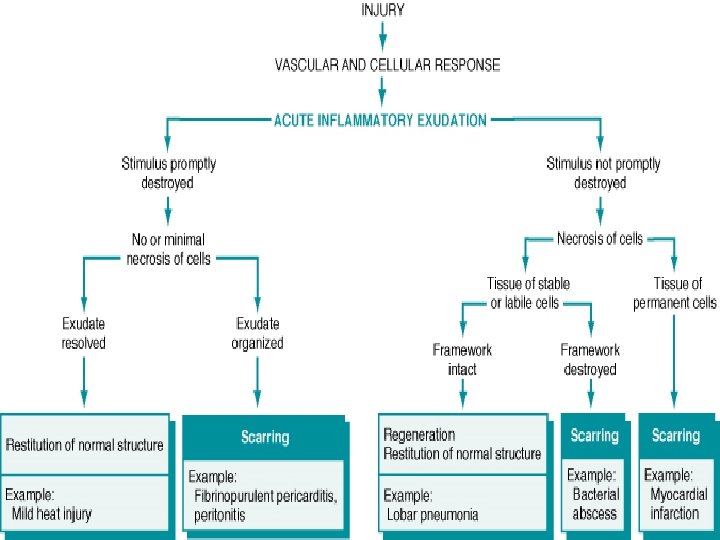
Repair & Regeneration • Repair begins early in inflammation • Regeneration refers to growth of cells and tissues to replace lost structure – as liver and kidney growth after partial hepatectomy and unilateral nephrectomy • Two processes: – Regeneration – Fibrosis

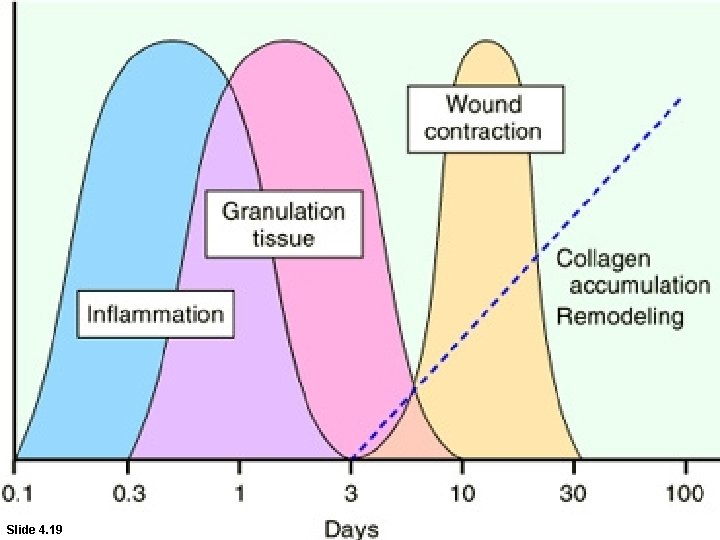
Healing • Healing is a fibroproliferative response that "patches" rather than restores a tissue. • It is a complex but orderly phenomenon involving a number of processes: – Induction of an inflammatory process in response to the initial injury, with removal of damaged and dead tissue – Proliferation and migration of parenchymal and connective tissue cells

Healing – Formation of new blood vessels (angiogenesis) and granulation tissue – Synthesis of ECM proteins and collagen deposition – Tissue remodeling – Wound contraction – Acquisition of wound strength



Healing • Healing is usually a tissue response – (1) to a wound (commonly in the skin) – (2) to inflammatory processes in internal organs – (3) to cell necrosis in organs incapable of regeneration

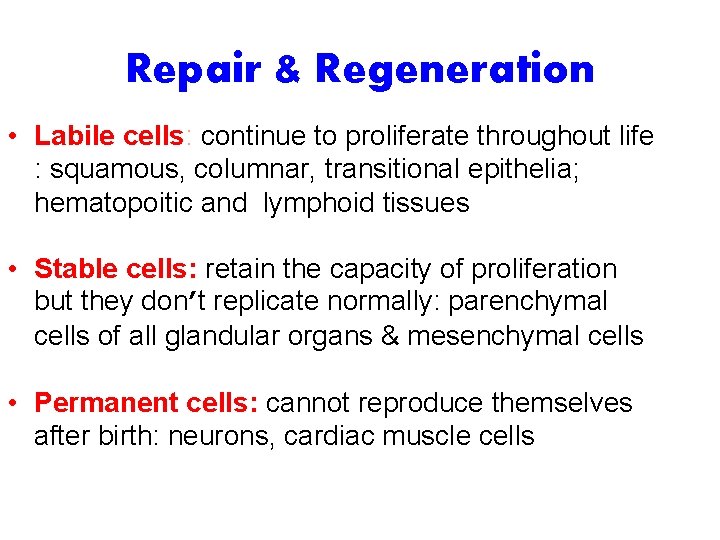
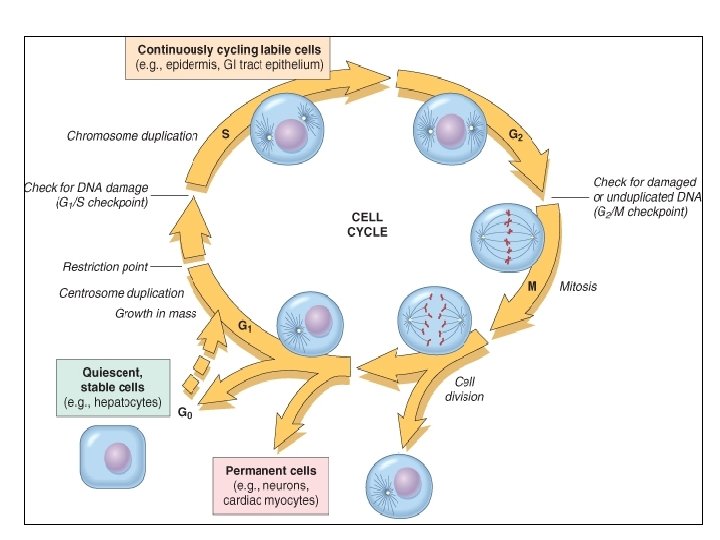
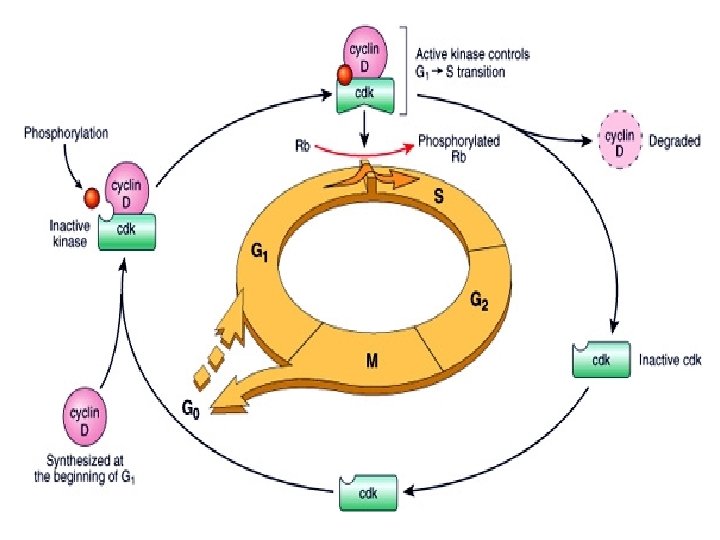
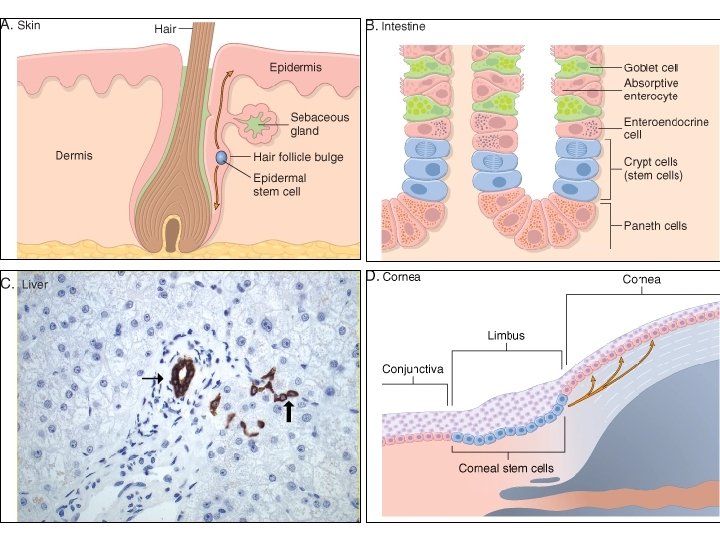
Repair & Regeneration • Labile cells: continue to proliferate throughout life : squamous, columnar, transitional epithelia; hematopoitic and lymphoid tissues • Stable cells: retain the capacity of proliferation but they don’t replicate normally: parenchymal cells of all glandular organs & mesenchymal cells • Permanent cells: cannot reproduce themselves after birth: neurons, cardiac muscle cells





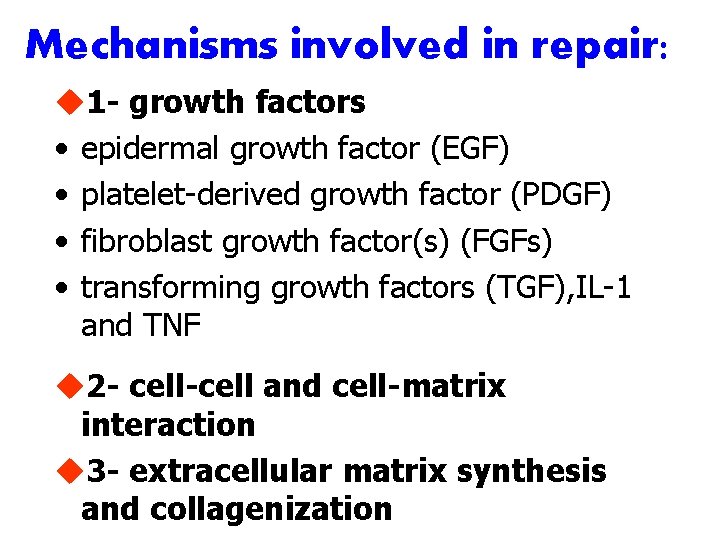
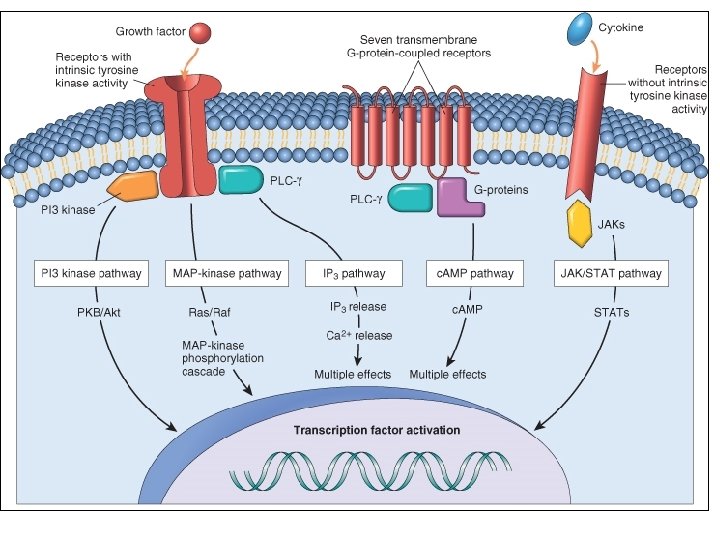
Mechanisms involved in repair: u 1 - growth factors • epidermal growth factor (EGF) • platelet-derived growth factor (PDGF) • fibroblast growth factor(s) (FGFs) • transforming growth factors (TGF), IL-1 and TNF u 2 - cell-cell and cell-matrix interaction u 3 - extracellular matrix synthesis and collagenization

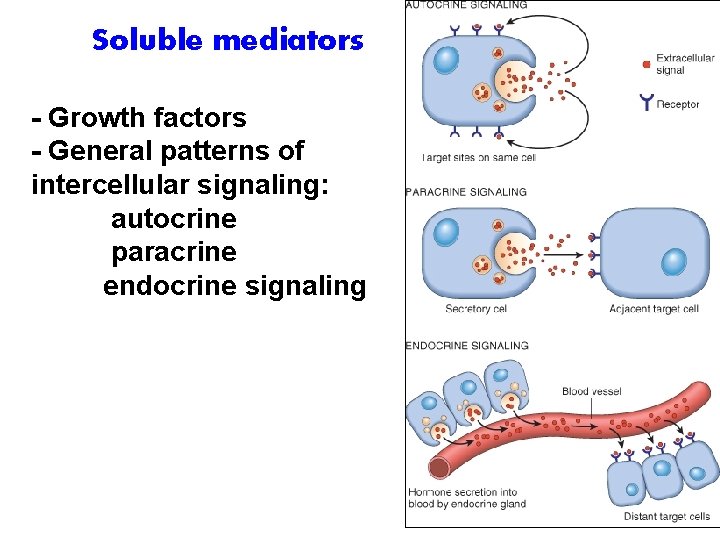
Soluble mediators - Growth factors - General patterns of intercellular signaling: autocrine paracrine endocrine signaling


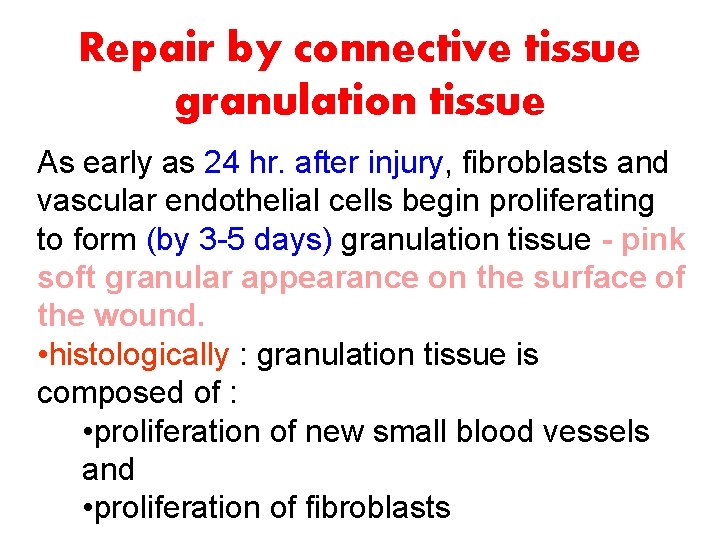
Repair by connective tissue granulation tissue As early as 24 hr. after injury, fibroblasts and vascular endothelial cells begin proliferating to form (by 3 -5 days) granulation tissue - pink soft granular appearance on the surface of the wound. • histologically : granulation tissue is composed of : • proliferation of new small blood vessels and • proliferation of fibroblasts

What is granulation tissue? • The term granulation tissue was used by ancient surgeons for the red, granular tissue filling the non healing wounds. • With the advent of microscopy, it was discovered that granulation tissue occurs in all wounds during healing, but that it may occur in chronic inflammation as well • It consists of fibroblasts surrounded by abundant extracellular matrix, newly formed blood vessels, and scattered macrophages and some other inflammatory cells.

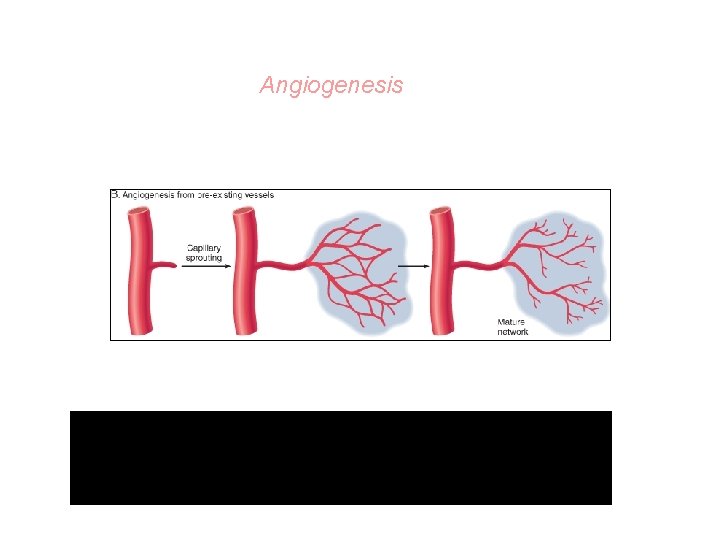
What are angioblasts? • Angioblasts are connective tissue cells that form new blood vessels. Angiogenesis is promoted by growth factors acting on angioblasts. • Several growth factors promote angiogenesis, but the most potent ones are VEGF and basic fibroblast growth factor (BFGF).

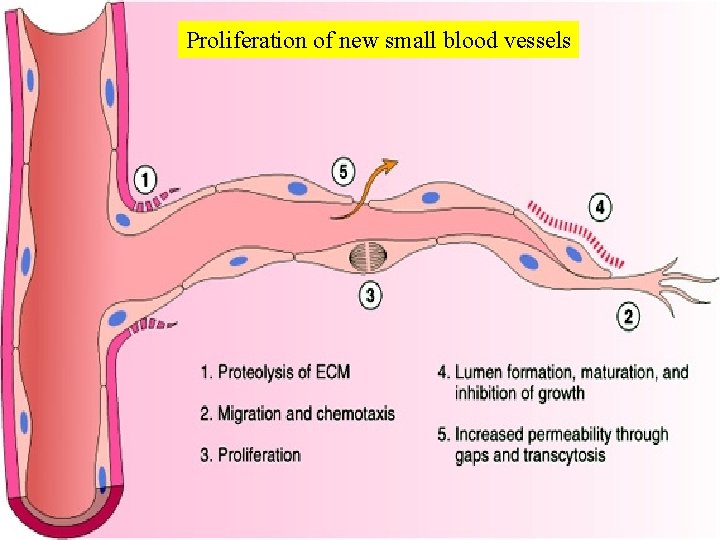
Proliferation of new small blood vessels

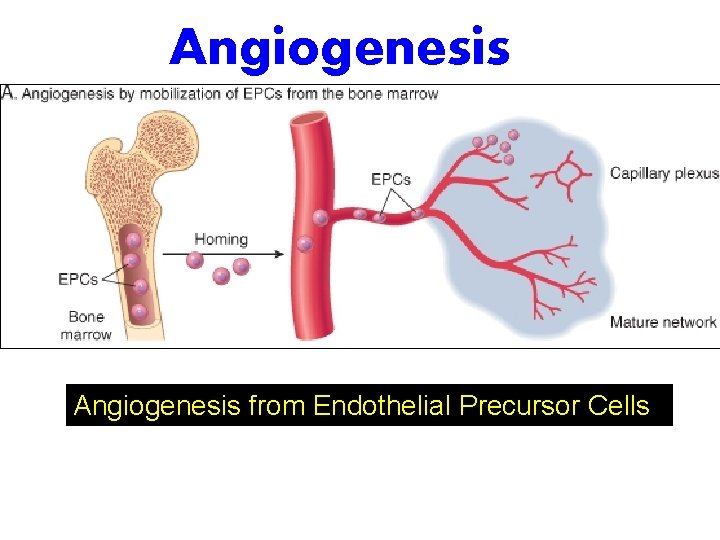
Angiogenesis from Endothelial Precursor Cells

Angiogenesis There is vasodilation and increased permeability of the existing vessels, degradation of ECM, and migration of endothelial cells.

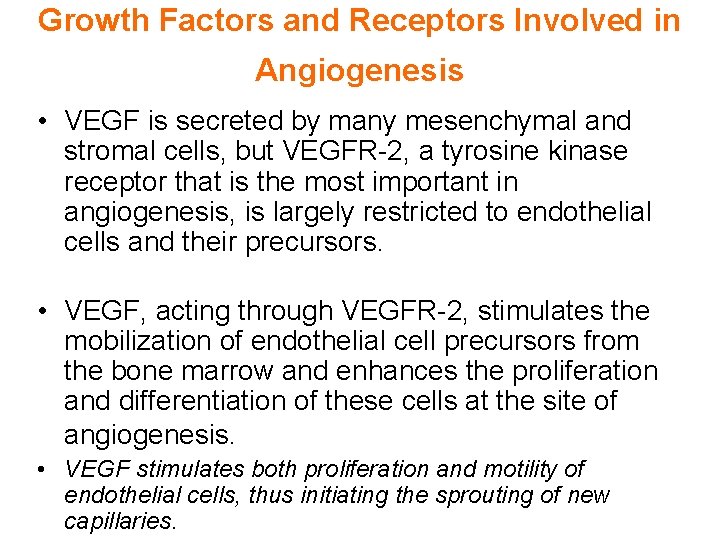
Growth Factors and Receptors Involved in Angiogenesis • VEGF is secreted by many mesenchymal and stromal cells, but VEGFR-2, a tyrosine kinase receptor that is the most important in angiogenesis, is largely restricted to endothelial cells and their precursors. • VEGF, acting through VEGFR-2, stimulates the mobilization of endothelial cell precursors from the bone marrow and enhances the proliferation and differentiation of these cells at the site of angiogenesis. • VEGF stimulates both proliferation and motility of endothelial cells, thus initiating the sprouting of new capillaries.

Growth Factors and Receptors Involved in Angiogenesis • Endothelial cell proliferation, differentiation, and migration can also be enhanced by FGF-2.

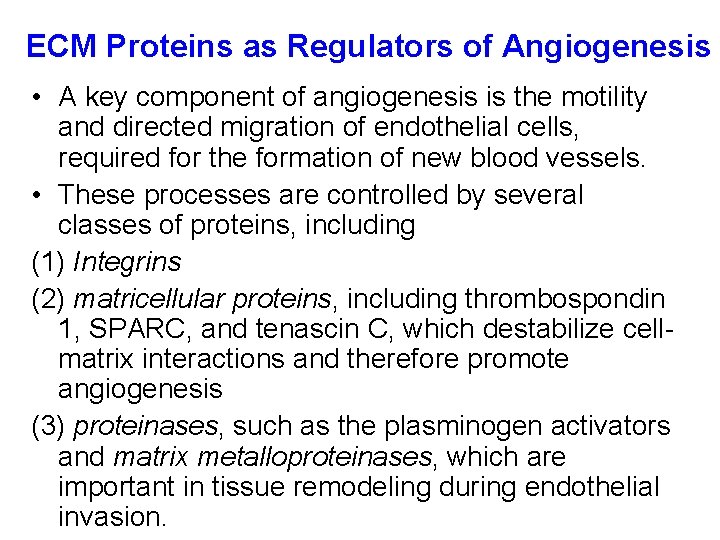
ECM Proteins as Regulators of Angiogenesis • A key component of angiogenesis is the motility and directed migration of endothelial cells, required for the formation of new blood vessels. • These processes are controlled by several classes of proteins, including (1) Integrins (2) matricellular proteins, including thrombospondin 1, SPARC, and tenascin C, which destabilize cellmatrix interactions and therefore promote angiogenesis (3) proteinases, such as the plasminogen activators and matrix metalloproteinases, which are important in tissue remodeling during endothelial invasion.

Repair by connective tissue granulation tissue Fibroblastic proliferation and synthesizing of proteoglycans and collagen. *myofibroblasts* Macrophages ridding the area of extracellular debris, fibrin and foreign matter Further healing: increased collagen, decreased active fibroblasts and new vessels(thrombosis and degeneration) At the end: scar (inactive fibroblasts, dense collagen, fragments of elastic tissue, extracellular matrix, few vessels).

SCAR FORMATION • Three processes that participate in the formation of a scar: (1) emigration and proliferation of fibroblasts in the site of injury, (2) deposition of ECM, and (3) tissue remodeling.

Slide 4. 19 W. B. Saunders Company items and derived items Copyright (c) 1999 by

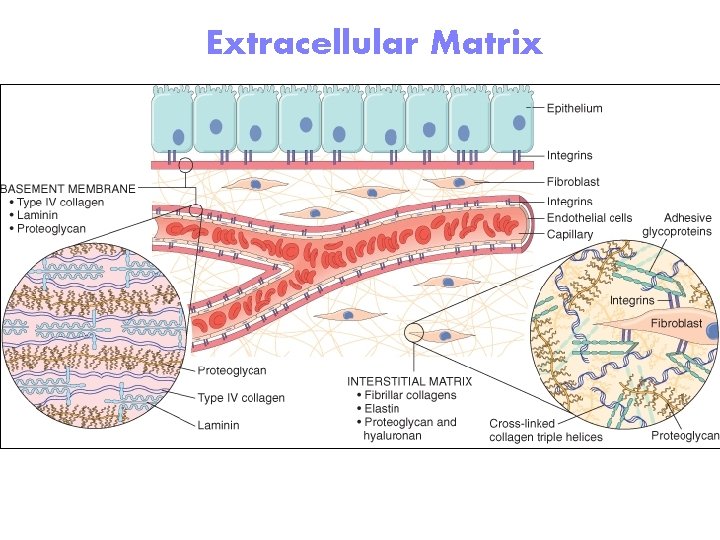
Extracellular Matrix

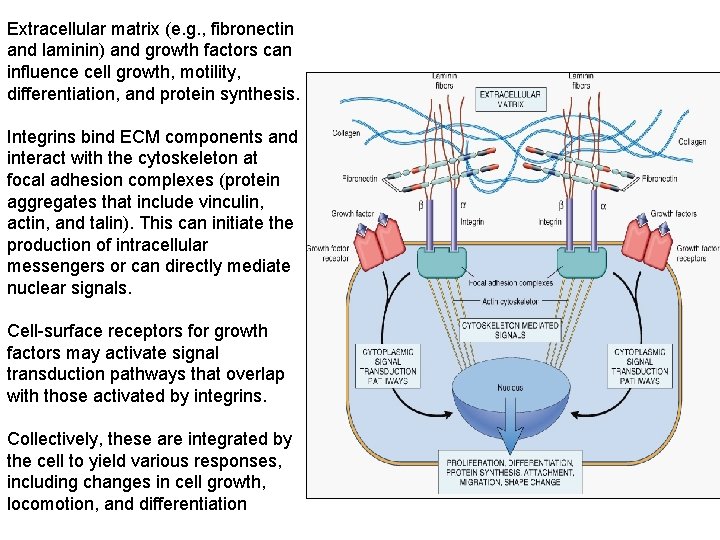
Extracellular matrix (e. g. , fibronectin and laminin) and growth factors can influence cell growth, motility, differentiation, and protein synthesis. Integrins bind ECM components and interact with the cytoskeleton at focal adhesion complexes (protein aggregates that include vinculin, actin, and talin). This can initiate the production of intracellular messengers or can directly mediate nuclear signals. Cell-surface receptors for growth factors may activate signal transduction pathways that overlap with those activated by integrins. Collectively, these are integrated by the cell to yield various responses, including changes in cell growth, locomotion, and differentiation

Wound healing


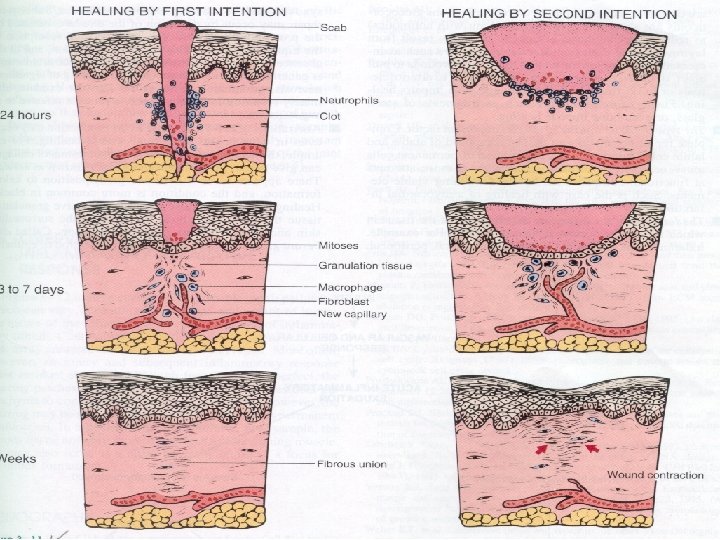
Wound healing 1. Primary union (healing by 1 st intention) clean surgical incision no significant bacterial contamination minimal loss of tissue clot, scab formation 24 hr. : neutrophils, mitotic activity of basal layer, thin epithelial layer day 3: macrophages, granulation tissue day 5: collagen bridges the incision, epidermis thickens 2 nd week: continued collagen and fibroblasts, blanching End of 1 st month: scar (cellular connective tissue, intact epidermis, lost appendages).

Wound healing 2. Secondary union (healing by 2 nd intention) Occurs when there is : more extensive loss of cells and tissue: infarction inflammatory ulceration abscess formation surface wound with large defect large tissue defect that must be filled Differs from primary healing: 1. inflammatory reaction is more intense 2. larger amounts of granulation tissue are formed 3. wound contraction (5 to 10%), ? myofibroblasts

HEALING BY SECOND INTENTION (WOUNDS WITH SEPARATED EDGES) • Inevitably, large tissue defects generate a larger fibrin clot that fills the defect and more necrotic debris and exudate that must be removed. Consequently the inflammatory reaction is more intense. • Much larger amounts of granulation tissue are formed.

What is the role of macrophages in wound healing? • Cleanup of debris. • Macrophages recruit other cells and are essential for the entry of fibroblasts and angioblasts into the wound. • Stimulation of matrix production. Macrophages are a potent source of growth factors and interleukins that stimulate fibroblasts and angioblasts to produce the extracellular matrix. • Remodeling of the scar. They secrete collagenases and other lytic enzymes that degrade collagen and other matrix components, thus restructuring the entire field. • At the same time, macrophages and fibroblasts, secrete tissue inhibitors of metalloproteinases (TIMPs), which counteract the action of lytic enzymes so that the remodeling of the scar proceeds in a regulated manner.

Difference between primary intention and secondary intention • The basic process of healing is the same in all wounds. In contrast to healing by primary intention, wounds healing by secondary intention – Require more time to close because the edges are far apart – Show a more prominent inflammatory reaction in and around the wound – Contain more copious granulation tissue inside the tissue defect

Which wounds heal by secondary intention? • Large, gaping wounds, as well those that are infected or contain foreign material

How do wounds heal by primary intention? • Clean surgical wounds heal by primary intention. Sequentially, the wound passes through several stages: – Hematoma (day 1) the wound is filled with blood and cell detritus. Influx of neutrophils, followed during the second day by an influx of macrophages, ensures that the extraneous material is removed. – Inflammation and early granulation tissue. (Days 2 -3) Macrophages stimulate the ingrowth of fibroblasts and angioblasts, which start forming collagen type III. Epidermal cells form a that seal off the defect.

– Fully developed granulation tissue. (Days 4 -6) Neovascularization reach its peak and the entire area seems swollen and red. In addition to numerous newly formed capillaries, the granulation tissue contains numerous fibroblasts that rapidly synthesize ECM molecules – Blanching. (Second week) Deposits of collagen compress the blood vessels and reduce the blood flow through the healing wound. .

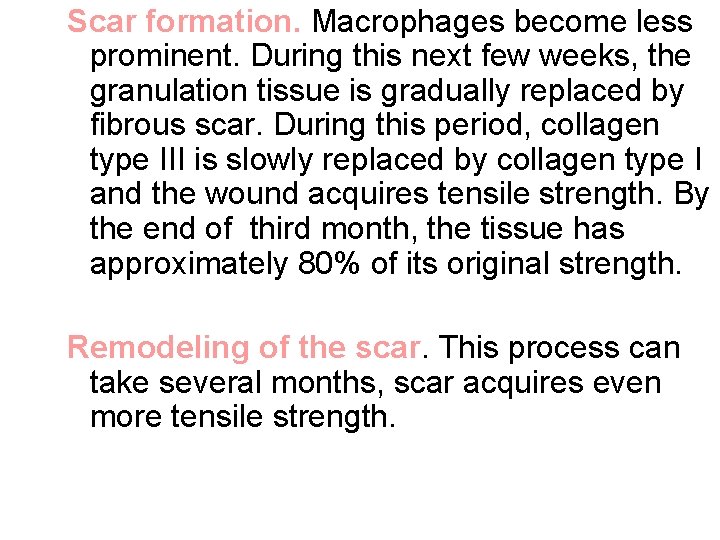
Scar formation. Macrophages become less prominent. During this next few weeks, the granulation tissue is gradually replaced by fibrous scar. During this period, collagen type III is slowly replaced by collagen type I and the wound acquires tensile strength. By the end of third month, the tissue has approximately 80% of its original strength. Remodeling of the scar. This process can take several months, scar acquires even more tensile strength.



Factors that influence healing • Not all of these events occur in every repair reaction. The repair process is influenced by many factors, including: • The tissue environment and the extent of tissue damage • The intensity and duration of the stimulus • Conditions that inhibit repair, such as the presence of foreign bodies or inadequate blood supply, • Various diseases that inhibit repair (diabetes in particular), and treatment with steroids.

Systemic factors that influence healing • Nutrition has profound effects on wound healing. Protein deficiency, for example, and particularly vitamin C deficiency, inhibit collagen synthesis and retard healing. • Metabolic status can change wound healing. Diabetes mellitus, is associated with delayed healing, as a consequence of the microangiopathy that is a frequent feature of this disease

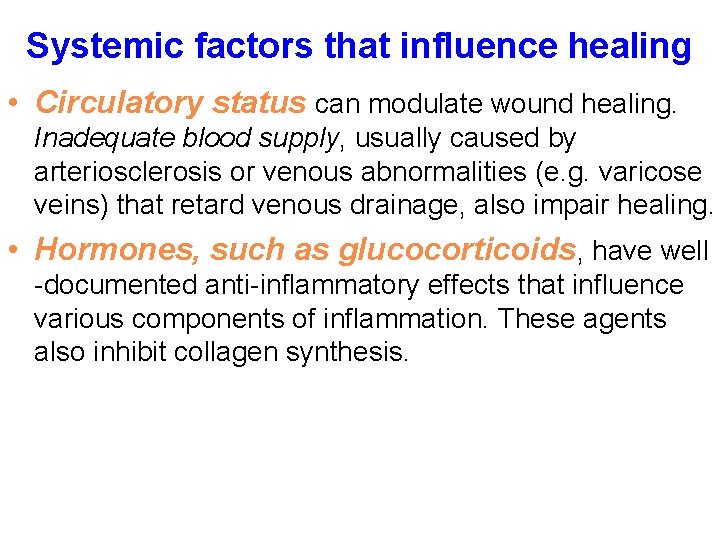
Systemic factors that influence healing • Circulatory status can modulate wound healing. Inadequate blood supply, usually caused by arteriosclerosis or venous abnormalities (e. g. varicose veins) that retard venous drainage, also impair healing. • Hormones, such as glucocorticoids, have well -documented anti-inflammatory effects that influence various components of inflammation. These agents also inhibit collagen synthesis.

Local factors that influence healing • Infection is the single most important cause of delay in healing because it results in persistent tissue injury and inflammation. • Mechanical factors, such as early motion of wounds, can delay healing, by compressing blood vessels and separating the edges of the wound.

Local factors that influence healing • Foreign bodies, such as unnecessary sutures or fragments of steel, glass, or even bone, constitute impediments to healing. • Size, location, and type of wound influence healing. Wounds in richly vascularized areas, such as the face, heal faster than those in poorly vascularized ones, such as the foot.

COMPLICATIONS IN CUTANEOUS WOUND HEALING • Complications in wound healing can arise from abnormalities in any of the basic components of the repair process. These aberrations can be grouped into three general categories: – (1) deficient scar formation – (2) excessive formation of the repair components – (3) formation of contractures.

Wound healing • Inadequate formation of granulation tissue or assembly of a scar can lead to two types of complications: – wound dehiscence – ulceration. Dehiscence or rupture of a wound is most common after abdominal surgery and is due to increased abdominal pressure. This mechanical stress on the abdominal wound can be generated by vomiting, coughing, or ileus. y

Wound healing Wounds can ulcerate because of inadequate vascularization during healing. For example, lower extremity wounds in individuals with atherosclerotic peripheral vascular disease typically ulcerate Nonhealing wounds also form in areas devoid of sensation. These neuropathic ulcers are occasionally seen in patients with diabetic peripheral neuropathy

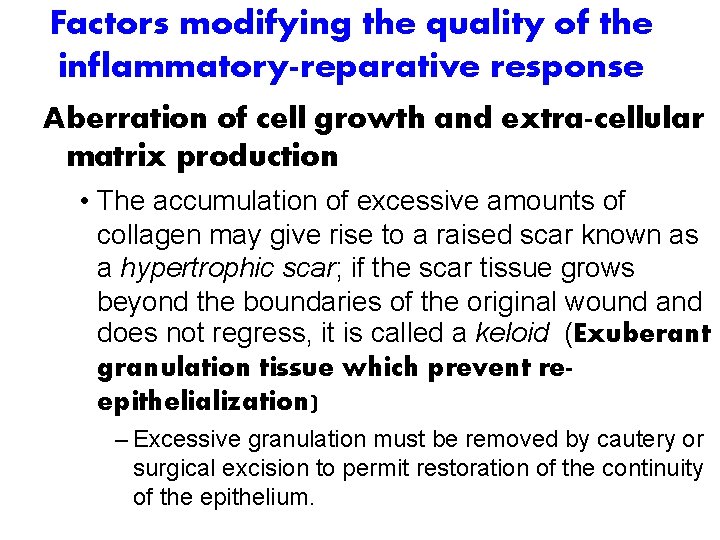
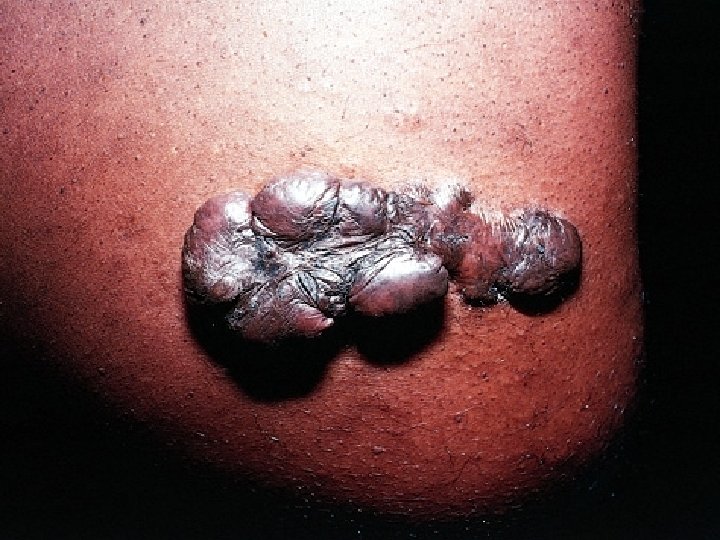
Factors modifying the quality of the inflammatory-reparative response Aberration of cell growth and extra-cellular matrix production • The accumulation of excessive amounts of collagen may give rise to a raised scar known as a hypertrophic scar; if the scar tissue grows beyond the boundaries of the original wound and does not regress, it is called a keloid (Exuberant granulation tissue which prevent reepithelialization) – Excessive granulation must be removed by cautery or surgical excision to permit restoration of the continuity of the epithelium.

What is a keloid? • Keloids are hyperplastic scars composed of irregularly deposited collagen. They may appear as bulging masses.


Wound Contraction • The feature that most clearly differentiates primary from secondary healing is the phenomenon of wound contraction, which occurs in large surface wounds.

Wound Contraction • The initial steps of wound contraction involve the formation of a network of actin-containing fibroblasts at the edge of the wound. • Permanent wound contraction requires the action of myofibroblastsaltered fibroblasts that have the ultrastructural characteristics of smooth muscle cells.

Wound Contraction • Contraction of these cells at the wound site decreases the gap between the dermal edges of the wound. • Substantial scar formation and thinning of the epidermis.

Wound Contraction • Contraction in the size of a wound is an important part of the normal healing process. • An exaggeration of this process is called a contracture and results in deformities of the wound and the surrounding tissues.

Wound Contraction • Contractures are particularly prone to develop on the palms, the soles, and the anterior aspect of the thorax. • Contractures are commonly seen after serious burns and can compromise the movement of joints.

What is wound contraction? • Reduction in size of the wounds healing by secondary intention occurs as a result of the action of myofibroblasts in the granulation tissue

What are contractures? • Contractures are deformities of extremities caused by irregular scars • most often related to extensive burns, • It limit the mobility ( part of the extremity usually cannot fully extend).

What is wound dehiscence? • Dehiscence (latin, split apart) is opening of a healing or partially heal, of its edges. Often, this occurs as a result of mechanical factors, infection or ischemic necrosis of the sutured edges.

Slide 4. 21 W. B. Saunders Company items and derived items Copyright (c) 1999 by

What is the most common causes of delayed wound healing? • The most common causes of delayed wound healing is infection, foreign bodies in the wound, mechanical factors, nutritional deficiencies or excess corticosteroids

THANK YOU!
 Gas turbine with regeneration
Gas turbine with regeneration Was the london docklands regeneration a success
Was the london docklands regeneration a success Objectives of artificial regeneration
Objectives of artificial regeneration Brayton cycle with regeneration pv diagram
Brayton cycle with regeneration pv diagram Fiat doblo dpf regeneration procedure
Fiat doblo dpf regeneration procedure Planarian regeneration
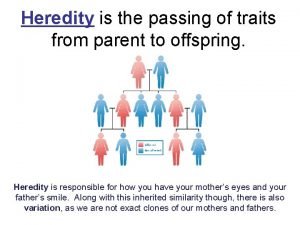
Planarian regeneration Heredity
Heredity Classification of gas turbine power plant
Classification of gas turbine power plant Cellules ciliées régénération
Cellules ciliées régénération Tissue regeneration
Tissue regeneration Nottingham redevelopment
Nottingham redevelopment Nucleotide excision repair
Nucleotide excision repair Mismatch repair
Mismatch repair Define wound healing
Define wound healing Local factors affecting wound healing
Local factors affecting wound healing Chapter 32 skin integrity and wound care
Chapter 32 skin integrity and wound care Chapter 48 skin integrity and wound care
Chapter 48 skin integrity and wound care Tidy and untidy wound
Tidy and untidy wound A car on a roller coaster track launched by a huge spring
A car on a roller coaster track launched by a huge spring Chapter 48 skin integrity and wound care
Chapter 48 skin integrity and wound care Concealed puncture wound
Concealed puncture wound Copyright
Copyright Additive pathology
Additive pathology Introduction to cryptology
Introduction to cryptology Cryptology and pathology
Cryptology and pathology Chris concepts
Chris concepts Systemic pathology questions and answers pdf
Systemic pathology questions and answers pdf Introduction and importance of seed pathology
Introduction and importance of seed pathology Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Tronsmo plant pathology and plant diseases download
Tronsmo plant pathology and plant diseases download Albugo eye
Albugo eye Crenation
Crenation Fish and shellfish pathology
Fish and shellfish pathology Leeds pathology tests and tubes
Leeds pathology tests and tubes Type of wound
Type of wound Wound tracking software
Wound tracking software Wound infection continuum
Wound infection continuum Pretibial laceration classification
Pretibial laceration classification Serosanguineous exudate
Serosanguineous exudate Levers and inclined planes
Levers and inclined planes What effect has peeta's comments had on the crowd
What effect has peeta's comments had on the crowd Doylestown wound center
Doylestown wound center Jacob's ladder wound closure
Jacob's ladder wound closure Slide is what kind of simple machine
Slide is what kind of simple machine Inclined plane wrapped around a post
Inclined plane wrapped around a post Shasta regional wound center
Shasta regional wound center Autolisis debridement
Autolisis debridement Wind water wound wonder drugs
Wind water wound wonder drugs Laceration types
Laceration types It is a type of inclined plane that is wound around a post
It is a type of inclined plane that is wound around a post Blackening in firearm injury
Blackening in firearm injury Amulet of wound closure
Amulet of wound closure What is wound certificate
What is wound certificate Bevelling incised wound
Bevelling incised wound Jackson's theory of thermal wounds
Jackson's theory of thermal wounds Wound classification surgery
Wound classification surgery The initiates archetype
The initiates archetype Contaminated wound
Contaminated wound Wound dehiscence
Wound dehiscence Obici physical therapy
Obici physical therapy Wound healing nutrition handout
Wound healing nutrition handout Mrsa wound
Mrsa wound Look alike sound alike medical terms
Look alike sound alike medical terms Concealed puncture wound
Concealed puncture wound Types of inflammation
Types of inflammation