Factors Involved in Wound Healing and Acute Wound
















- Slides: 19

Factors Involved in Wound Healing and Acute Wound Failure Michelle Suri The University of Manitoba

Definitions l Acute wound l l Traumatic loss of normal structure and function to recently injured tissue after noxious insult Acute wound healing l The highly regulated process of cellular, humoral and molecular events activated at the time of acute injury and resulting in a time – dependent but predictible and orderly pattern of tissue repair

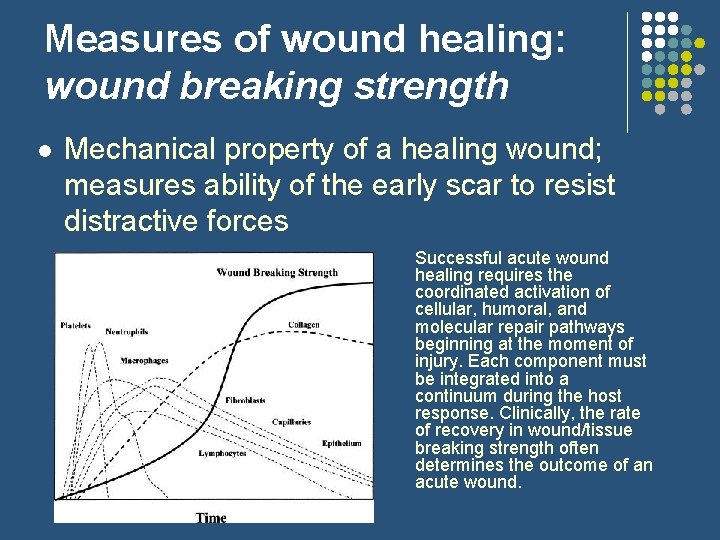
Measures of wound healing: wound breaking strength l Mechanical property of a healing wound; measures ability of the early scar to resist distractive forces Successful acute wound healing requires the coordinated activation of cellular, humoral, and molecular repair pathways beginning at the moment of injury. Each component must be integrated into a continuum during the host response. Clinically, the rate of recovery in wound/tissue breaking strength often determines the outcome of an acute wound.

Measures of wound healing (cont’d) : tensile strength l l Normalizes breaking strength to the surface area of the wound edge Tensile strength = wound breaking strength / surface area of the wound edge

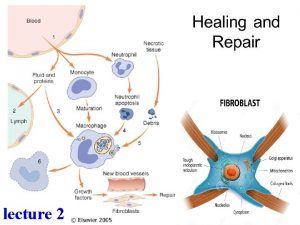
Stages of Wound Healing I. Hemostasis (the leak is plugged) l l l Occurs immediately, within the first 24 hrs Formation of primary platelet plug; this fills the tissue defect left by the injury Vasospasm – reduces blood loss by decreasing vessel diameter Clotting cascade activated Activated platelets release cytokines which increase vascular permeability and facilitate the next stage of wound healing, diapedesis of leukocytes into the wound site

Stages of wound healing 2. Inflammation (the dirt is removed) l l Migration of cells into the clot Two phases: l (1) Early l Influx neutrophils § l cleanse the wound of foreign material (2) Late l Macrophages predominate § § § occurs after 24 hrs Debris degradation, microorganism ingestion, secrete growth factors, cytokines, and enzymes involved in NO production NO – NB factor in wound healing due to affects on vascular reactivity, macrophages and collagen production

Stages of wound healing 3. Proliferation (the hole is patched with permanent material) l l Grossly exhibited by the formation of granulation tissue, which is granular, highly vascular and red; steps involved : (1) Fibroplasia (the workers enter the construction site) l Increase in number of fibroblasts l l (2) Extracellular matrix formation (the workers lay down the scaffolding to patch the hole) l l Fibronectin and hyaluronic acid are laid down by fibroblasts and facilitates the migration of macrophages and more fibroblasts, as well as providing a scaffold for new capillary formation (3) Collagen formation (the hole is plugged with permanent material) l l Sythensize structural proteins, reorganize wound matrix and promote wound contraction Initially secreted by fibroblasts as procollagens Converted to collagen III and finally collagen I The deposition and remodeling of collagen gradually increases the tensile strength of the wound (4) Angiogensis (the water supply is hooked up) l Endothelial cell mitogenesis and migration

Stages of wound healing 4. remodelling (the patch is made pretty) l l Process occurs over months and years by a slow reduction of cellular content and vascularity The wound regains 3 -5% of its original strength at 2 weeks; 15%, at 3 weeks; 35%, at 1 month; and increases to a final strength of 80%, after several months

Acute wound failure l l l Occurs when the load placed across the wound exceeds the resistive capacity of the suture line and provisional matrix; the acute wound is totally dependant on the suture until breaking strengths increase Usually due to an abnormality in the degree or duration of the sequential components of tissue repair The result of acute wound failure is either an incisional hernia or complete dehiscence

Acute wound failure – disruption in the stages of healing l I. Hemostasis l l l II. Inflammtion l l l Tensile strength of wound is basically zero Any delay or disruption, such that occurs in wound infection or foreign body prescence, can prolong this phase III. Proliferation l l Platelet dysfunction or poor surgical technique may lead to dysfunction at this stage Hematoma formation cause mechanical disruption Decreased wound breaking strength can be caused by a prolonged inflammatory phase Hypothesiszed that delays in recruitment of fascial fibroblasts may cause delays in fascial repair Abnormalities in collagen formation have been noted in pt’s with wound failure – increased collagen III IV. Remodelling l Hypertrophic or keloid scarring with over-proliferation of ECM and collagen, and decreased remodelling

Acute wound failure l There are many risk factors that may contribute to the process of wound failure; each of these factors may cause failure in a single or multiple stages of the wound healing process.

Acute wound failure – systemic factors that delay wound healing l Specific patient characteristics may contribute to delayed healing l l l l l Obesity CV disease Respiratory disease Metabolic disease Endocrine disease Renal and hepatic failure DM Old age Malignancy + assoc risks – antimetabolites, cytotoxic agents and steroids, radiation Critically ill patients have many abnormalities that predispose them to delayed healing l l l Tissue hypoxia Disrupted tissue perfusion Hypothermia and pain Trauma and burns Sepsis Poor nutrition

Acute wound failure – mechanical factors l Most often due to tissue failure with the suture pulling through adjacent tissue and not suture fracture or knot slippage l l Type of abdominal wall closure l l Tissue failure occurs at the wound edge, where proteases activated during normal tissue repair result in a loss of tissue integrity Optimal suture length-to-wound length (SL-to-WL) ratio 4: 1 with 1 cm bites followed by 1 cm of progress between each bite Should provide adequate approximation with minimal tension to reduce ischemia, which can also affect tissue integrity Mass closure has also been found to be as effective as layered closures, and interrupted closures Increased intra-abdominal pressure l l l Abdominal fluid collection Distended bowel Lumenal gas The mechanism by which an acute wound provisional matrix melds with uninjured tissue at the wound border is incompletely understood. Clinical and laboratory experience suggests that many acute wounds mechanically fail at the immature scar–to–normal tissue interface. During the first 3 to 5 days of repair, an acute wound is most likely to fail within the provisional matrix itself (thick arrow). After that, mechanical failure is more likely to occur at the early scar–to–wound edge interface (thin arrow).

Incisional Hernias l l l 11% to 20% of abdominal wall fascial closures lead to ventral incisional hernia formation Recurrence rate approximately 24 -58% Etiology l Biological factors l l Deep wound infection Hypoperfusion (shock) Malnutrition Mechanical factors l l l Closure under tension Suture fracture Knot slippage Fascial tearing Suboptimal SL to SW ratio

Dehiscence l Results when a wound fails to heal in apposition, with subsequent disruption of suture line, both in the superficial and deep layers

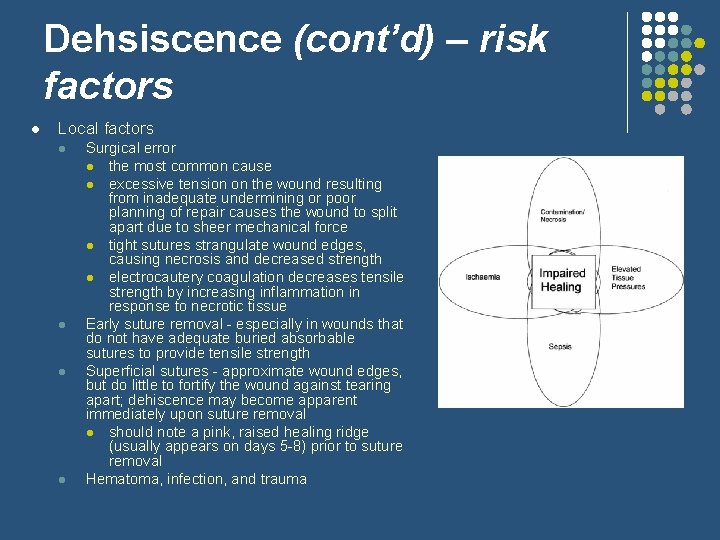
Dehsiscence (cont’d) – risk factors l Local factors l l Surgical error l the most common cause l excessive tension on the wound resulting from inadequate undermining or poor planning of repair causes the wound to split apart due to sheer mechanical force l tight sutures strangulate wound edges, causing necrosis and decreased strength l electrocautery coagulation decreases tensile strength by increasing inflammation in response to necrotic tissue Early suture removal - especially in wounds that do not have adequate buried absorbable sutures to provide tensile strength Superficial sutures - approximate wound edges, but do little to fortify the wound against tearing apart; dehiscence may become apparent immediately upon suture removal l should note a pink, raised healing ridge (usually appears on days 5 -8) prior to suture removal Hematoma, infection, and trauma

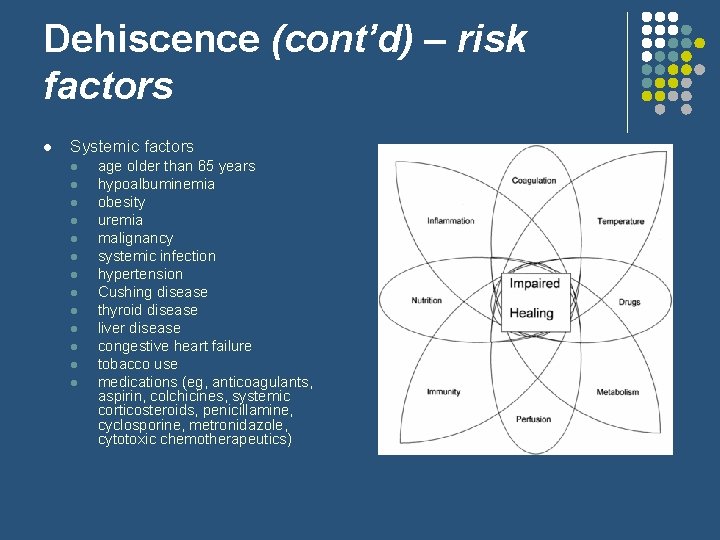
Dehiscence (cont’d) – risk factors l Systemic factors l l l l age older than 65 years hypoalbuminemia obesity uremia malignancy systemic infection hypertension Cushing disease thyroid disease liver disease congestive heart failure tobacco use medications (eg, anticoagulants, aspirin, colchicines, systemic corticosteroids, penicillamine, cyclosporine, metronidazole, cytotoxic chemotherapeutics)

Dehiscence (cont’d) Prevention l l l Avoiding crushing of tissue, excessive electrocautery, ineffective hemostasis, dead space in the wound, excessive wound tension, and overly tight sutures Buried absorbable sutures to provide tensile strength Tensile strength retention properties of suture material l l l polydioxanone (PDS II) - synthetic absorbable monofilament suture with low reactivity and prolonged retention of tensile strength retains 70% of its strength at 2 weeks, 50%, at 4 weeks, and 25%, at 6 weeks associated with significantly less scar spreading in high-tension areas when compared with a polyglycolic acid (Dexon) suture Patients should be instructed to avoid stretching or trauma to the wound area Wound immobilization with Steri-Strips and a dressing provides some protection and serves as a reminder to the patient Experiemental rat models involving injection of fibroproliferative growth factor before making an incision have shown a reduction in fascial wound failure and incisional hernia formation

Dehsicence (cont’d) treatment l l Wounds may be resutured in cases due to premature suture removal or trauma without evidence of infection Devitalized tissue may need to be removed Dehiscence due to hematoma may be resutured in uncomplicated cases after complete removal of the hematoma l In cases of delayed hematoma or infection, the wound is best treated by allowing it to heal by second intention granulation Delayed primary closure l Only surgical technique known to accelerate incisional healing l Dermal wound closed 3 to 5 days after granulation tissue formed; allows maximal wound breaking strength to form in a minimum amount of time l Theory behind the success l l Cellular and molecular elements of tissue repair are activated before wound closure Scar revision is best planned after adequate healing has occurred, usually no sooner than 8 weeks
 Systemic factors affecting wound healing
Systemic factors affecting wound healing Inflammation fever
Inflammation fever Factors affecting wound healing ppt
Factors affecting wound healing ppt Concealed puncture wounds meaning
Concealed puncture wounds meaning Wound healing nutrition handout
Wound healing nutrition handout Rank and wakefield classification of wounds
Rank and wakefield classification of wounds Primary union wound healing
Primary union wound healing Pt factors involved
Pt factors involved Three categories of opportunity analysis canvas
Three categories of opportunity analysis canvas Hroc target
Hroc target Enaahtig north healing lodge
Enaahtig north healing lodge Institute of health and healing
Institute of health and healing Doing good and healing all
Doing good and healing all Abiotic
Abiotic Abiotic vs biotic factors
Abiotic vs biotic factors Abiotic factors and biotic factors
Abiotic factors and biotic factors Factors of 18
Factors of 18 Highest common factor
Highest common factor Factors of 8
Factors of 8 What is quantum healing
What is quantum healing