Prime Healthcare Shasta Regional Medical Center Wound Care
















- Slides: 36

Prime Healthcare Shasta Regional Medical Center Wound Care Clinic The Art & Science of Diabetes Symposium 2017

Prime Healthcare Shasta Regional Medical Center Wound Care Clinic

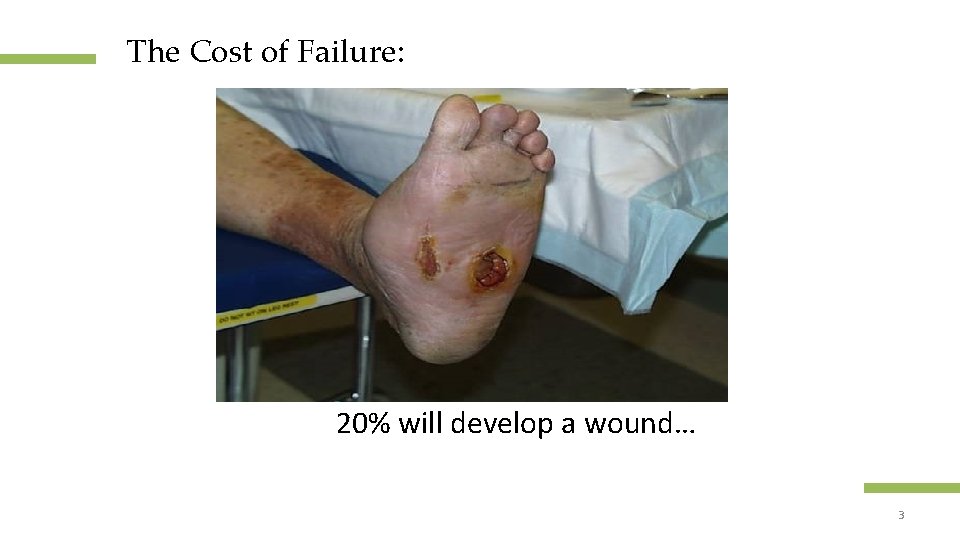
The Cost of Failure: 20% will develop a wound… Confidential and proprietary information 3 © 2013 Healogics, Inc. All Rights Reserved

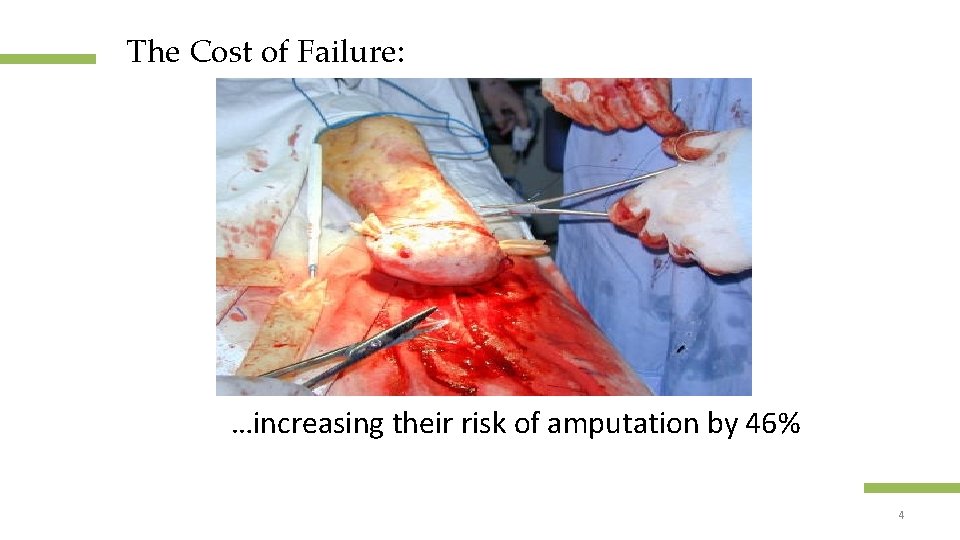
The Cost of Failure: …increasing their risk of amputation by 46% Confidential and proprietary information 4 © 2013 Healogics, Inc. All Rights Reserved

The Cost of Failure: … 27% require a 2 nd amputation within the year Confidential and proprietary information 5 © 2013 Healogics, Inc. All Rights Reserved

The Cost of Failure: …and 50% are dead 5 years later Confidential and proprietary information 6 © 2013 Healogics, Inc. All Rights Reserved

By the Numbers… • Chronic wounds affect 6. 5 million Americans/ year at a treatment cost of $25 billion per year • Additional $39 billion in lost wages/ year • $15. 3 billion estimated expense on wound care products in 2010 (the cost of “success”? ) Confidential and proprietary information 7 © 2013 Healogics, Inc. All Rights Reserved

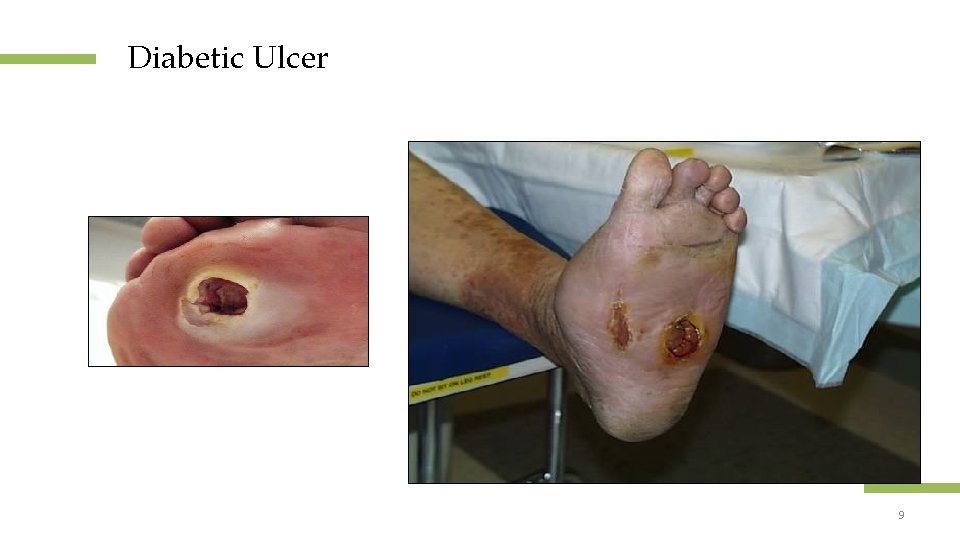
Diabetic Ulcer Location: plantar aspect of the foot beneath a bony prominence. Appearance: ill-defined borders, prominent callus, and palpable pulses. Confidential and proprietary information 8 © 2013 Healogics, Inc. All Rights Reserved

Diabetic Ulcer Confidential and proprietary information 9 © 2013 Healogics, Inc. All Rights Reserved


DFU- Management Principles • • Off-loading Debridement/ dressing selection (clean, moist wound bed) Evaluate and correct ischemia/osteomyelitis Adjunctive therapy • Skin substitutes • HBOT Confidential and proprietary information 11 © 2013 Healogics, Inc. All Rights Reserved

Skin Subsitutes • Bio-Engineered – Apligraf – Dermagraft • Collagen Wound Matrix – Oasis – Puraply • Amniotic Membrane Allografts – Grafix – Epi. Fix Confidential and proprietary information 12 © 2013 Healogics, Inc. All Rights Reserved

Prime Healthcare Shasta Regional Medical Center Wound Care Clinic

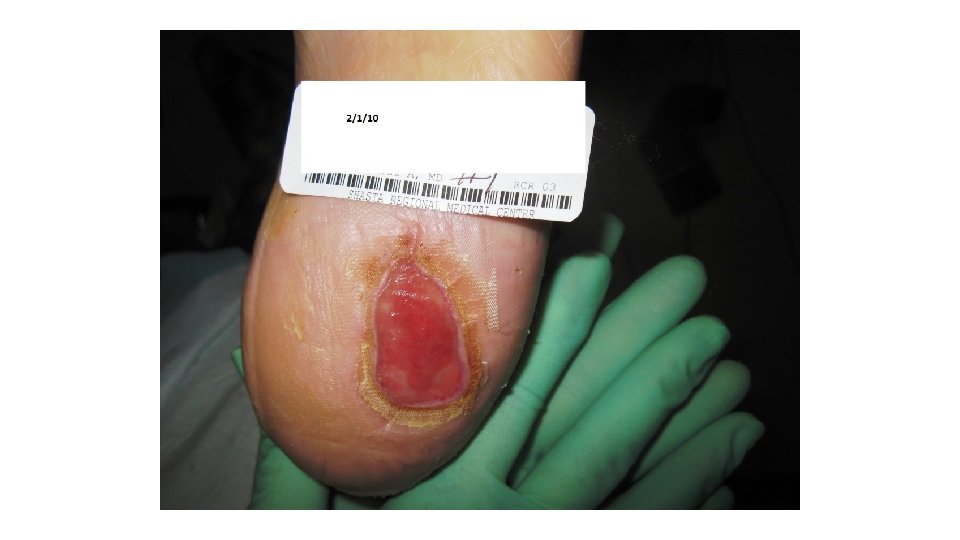
Case Study’s First visit to the Wound Center 11/21/14

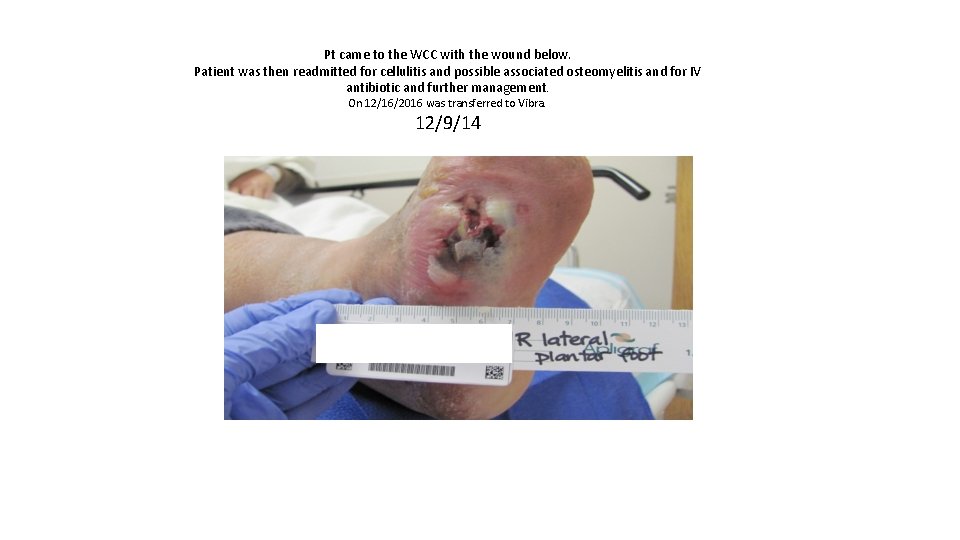
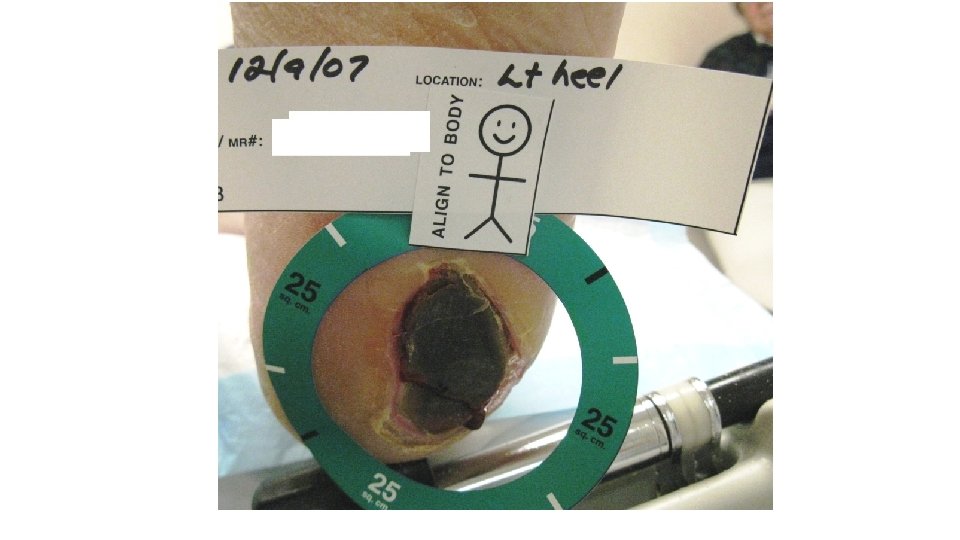
Pt came to the WCC with the wound below. Patient was then readmitted for cellulitis and possible associated osteomyelitis and for IV antibiotic and further management. On 12/16/2016 was transferred to Vibra. 12/9/14

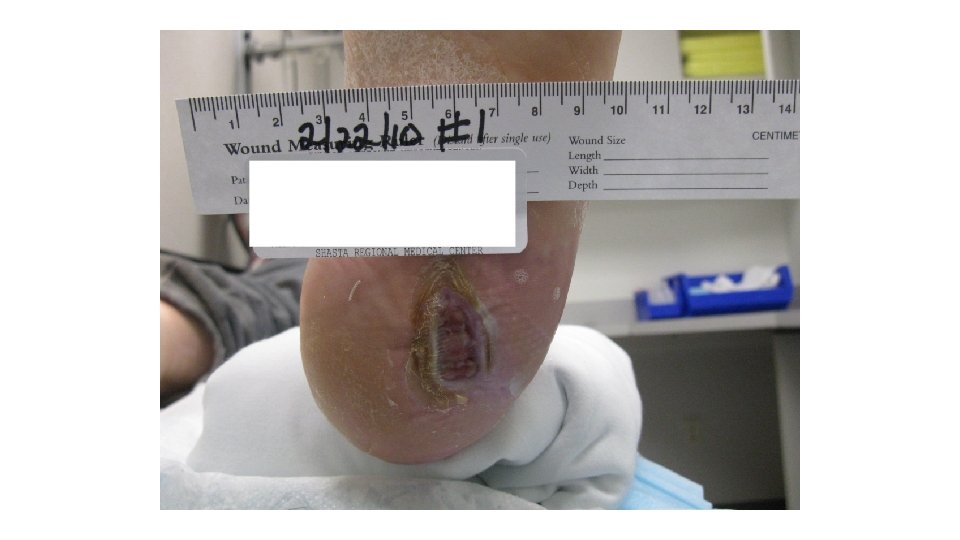
• 5/5/2015 - Again admitted to the hospital with DFU and Cellulitis of the right foot. • 8/20/15 – Admitted for heart failure • 2/8/16 – Sepsis from DFU right foot, pt refuses BKA.

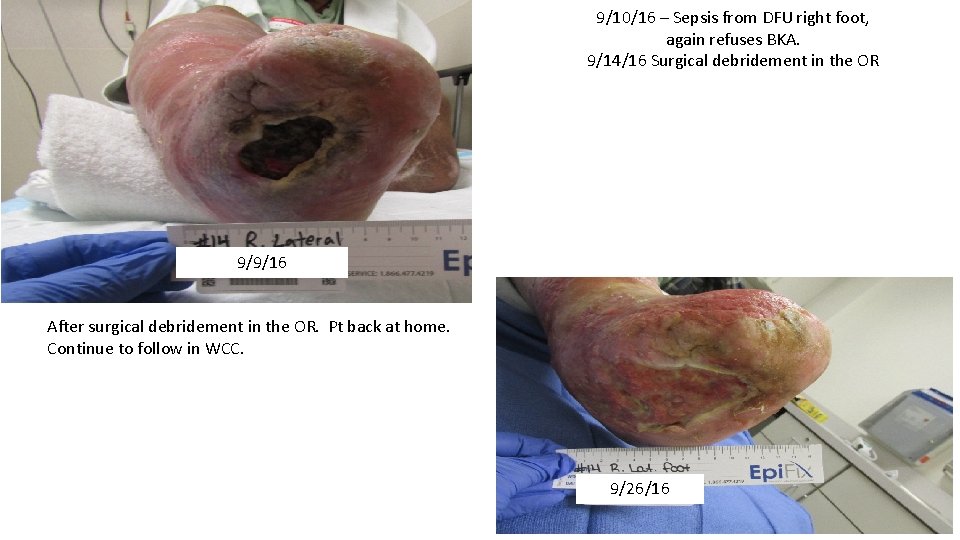
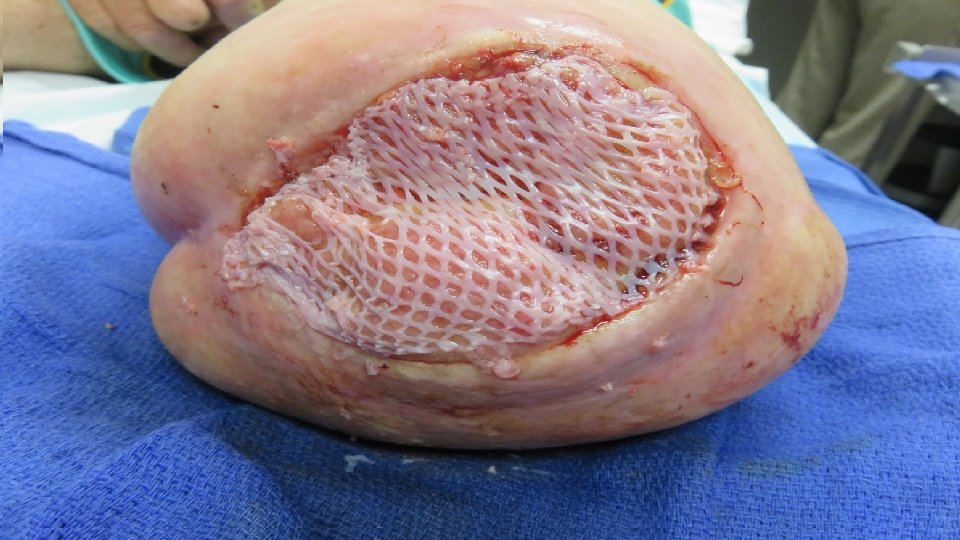
9/10/16 – Sepsis from DFU right foot, again refuses BKA. 9/14/16 Surgical debridement in the OR 9/9/16 After surgical debridement in the OR. Pt back at home. Continue to follow in WCC. 9/26/16

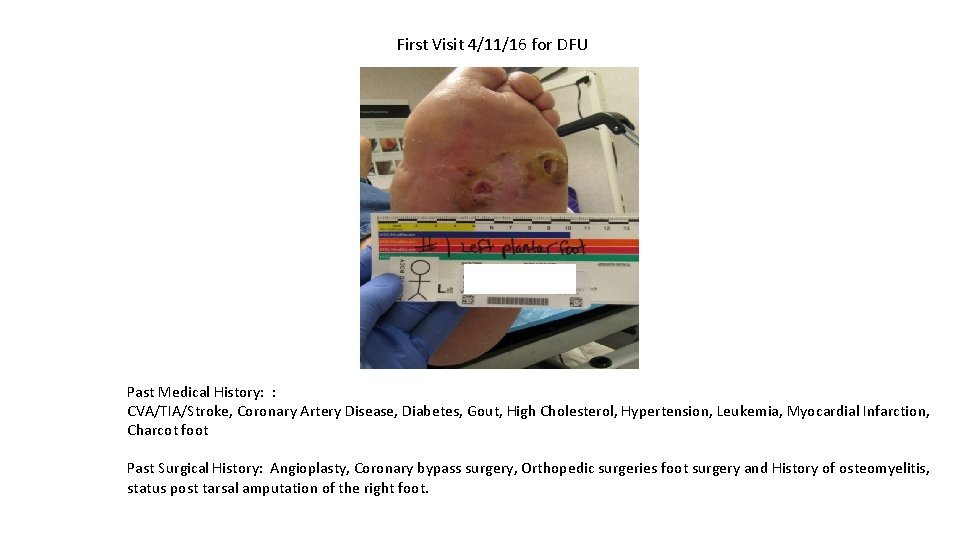
First Visit 4/11/16 for DFU Past Medical History: : CVA/TIA/Stroke, Coronary Artery Disease, Diabetes, Gout, High Cholesterol, Hypertension, Leukemia, Myocardial Infarction, Charcot foot Past Surgical History: Angioplasty, Coronary bypass surgery, Orthopedic surgeries foot surgery and History of osteomyelitis, status post tarsal amputation of the right foot.

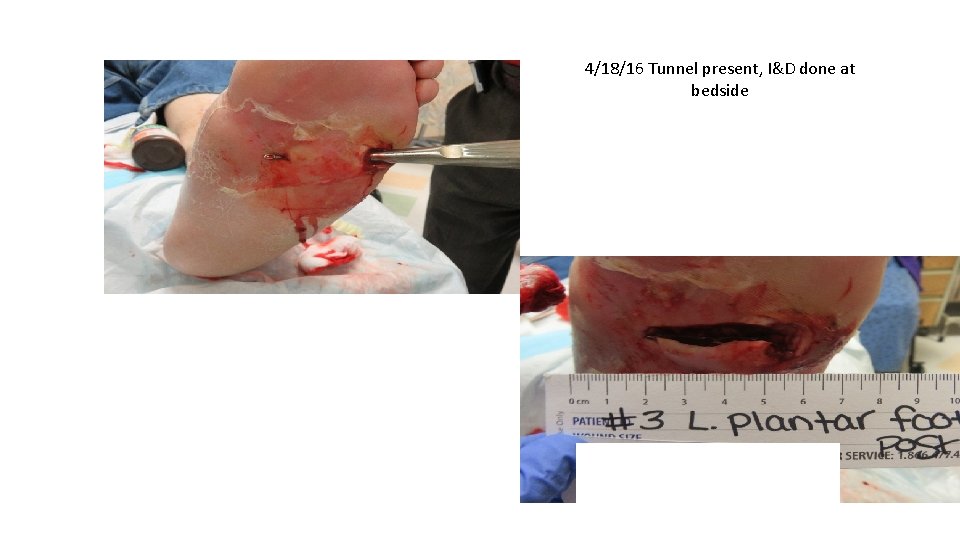
4/18/16 Tunnel present, I&D done at bedside

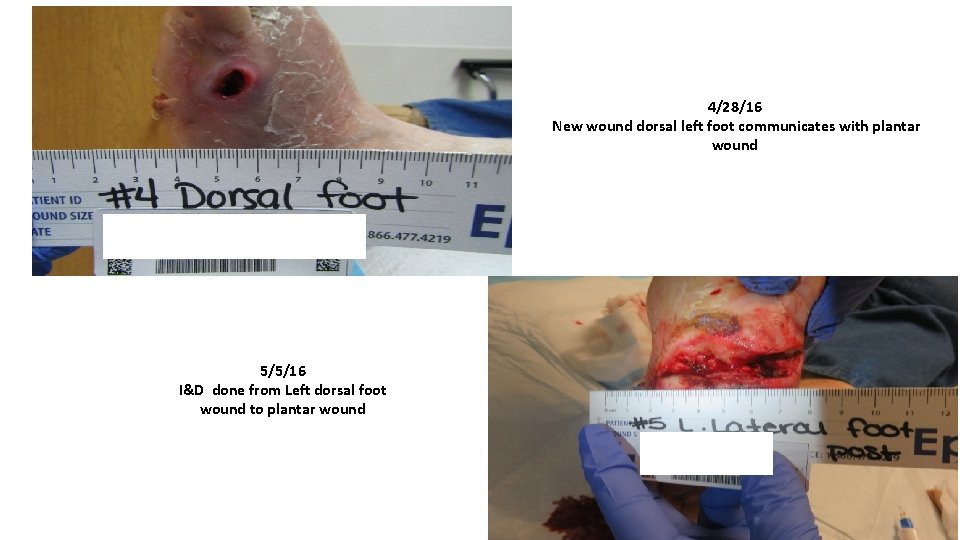
4/28/16 New wound dorsal left foot communicates with plantar wound 5/5/16 I&D done from Left dorsal foot wound to plantar wound

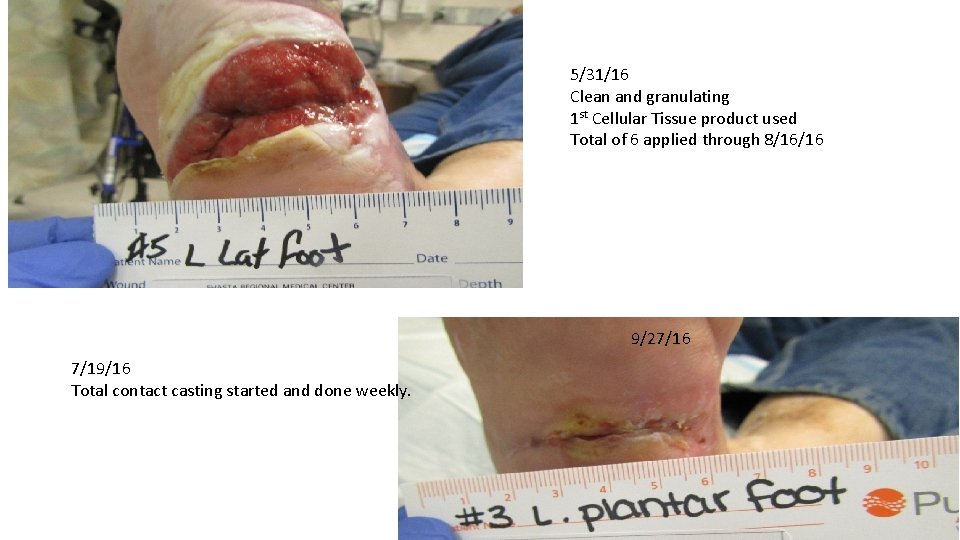
5/31/16 Clean and granulating 1 st Cellular Tissue product used Total of 6 applied through 8/16/16 9/27/16 7/19/16 Total contact casting started and done weekly.

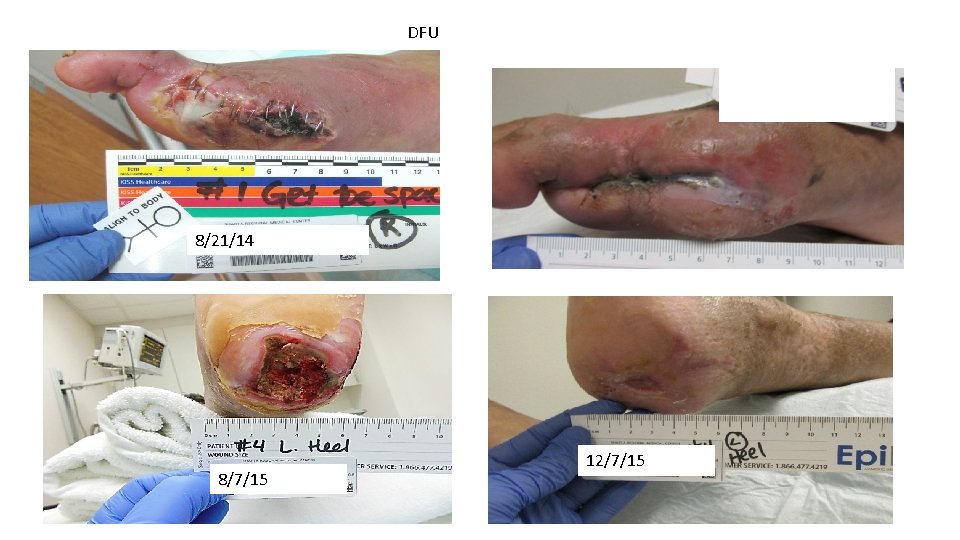
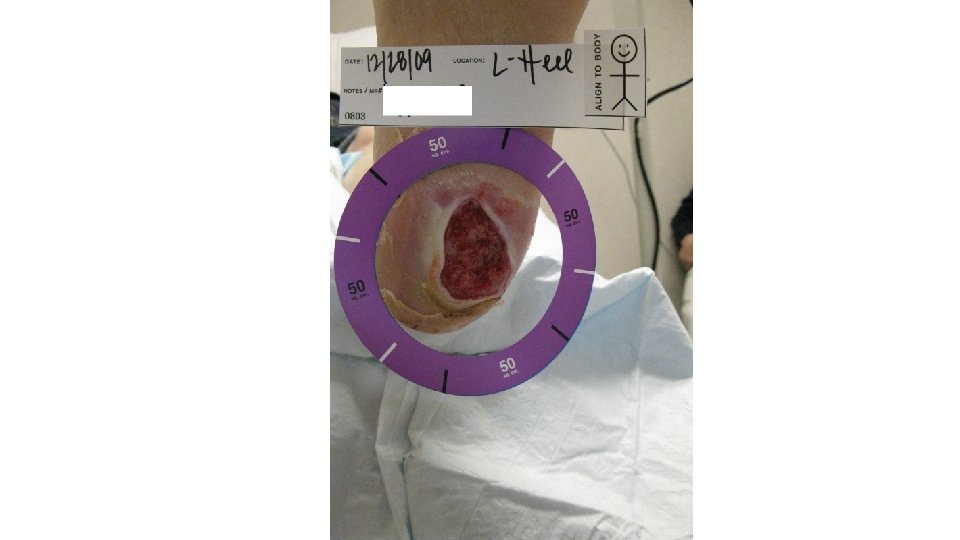
DFU 8/21/14 8/7/15 12/7/15


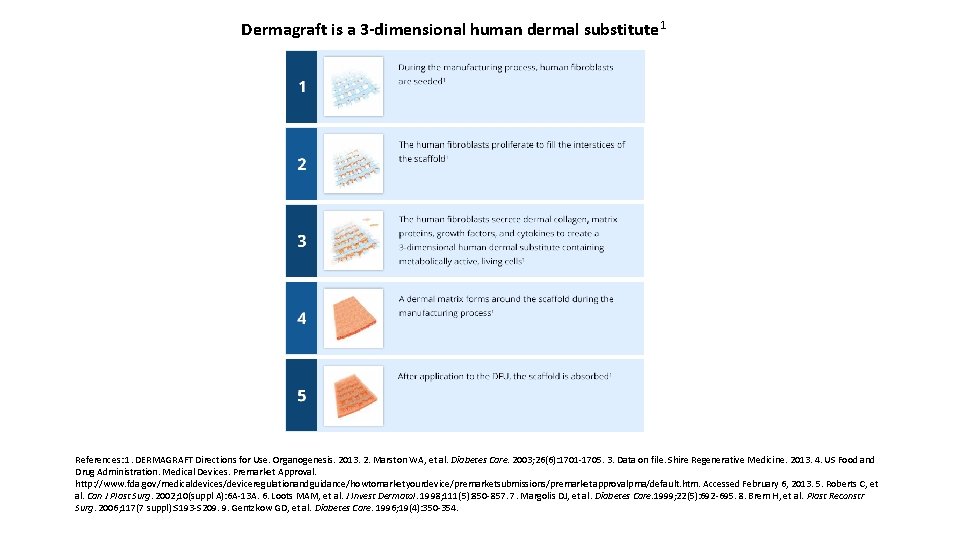
Dermagraft is a 3 -dimensional human dermal substitute 1 References: 1. DERMAGRAFT Directions for Use. Organogenesis. 2013. 2. Marston WA, et al. Diabetes Care. 2003; 26(6): 1701 -1705. 3. Data on file. Shire Regenerative Medicine. 2013. 4. US Food and Drug Administration. Medical Devices. Premarket Approval. http: //www. fda. gov/medicaldevices/deviceregulationandguidance/howtomarketyourdevice/premarketsubmissions/premarketapprovalpma/default. htm. Accessed February 6, 2013. 5. Roberts C, et al. Can J Plast Surg. 2002; 10(suppl A): 6 A-13 A. 6. Loots MAM, et al. J Invest Dermatol. 1998; 111(5): 850 -857. 7. Margolis DJ, et al. Diabetes Care. 1999; 22(5): 692 -695. 8. Brem H, et al. Plast Reconstr Surg. 2006; 117(7 suppl): S 193 -S 209. 9. Gentzkow GD, et al. Diabetes Care. 1996; 19(4): 350 -354.

• Chronic diabetic foot ulcers (DFUs) demonstrate impaired healing that results from multiple pathological factors (eg, vascular insufficiency, altered cellular activity, and a dysfunctional extracellular matrix), leading to a deficient wound bed, which often fails to respond to conventional therapy alone 5 -7 • Dermagraft helps to restore the compromised DFU dermal bed to facilitate healing by providing a substrate over which the patient’s own epithelial cells can migrate to close the wound 5 – Composed of human fibroblasts, an extracellular matrix, and a bioabsorbable polyglactin mesh scaffold 1 – Cryopreserved, 3 -dimensional, human dermal substitute 1 – Allows for serial applications without the need for removal of the product from the wound 1 References: 1. DERMAGRAFT Directions for Use. Organogenesis. 2013. 2. Marston WA, et al. Diabetes Care. 2003; 26(6): 1701 -1705. 3. Data on file. Shire Regenerative Medicine. 2013. 4. US Food and Drug Administration. Medical Devices. Premarket Approval. http: //www. fda. gov/medicaldevices/deviceregulationandguidance/howtomarketyourdevice/premarketsubmissions/premarketapprovalpma/default. htm. Accessed February 6, 2013. 5. Roberts C, et al. Can J Plast Surg. 2002; 10(suppl A): 6 A-13 A. 6. Loots MAM, et al. J Invest Dermatol. 1998; 111(5): 850 -857. 7. Margolis DJ, et al. Diabetes Care. 1999; 22(5): 692 -695. 8. Brem H, et al. Plast Reconstr Surg. 2006; 117(7 suppl): S 193 -S 209. 9. Gentzkow GD, et al. Diabetes Care. 1996; 19(4): 350 -354.

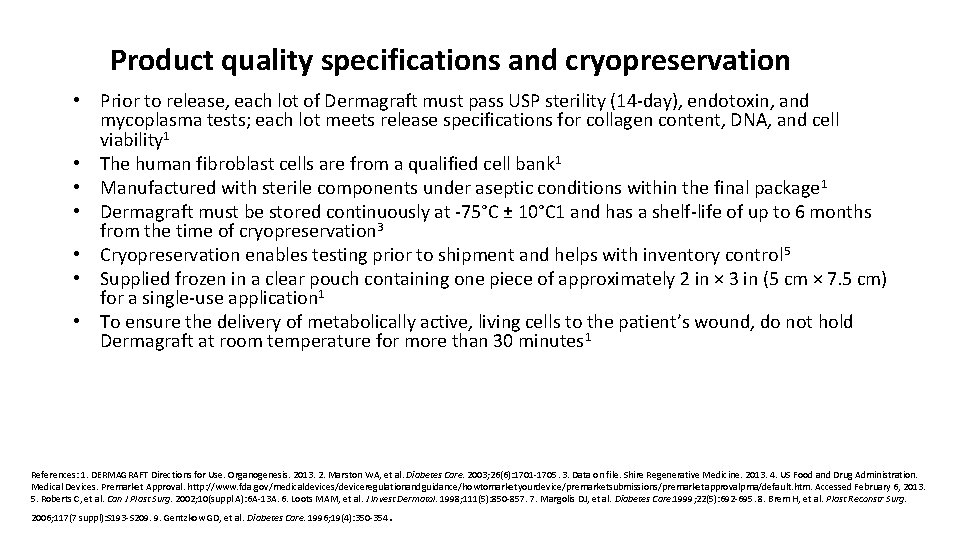
Product quality specifications and cryopreservation • Prior to release, each lot of Dermagraft must pass USP sterility (14 -day), endotoxin, and mycoplasma tests; each lot meets release specifications for collagen content, DNA, and cell viability 1 • The human fibroblast cells are from a qualified cell bank 1 • Manufactured with sterile components under aseptic conditions within the final package 1 • Dermagraft must be stored continuously at -75°C ± 10°C 1 and has a shelf-life of up to 6 months from the time of cryopreservation 3 • Cryopreservation enables testing prior to shipment and helps with inventory control 5 • Supplied frozen in a clear pouch containing one piece of approximately 2 in × 3 in (5 cm × 7. 5 cm) for a single-use application 1 • To ensure the delivery of metabolically active, living cells to the patient’s wound, do not hold Dermagraft at room temperature for more than 30 minutes 1 References: 1. DERMAGRAFT Directions for Use. Organogenesis. 2013. 2. Marston WA, et al. Diabetes Care. 2003; 26(6): 1701 -1705. 3. Data on file. Shire Regenerative Medicine. 2013. 4. US Food and Drug Administration. Medical Devices. Premarket Approval. http: //www. fda. gov/medicaldevices/deviceregulationandguidance/howtomarketyourdevice/premarketsubmissions/premarketapprovalpma/default. htm. Accessed February 6, 2013. 5. Roberts C, et al. Can J Plast Surg. 2002; 10(suppl A): 6 A-13 A. 6. Loots MAM, et al. J Invest Dermatol. 1998; 111(5): 850 -857. 7. Margolis DJ, et al. Diabetes Care. 1999; 22(5): 692 -695. 8. Brem H, et al. Plast Reconstr Surg. 2006; 117(7 suppl): S 193 -S 209. 9. Gentzkow GD, et al. Diabetes Care. 1996; 19(4): 350 -354 .










 Shasta regional wound center
Shasta regional wound center Doylestown hospital wound care center
Doylestown hospital wound care center Aileen takahashi md torrance ca
Aileen takahashi md torrance ca Mercy regional medical center lorain
Mercy regional medical center lorain Unm sandoval regional medical center billing
Unm sandoval regional medical center billing Healthcare and the healthcare team chapter 2
Healthcare and the healthcare team chapter 2 Sports medicine meaning
Sports medicine meaning Gbmc infoweb
Gbmc infoweb Torrance memorial hospital medical records
Torrance memorial hospital medical records Cartersville medical center medical records
Cartersville medical center medical records Jeff bezos prime video prime
Jeff bezos prime video prime Wound discharge colors
Wound discharge colors Chapter 32 skin integrity and wound care
Chapter 32 skin integrity and wound care Chapter 48 skin integrity and wound care
Chapter 48 skin integrity and wound care Chapter 48 skin integrity and wound care
Chapter 48 skin integrity and wound care Transient contact
Transient contact Wound care pretest
Wound care pretest Wound care near freedom
Wound care near freedom Aqucel foam
Aqucel foam Chapter 48 skin integrity and wound care
Chapter 48 skin integrity and wound care Primary secondary tertiary medical care
Primary secondary tertiary medical care Medical internet of things and big data in healthcare
Medical internet of things and big data in healthcare Healthcare cisco smartnet total care support
Healthcare cisco smartnet total care support Uhc medication prior authorization form
Uhc medication prior authorization form Roanoke regional loan center
Roanoke regional loan center Regional center for border health
Regional center for border health Regional center of cordillera administrative region
Regional center of cordillera administrative region High plains climate
High plains climate Sc acis
Sc acis Joint regional intelligence center
Joint regional intelligence center Regional center consumers
Regional center consumers Center for regional udvikling
Center for regional udvikling Northwest regional data center
Northwest regional data center Western regional training center
Western regional training center New york regional census center
New york regional census center Ptal california medical board
Ptal california medical board Hepburn osteometric board
Hepburn osteometric board