Chapter 16 Repair Systems 16 1 Introduction mismatch






- Slides: 32

Chapter 16 Repair Systems

16. 1 Introduction • mismatch repair (MMR) – A type of repair that corrects mispaired bases, typically immediately following replication. Figure 16. 01: Repair genes can be classified into pathways that use different mechanisms to reverse or bypass damage to DNA. – The process preferentially corrects the sequence of the daughter strand by distinguishing the daughter strand parental strand, sometimes on the basis of their states of methylation.

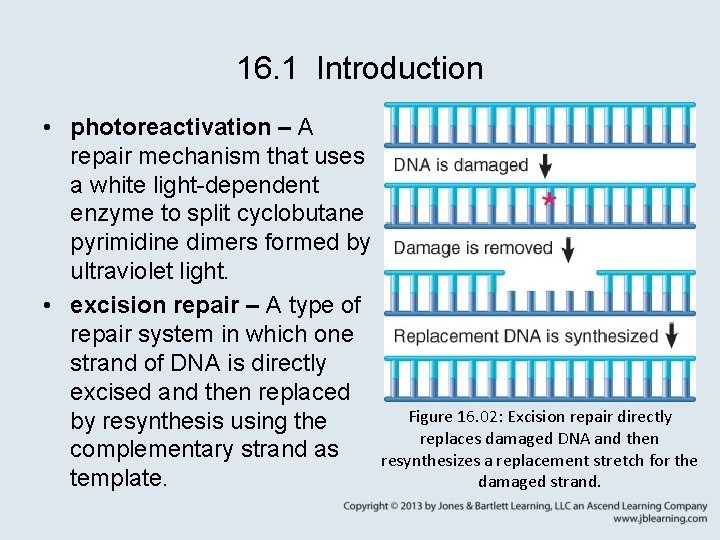
16. 1 Introduction • photoreactivation – A repair mechanism that uses a white light-dependent enzyme to split cyclobutane pyrimidine dimers formed by ultraviolet light. • excision repair – A type of repair system in which one strand of DNA is directly excised and then replaced Figure 16. 02: Excision repair directly by resynthesis using the replaces damaged DNA and then complementary strand as resynthesizes a replacement stretch for the template. damaged strand.

16. 1 Introduction • base excision repair (BER) – A pathway of excision repair that recognizes damage to single bases, such as deaminiation or alkylation, and either repairs the base alone (short-patch repair) or replaces 2– 10 nucleotides (long-patch repair).

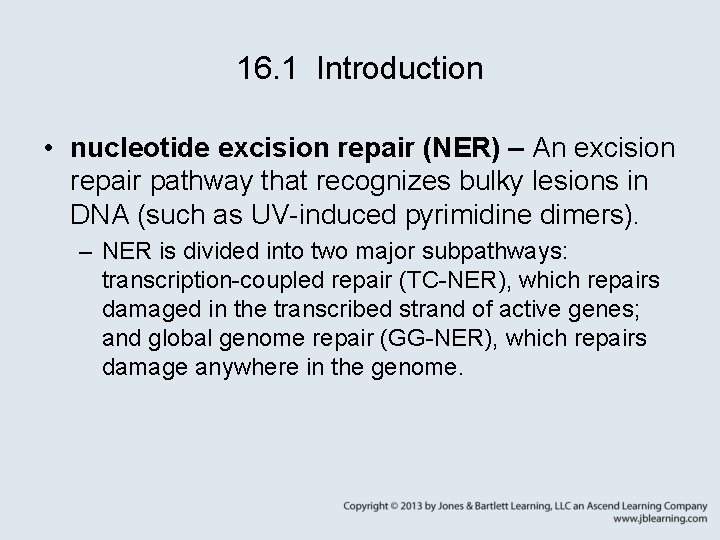
16. 1 Introduction • nucleotide excision repair (NER) – An excision repair pathway that recognizes bulky lesions in DNA (such as UV-induced pyrimidine dimers). – NER is divided into two major subpathways: transcription-coupled repair (TC-NER), which repairs damaged in the transcribed strand of active genes; and global genome repair (GG-NER), which repairs damage anywhere in the genome.

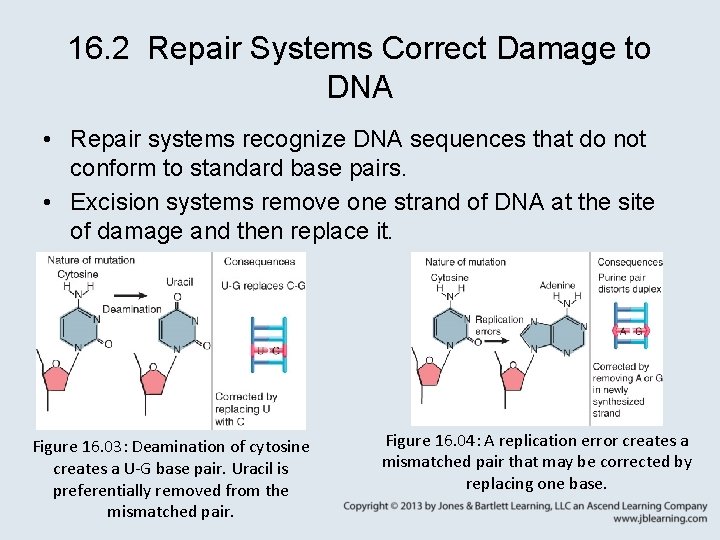
16. 2 Repair Systems Correct Damage to DNA • Repair systems recognize DNA sequences that do not conform to standard base pairs. • Excision systems remove one strand of DNA at the site of damage and then replace it. Figure 16. 03: Deamination of cytosine creates a U-G base pair. Uracil is preferentially removed from the mismatched pair. Figure 16. 04: A replication error creates a mismatched pair that may be corrected by replacing one base.

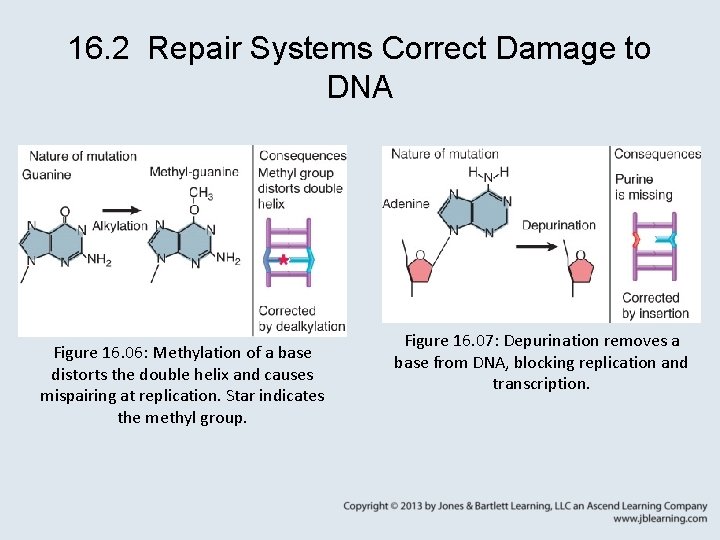
16. 2 Repair Systems Correct Damage to DNA Figure 16. 06: Methylation of a base distorts the double helix and causes mispairing at replication. Star indicates the methyl group. Figure 16. 07: Depurination removes a base from DNA, blocking replication and transcription.

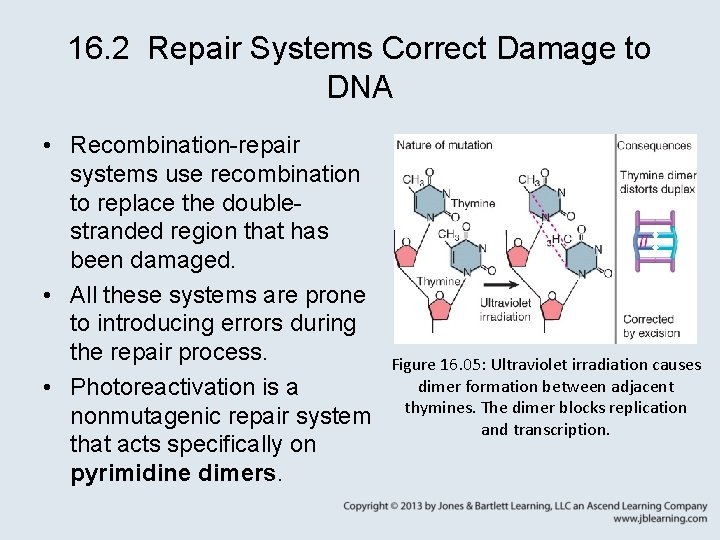
16. 2 Repair Systems Correct Damage to DNA • Recombination-repair systems use recombination to replace the doublestranded region that has been damaged. • All these systems are prone to introducing errors during the repair process. • Photoreactivation is a nonmutagenic repair system that acts specifically on pyrimidine dimers. Figure 16. 05: Ultraviolet irradiation causes dimer formation between adjacent thymines. The dimer blocks replication and transcription.

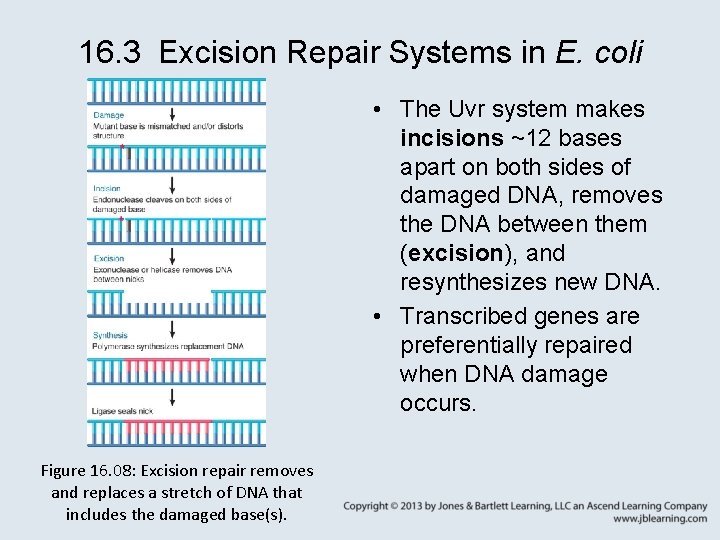
16. 3 Excision Repair Systems in E. coli • The Uvr system makes incisions ~12 bases apart on both sides of damaged DNA, removes the DNA between them (excision), and resynthesizes new DNA. • Transcribed genes are preferentially repaired when DNA damage occurs. Figure 16. 08: Excision repair removes and replaces a stretch of DNA that includes the damaged base(s).

16. 4 Eukaryotic Nucleotide Excision Repair Pathways • Xeroderma pigmentosum (XP) is a human disease caused by mutations in any one of several nucleotide excision repair genes. • Numerous proteins, including XP products and the transcription factor TFIIH, are involved in eukaryotic nucleotide excision repair.

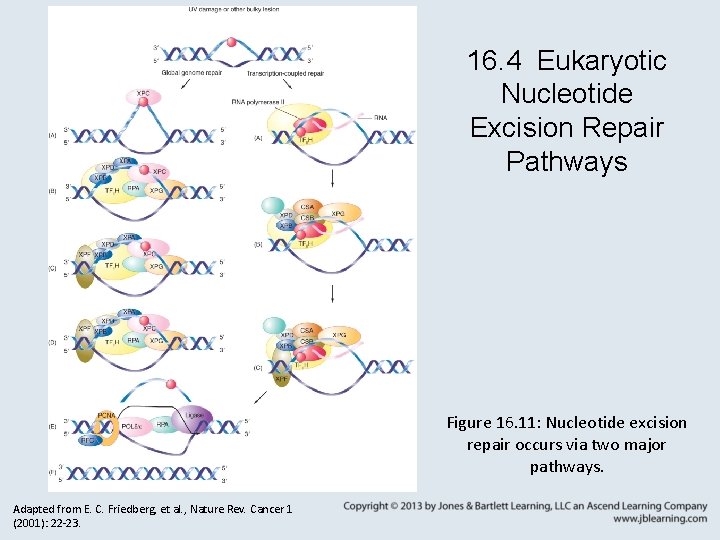
16. 4 Eukaryotic Nucleotide Excision Repair Pathways Figure 16. 11: Nucleotide excision repair occurs via two major pathways. Adapted from E. C. Friedberg, et al. , Nature Rev. Cancer 1 (2001): 22 -23.

16. 4 Eukaryotic Nucleotide Excision Repair Pathways • Global genome repair recognizes damage anywhere in the genome. • Transcriptionally active genes are preferentially repaired via transcription-coupled repair. • Global genome repair and transcription-coupled repair differ in their mechanisms of damage recognition (XPC vs. RNA polymerase II).

16. 4 Eukaryotic Nucleotide Excision Repair Pathways • TFIIH provides the link to a complex of repair enzymes. • Mutations in the XPD component of TFIIH cause three types of human diseases.

16. 5 Base Excision Repair Systems Require Glycosylases • Base excision repair is triggered by directly removing a damaged base from DNA. • Base removal triggers the removal and replacement of a stretch of polynucleotides. • The nature of the base removal reaction determines which of two pathways for excision repair is activated. • The polδ/ε pathway replaces a long polynucleotide stretch; the polβ pathway replaces a short stretch.

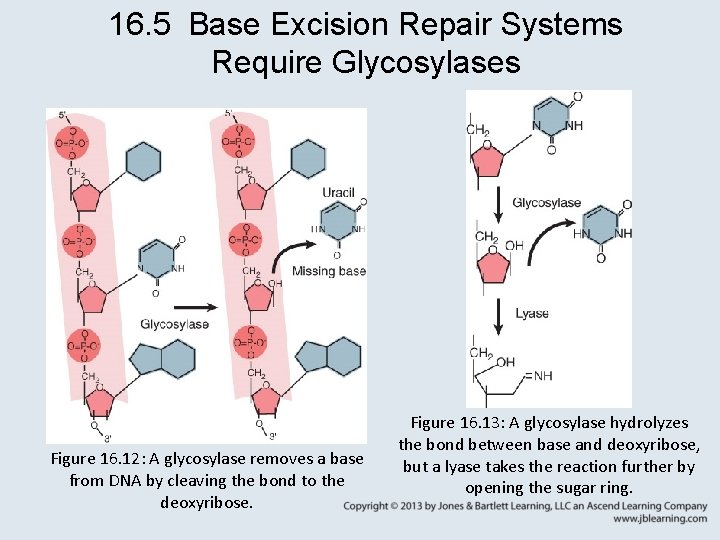
16. 5 Base Excision Repair Systems Require Glycosylases • Uracil and alkylated bases are recognized by glycosylases and removed directly from DNA. • Glycosylases and photolyase (a lyase) act by flipping the base out of the double helix, where, depending on the reaction, it is either removed or modified and returned to the helix.

16. 5 Base Excision Repair Systems Require Glycosylases Figure 16. 12: A glycosylase removes a base from DNA by cleaving the bond to the deoxyribose. Figure 16. 13: A glycosylase hydrolyzes the bond between base and deoxyribose, but a lyase takes the reaction further by opening the sugar ring.

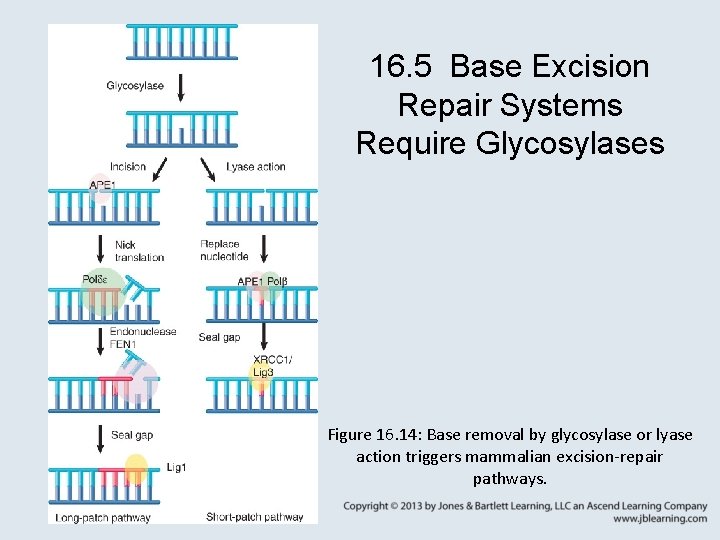
16. 5 Base Excision Repair Systems Require Glycosylases Figure 16. 14: Base removal by glycosylase or lyase action triggers mammalian excision-repair pathways.

16. 6 Error-Prone Repair and Translesion Synthesis • Damaged DNA that has not been repaired causes DNA polymerase III to stall during replication. • DNA polymerase V (coded by umu. CD) or DNA polymerase IV (coded by din. B) can synthesize a complement to the damaged strand. • The DNA synthesized by repair DNA polymerases often has errors in its sequence (error-prone synthesis).

16. 7 Controlling the Direction of Mismatch Repair • mutator – A mutation or a mutated gene that increases the basal level of mutation. – Such genes often code for proteins that are involved in repairing damaged DNA. • The mut genes code for a mismatch repair system that deals with mismatched base pairs.

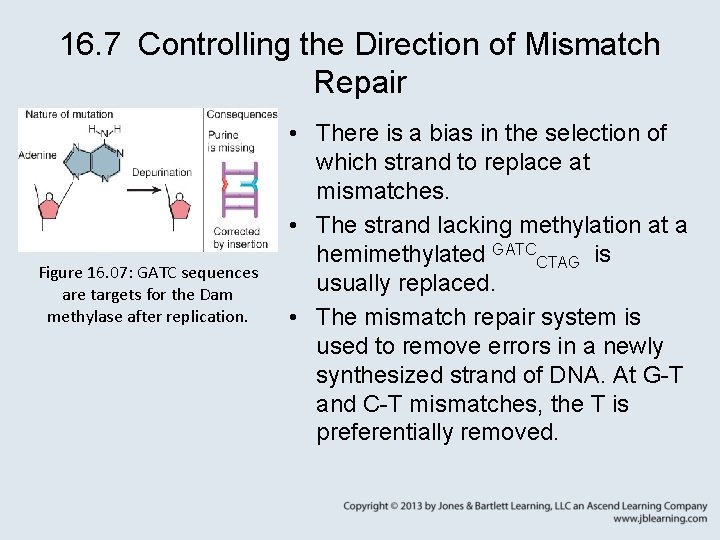
16. 7 Controlling the Direction of Mismatch Repair Figure 16. 07: GATC sequences are targets for the Dam methylase after replication. • There is a bias in the selection of which strand to replace at mismatches. • The strand lacking methylation at a hemimethylated GATCCTAG is usually replaced. • The mismatch repair system is used to remove errors in a newly synthesized strand of DNA. At G-T and C-T mismatches, the T is preferentially removed.

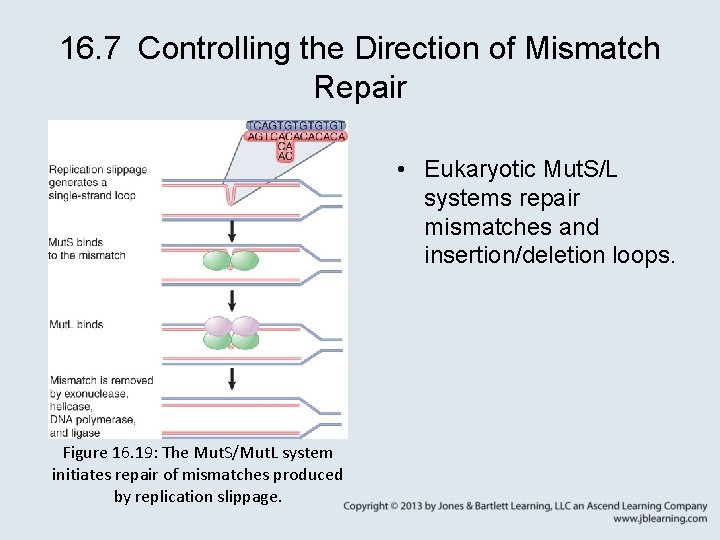
16. 7 Controlling the Direction of Mismatch Repair • Eukaryotic Mut. S/L systems repair mismatches and insertion/deletion loops. Figure 16. 19: The Mut. S/Mut. L system initiates repair of mismatches produced by replication slippage.

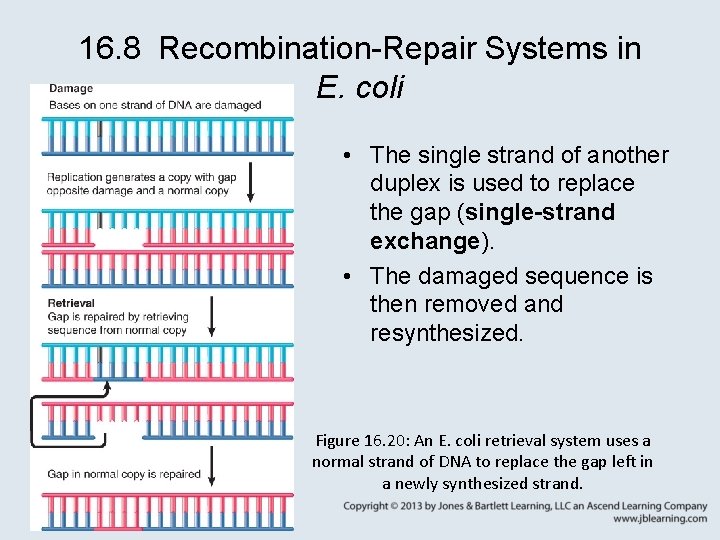
16. 8 Recombination-Repair Systems in E. coli • The rec genes of E. coli code for the principal recombination-repair system. • The recombination-repair system functions when replication leaves a gap in a newly synthesized strand that is opposite a damaged sequence.

16. 8 Recombination-Repair Systems in E. coli • The single strand of another duplex is used to replace the gap (single-strand exchange). • The damaged sequence is then removed and resynthesized. Figure 16. 20: An E. coli retrieval system uses a normal strand of DNA to replace the gap left in a newly synthesized strand.

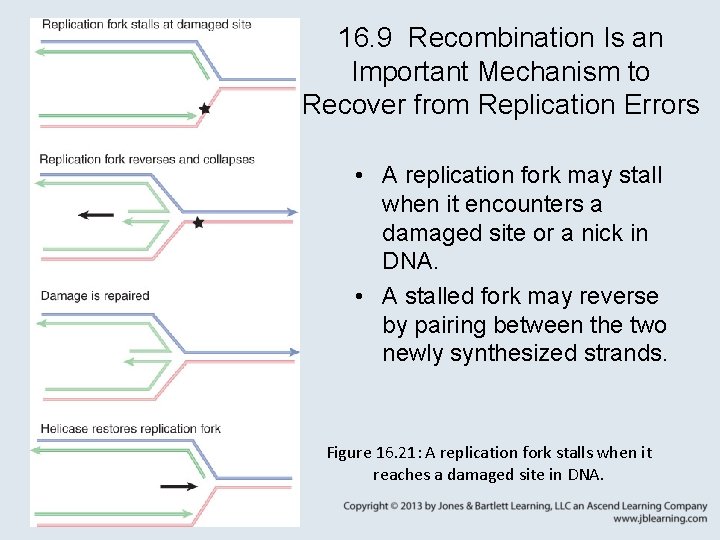
16. 9 Recombination Is an Important Mechanism to Recover from Replication Errors • A replication fork may stall when it encounters a damaged site or a nick in DNA. • A stalled fork may reverse by pairing between the two newly synthesized strands. Figure 16. 21: A replication fork stalls when it reaches a damaged site in DNA.

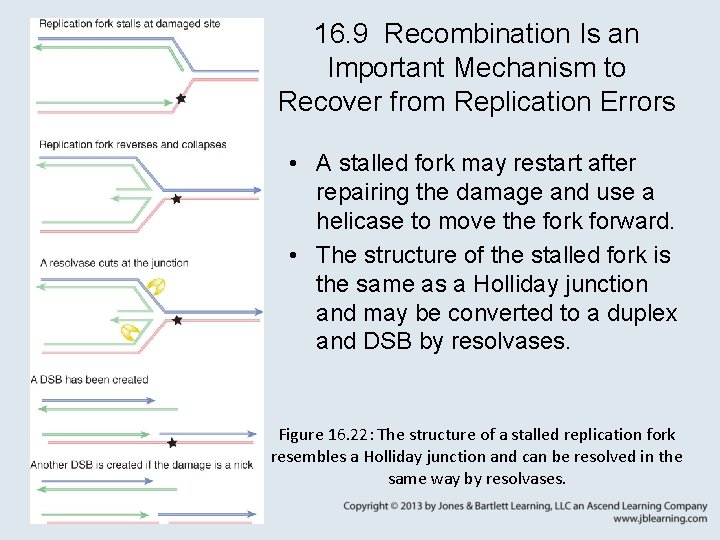
16. 9 Recombination Is an Important Mechanism to Recover from Replication Errors • A stalled fork may restart after repairing the damage and use a helicase to move the fork forward. • The structure of the stalled fork is the same as a Holliday junction and may be converted to a duplex and DSB by resolvases. Figure 16. 22: The structure of a stalled replication fork resembles a Holliday junction and can be resolved in the same way by resolvases.

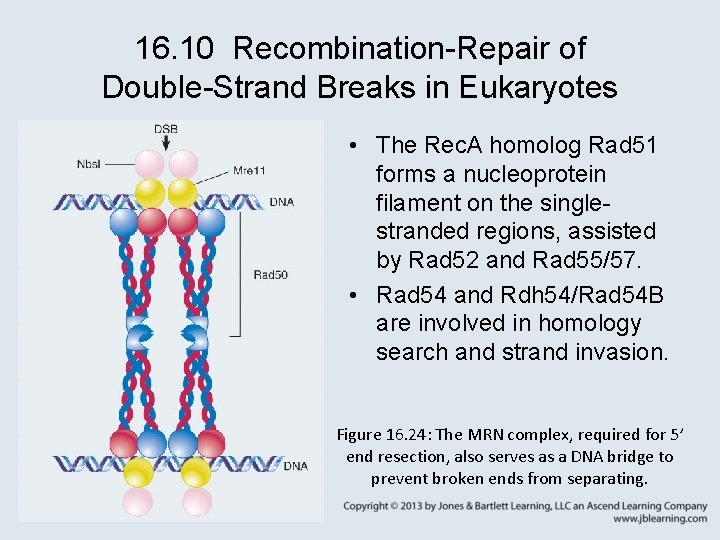
16. 10 Recombination-Repair of Double-Strand Breaks in Eukaryotes • The yeast RAD mutations, identified by radiationsensitive phenotypes, are in genes that code for repair systems. • The RAD 52 group of genes is required for recombination repair. • The MRX (yeast) or MRN (mammals) complex is required to form a single-stranded region at each DNA end.

16. 10 Recombination-Repair of Double-Strand Breaks in Eukaryotes • The Rec. A homolog Rad 51 forms a nucleoprotein filament on the singlestranded regions, assisted by Rad 52 and Rad 55/57. • Rad 54 and Rdh 54/Rad 54 B are involved in homology search and strand invasion. Figure 16. 24: The MRN complex, required for 5’ end resection, also serves as a DNA bridge to prevent broken ends from separating.

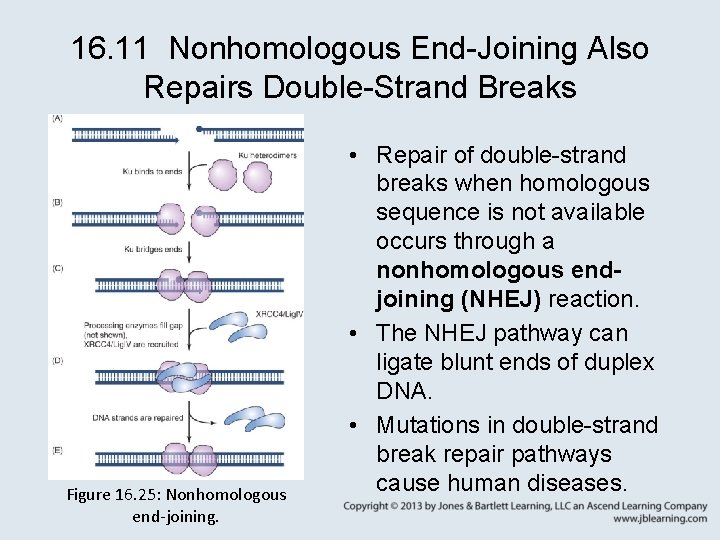
16. 11 Nonhomologous End-Joining Also Repairs Double-Strand Breaks Figure 16. 25: Nonhomologous end-joining. • Repair of double-strand breaks when homologous sequence is not available occurs through a nonhomologous endjoining (NHEJ) reaction. • The NHEJ pathway can ligate blunt ends of duplex DNA. • Mutations in double-strand break repair pathways cause human diseases.

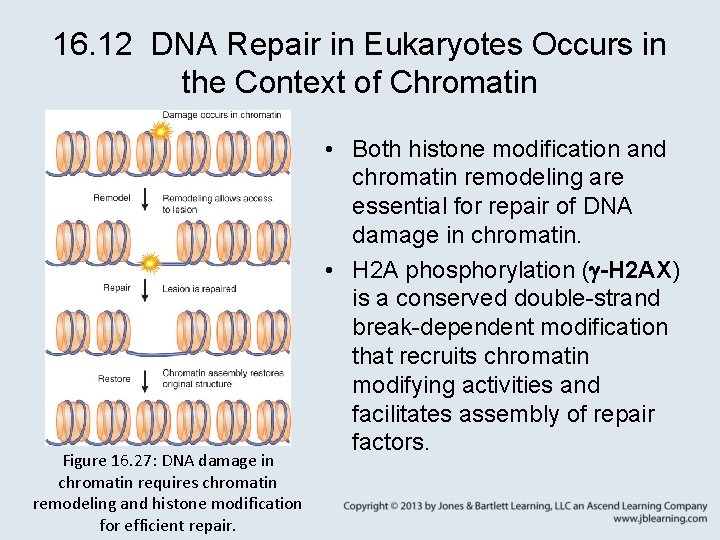
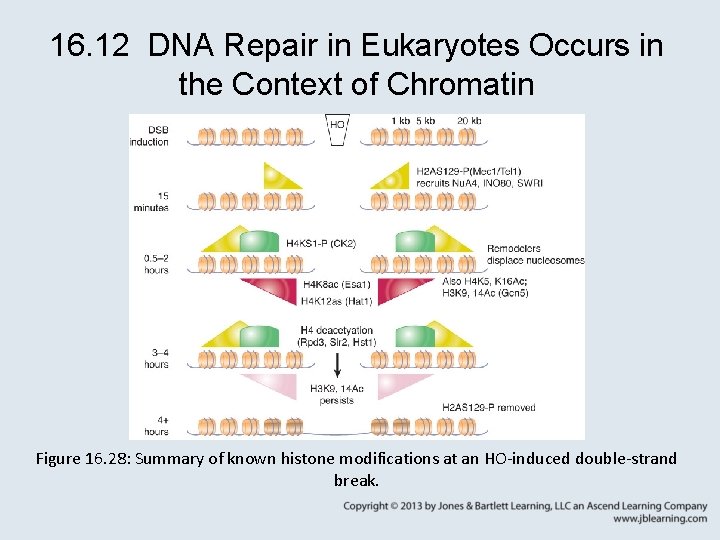
16. 12 DNA Repair in Eukaryotes Occurs in the Context of Chromatin Figure 16. 27: DNA damage in chromatin requires chromatin remodeling and histone modification for efficient repair. • Both histone modification and chromatin remodeling are essential for repair of DNA damage in chromatin. • H 2 A phosphorylation ( -H 2 AX) is a conserved double-strand break-dependent modification that recruits chromatin modifying activities and facilitates assembly of repair factors.

16. 12 DNA Repair in Eukaryotes Occurs in the Context of Chromatin • Different patterns of histone modifications may distinguish stages of repair or different pathways of repair. • Remodelers and chaperones are required to reset chromatin structure after completion of repair.

16. 12 DNA Repair in Eukaryotes Occurs in the Context of Chromatin Figure 16. 28: Summary of known histone modifications at an HO-induced double-strand break.

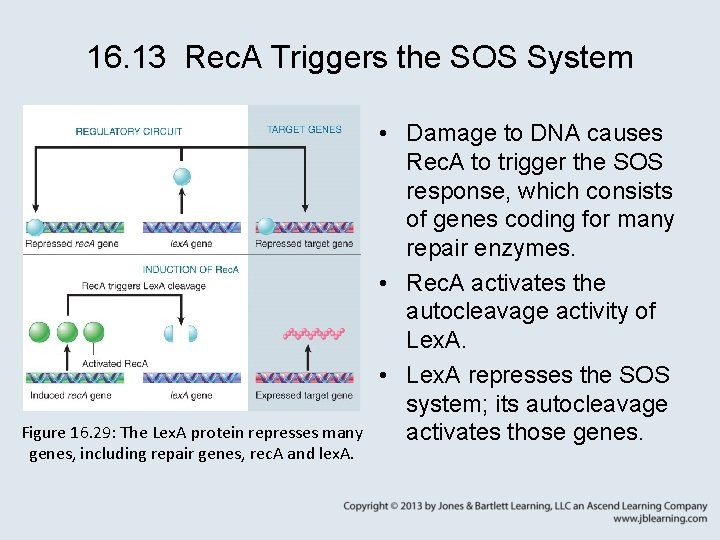
16. 13 Rec. A Triggers the SOS System Figure 16. 29: The Lex. A protein represses many genes, including repair genes, rec. A and lex. A. • Damage to DNA causes Rec. A to trigger the SOS response, which consists of genes coding for many repair enzymes. • Rec. A activates the autocleavage activity of Lex. A. • Lex. A represses the SOS system; its autocleavage activates those genes.