NAMING Organic Chemistry IUPAC L O recognize the









- Slides: 43

NAMING Organic Chemistry IUPAC

L. O -recognize the common functional groups -understand be able to use the IUPAC nomenclature -understand the formation of homologous series and the similarity of properties -understand why isomerism occurs and be able to draw isomers for given molecular formulas -understand the formation of sigma and pi bonds and know their occurrence and the implication for molecular shapes -understand the concept of saturation and apply it to the alkanes as a ‘base’ homologous series -recognise the importance of alkanes as fuels, their combustion products and the environmental effects of burning hydrocarbon fuels -recall details of the fractionation of oil and the uses of the fractions -understand homolytic bond fission and the formation of free radicals -understand catalytic cracking and thermal cracking in terms of a free radical process -know and understand the mechanism of photochemical chlorination

What is ORGANIC 2 min https: //www. youtube. com/watch? v=s. DTys. JFogwg Teravalencey Catenation Isomerization

Organic Chem Deals with compounds of carbon EXCEPT (memorize this exclusions NOW): 1) CO 2 and CO(g) 2) ionic compounds with carbonate or CO 32 - like Ca. CO 3 3) CN – or Cyanides 4) Carbides like Ca 2 C 5) Pure carbon in diamond, graphite and graphine allotropes: or C(s) diamond or C(s) Graphite or C(s) Graphene

Fossil Fuels • The major source of carbon compounds is still living or previously living things, such as plants, animals and all types of fossil fuels. • Fossil fuels are chemical potential energy. The original source of this energy is the sun.

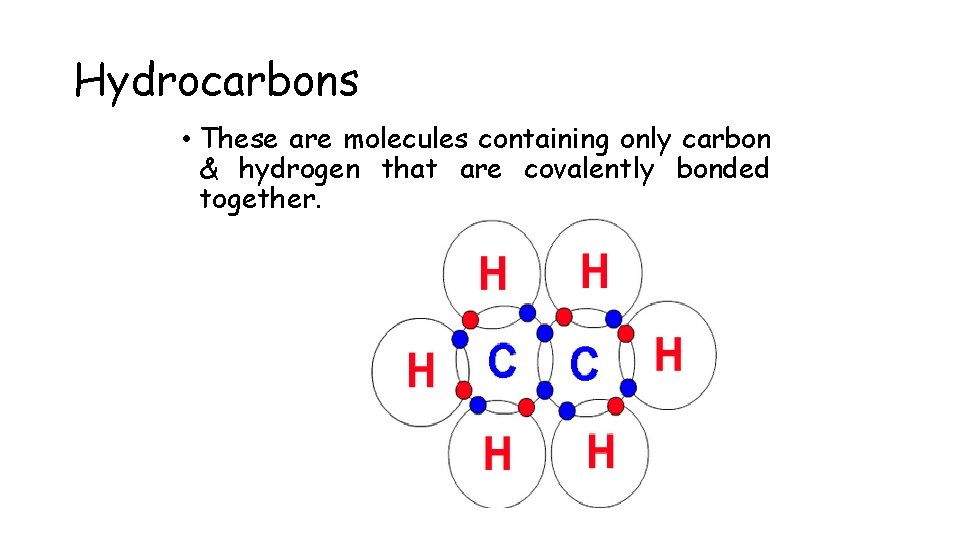
Hydrocarbons • These are molecules containing only carbon & hydrogen that are covalently bonded together.

Just One element gives rise to 90% of all compounds known Catenation • The linkage of atoms of the same elements to form longer chain is called • Carbon due to its tetravalent nature has the unique property to form bonds with other atoms of carbon forming a long chain. Straight chains Branched chains Cyclic rings Aromatic rings


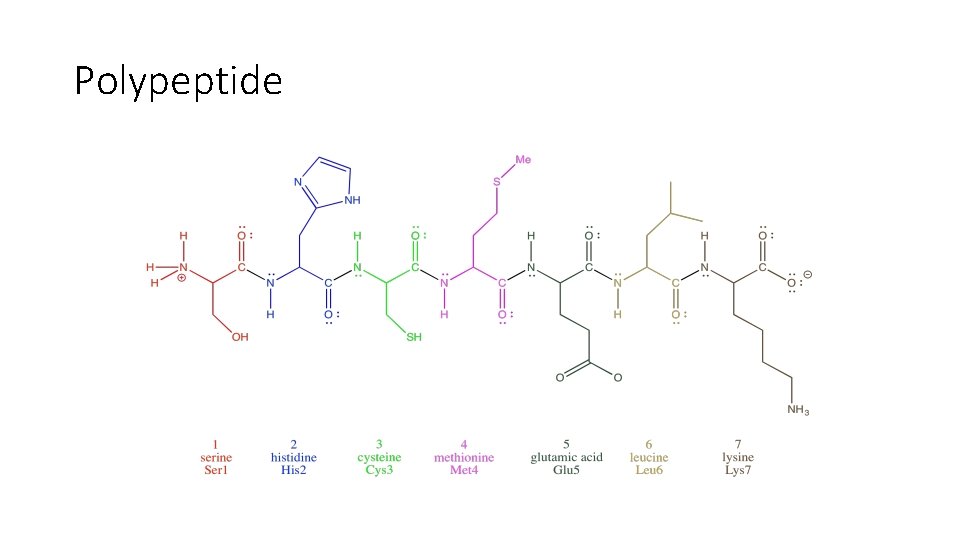
Polypeptide

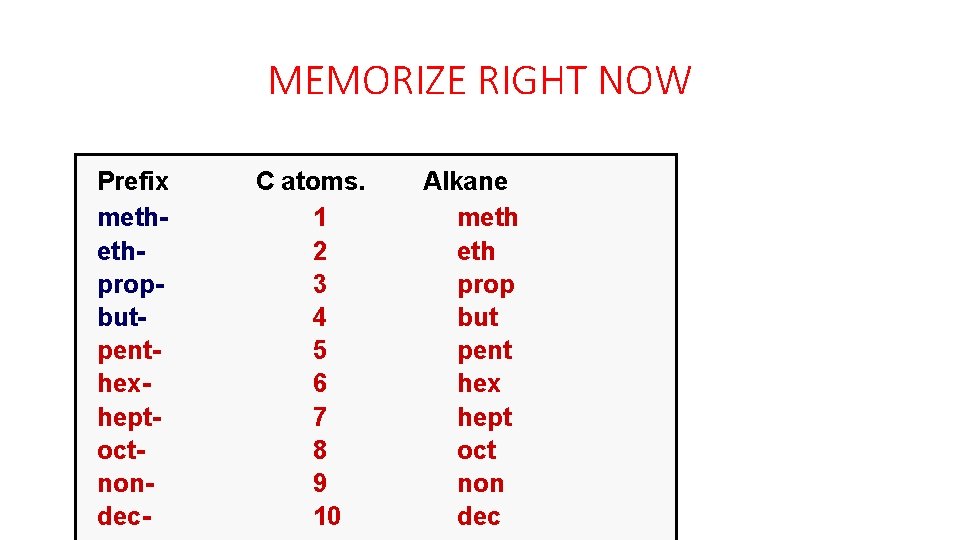
MEMORIZE RIGHT NOW Prefix methethpropbutpenthexheptoctnondec- C atoms. 1 2 3 4 5 6 7 8 9 10 Alkane meth prop but pent hex hept oct non dec

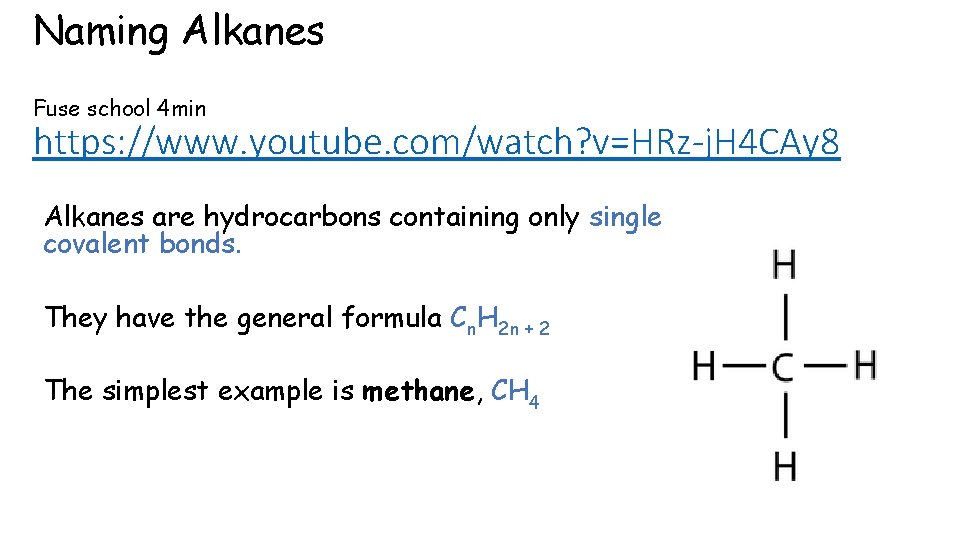
Naming Alkanes Fuse school 4 min https: //www. youtube. com/watch? v=HRz-j. H 4 CAy 8 Alkanes are hydrocarbons containing only single covalent bonds. They have the general formula Cn. H 2 n + 2 The simplest example is methane, CH 4

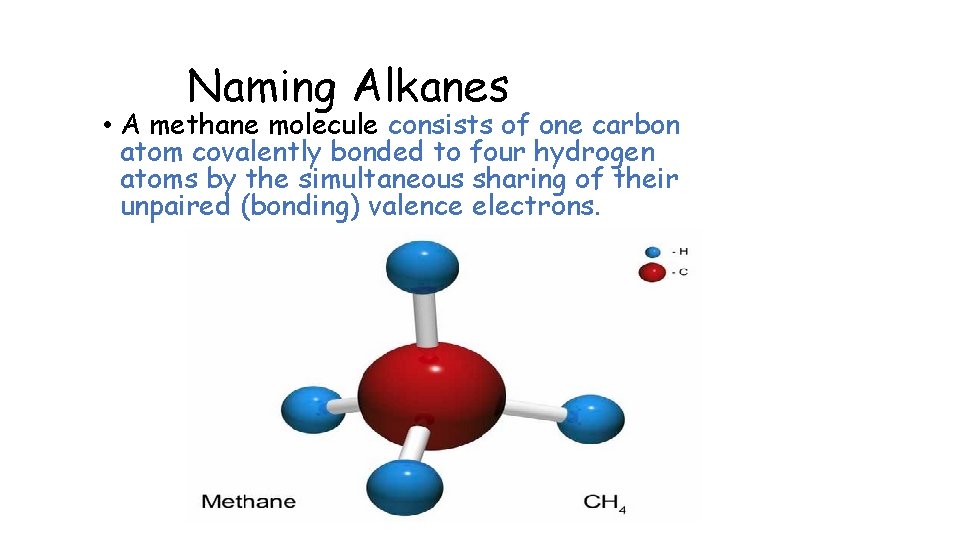
Naming Alkanes • A methane molecule consists of one carbon atom covalently bonded to four hydrogen atoms by the simultaneous sharing of their unpaired (bonding) valence electrons.

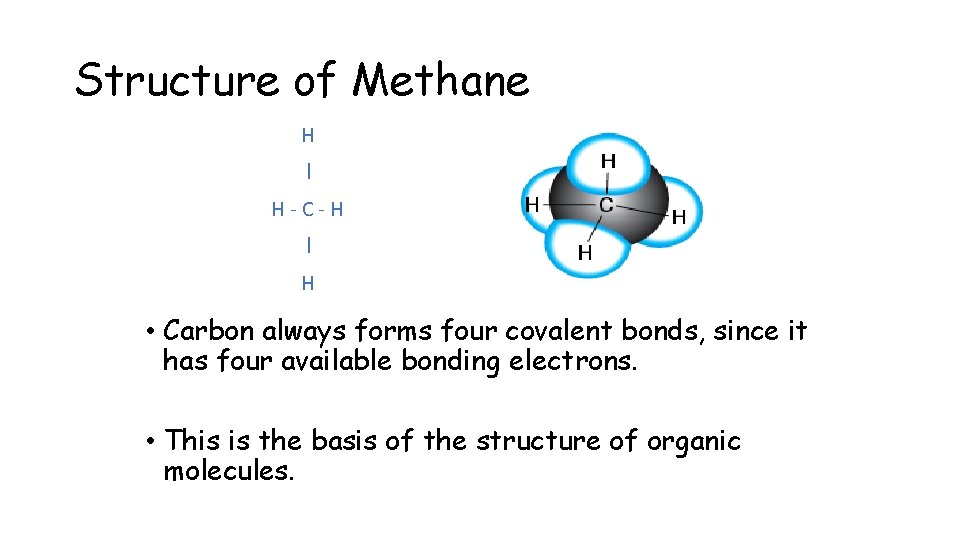
Structure of Methane H I H-C-H I H • Carbon always forms four covalent bonds, since it has four available bonding electrons. • This is the basis of the structure of organic molecules.

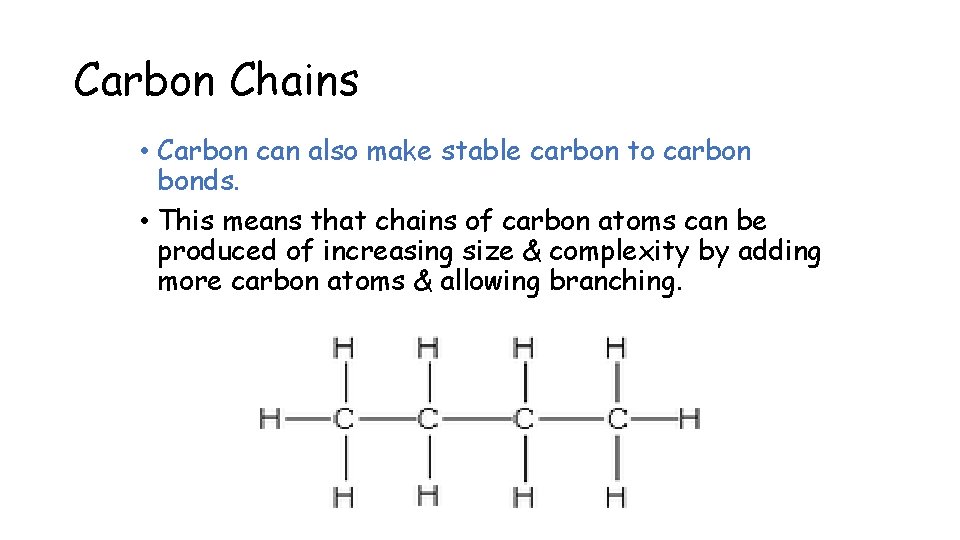
Carbon Chains • Carbon can also make stable carbon to carbon bonds. • This means that chains of carbon atoms can be produced of increasing size & complexity by adding more carbon atoms & allowing branching.

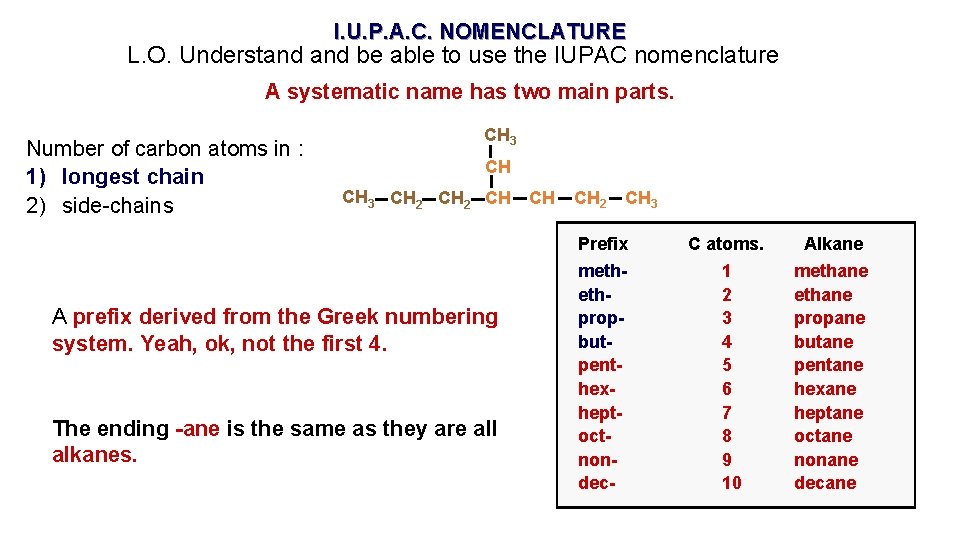
I. U. P. A. C. NOMENCLATURE L. O. Understand be able to use the IUPAC nomenclature A systematic name has two main parts. Number of carbon atoms in : 1) longest chain 2) side-chains CH 3 CH 2 CH A prefix derived from the Greek numbering system. Yeah, ok, not the first 4. The ending -ane is the same as they are all alkanes. CH CH 2 CH 3 Prefix methethpropbutpenthexheptoctnondec- C atoms. 1 2 3 4 5 6 7 8 9 10 Alkane methane propane butane pentane hexane heptane octane nonane decane

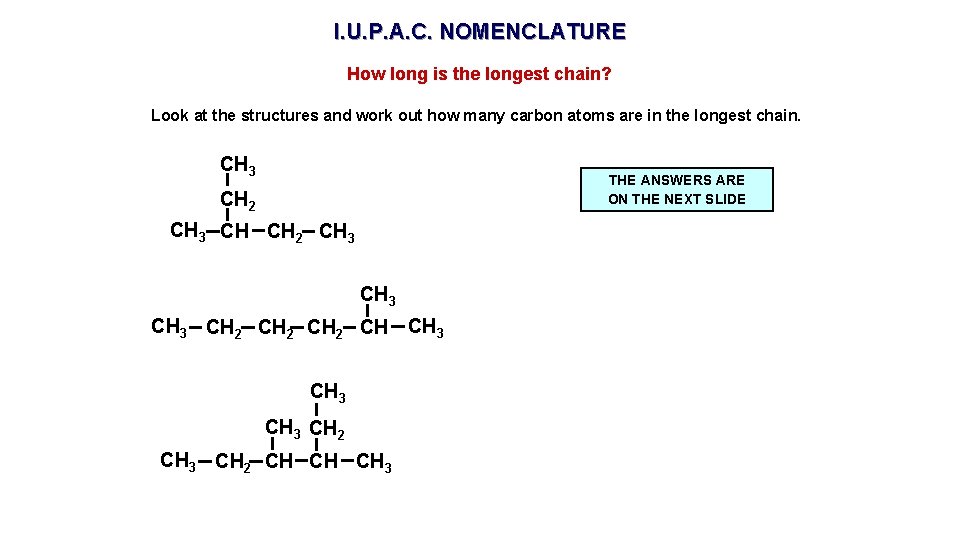
I. U. P. A. C. NOMENCLATURE How long is the longest chain? Look at the structures and work out how many carbon atoms are in the longest chain. CH 3 THE ANSWERS ARE ON THE NEXT SLIDE CH 2 CH 3 CH 2 CH CH 3 CH 2 CH CH CH 3

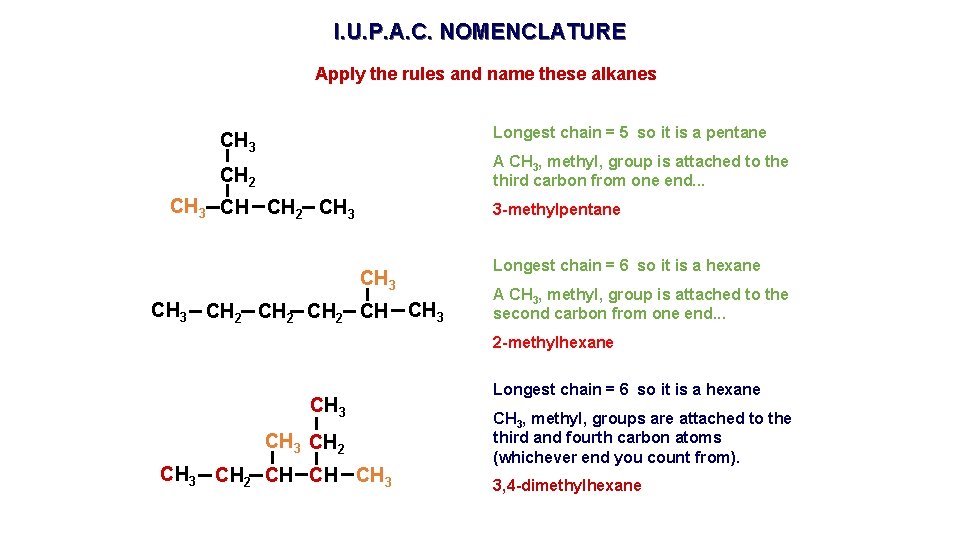
I. U. P. A. C. NOMENCLATURE Apply the rules and name these alkanes Longest chain = 5 so it is a pentane CH 3 A CH 3, methyl, group is attached to the third carbon from one end. . . CH 2 CH 3 CH CH 2 CH 3 3 -methylpentane CH 3 CH 2 CH CH 3 Longest chain = 6 so it is a hexane A CH 3, methyl, group is attached to the second carbon from one end. . . 2 -methylhexane CH 3 CH 2 CH CH CH 3 Longest chain = 6 so it is a hexane CH 3, methyl, groups are attached to the third and fourth carbon atoms (whichever end you count from). 3, 4 -dimethylhexane

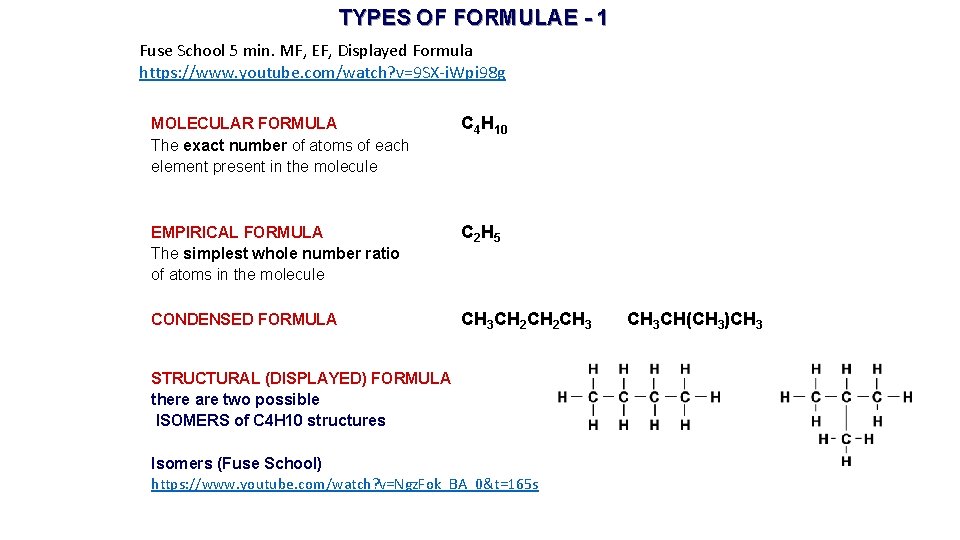
TYPES OF FORMULAE - 1 Fuse School 5 min. MF, EF, Displayed Formula https: //www. youtube. com/watch? v=9 SX-i. Wpi 98 g MOLECULAR FORMULA The exact number of atoms of each element present in the molecule C 4 H 10 EMPIRICAL FORMULA The simplest whole number ratio of atoms in the molecule C 2 H 5 CONDENSED FORMULA CH 3 CH 2 CH 3 STRUCTURAL (DISPLAYED) FORMULA there are two possible ISOMERS of C 4 H 10 structures Isomers (Fuse School) https: //www. youtube. com/watch? v=Ngz. Fok_BA_0&t=165 s CH 3 CH(CH 3)CH 3

Alkanes - Nomenclature 1. The parent name, the longest continuous chain. 2. The suffix indicates what functional group is present. 3. The prefix tells us the identity, location, and number of substituents attached to the carbon chain.

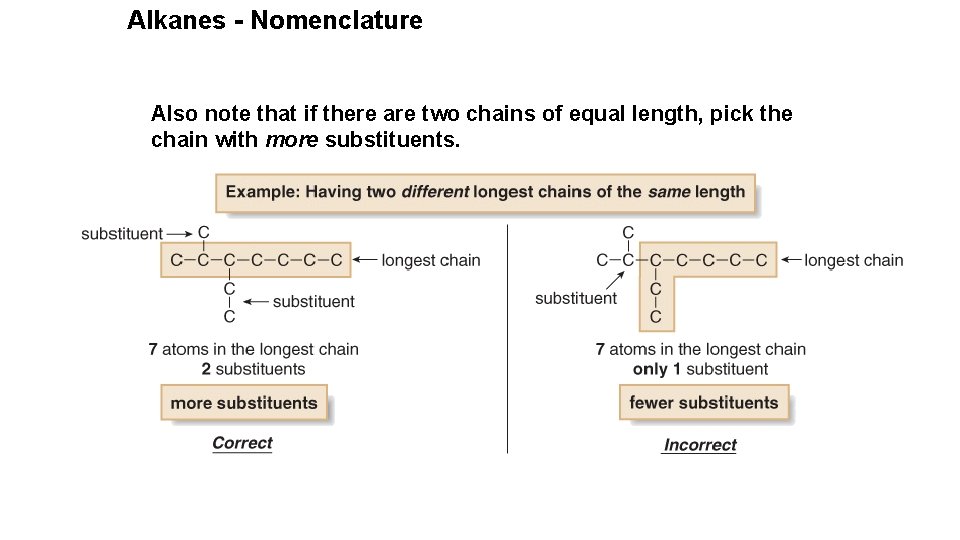
Alkanes - Nomenclature Also note that if there are two chains of equal length, pick the chain with more substituents.

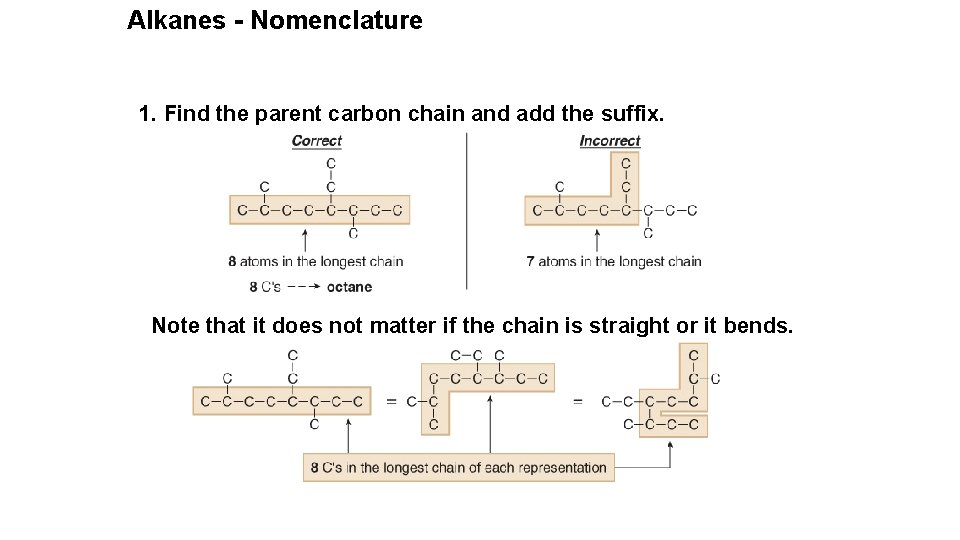
Alkanes - Nomenclature 1. Find the parent carbon chain and add the suffix. Note that it does not matter if the chain is straight or it bends.

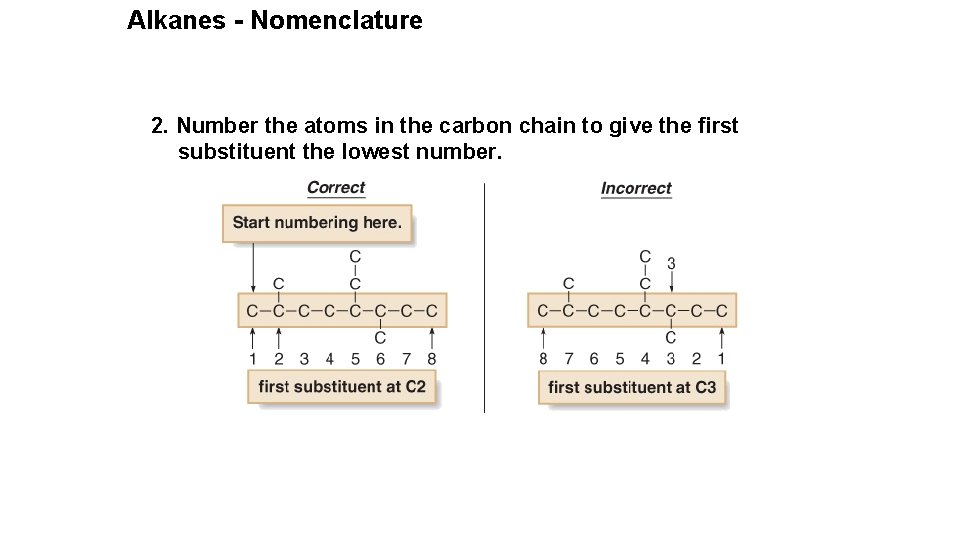
Alkanes - Nomenclature 2. Number the atoms in the carbon chain to give the first substituent the lowest number.

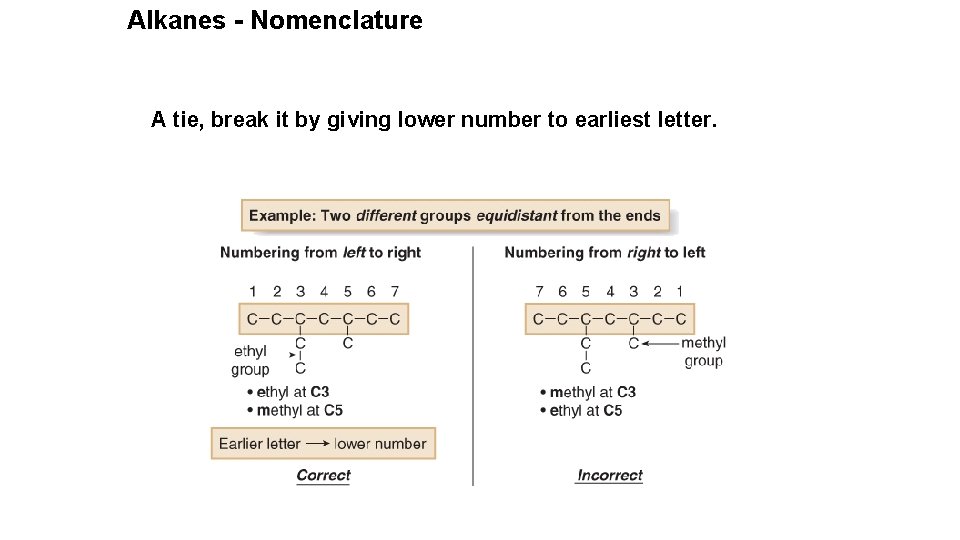
Alkanes - Nomenclature A tie, break it by giving lower number to earliest letter.

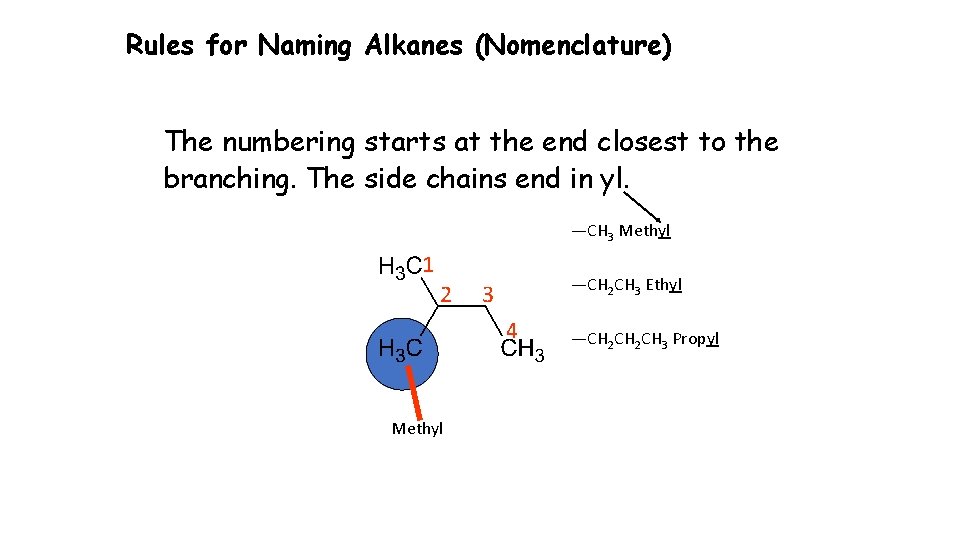
Rules for Naming Alkanes (Nomenclature) The numbering starts at the end closest to the branching. The side chains end in yl. —CH 3 Methyl 1 2 —CH 2 CH 3 Ethyl 3 4 Methyl —CH 2 CH 3 Propyl

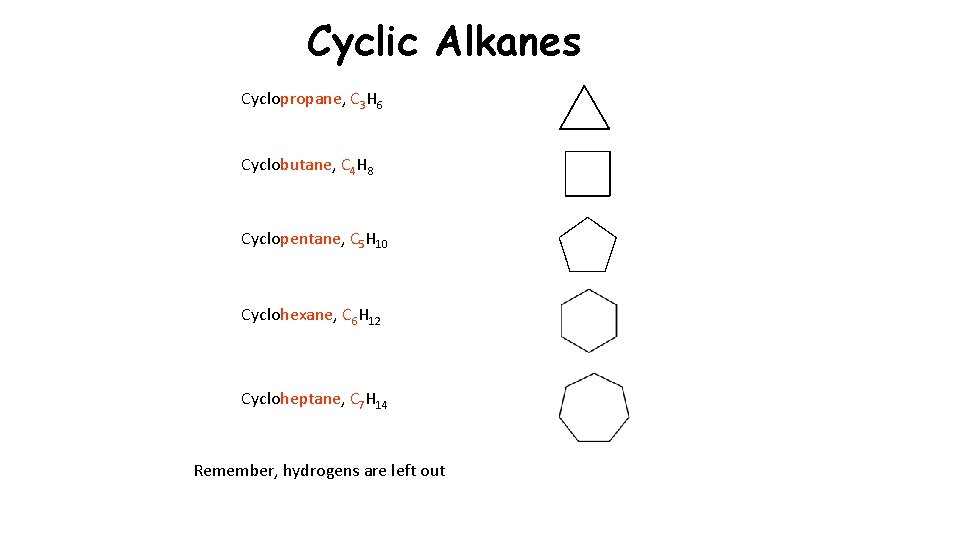
Cyclic Alkanes Cyclopropane, C 3 H 6 Cyclobutane, C 4 H 8 Cyclopentane, C 5 H 10 Cyclohexane, C 6 H 12 Cycloheptane, C 7 H 14 Remember, hydrogens are left out

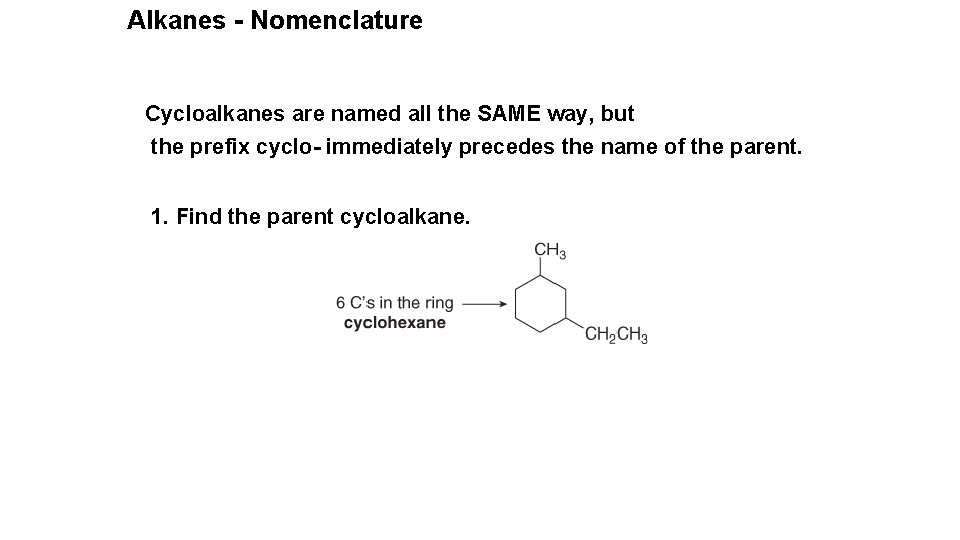
Alkanes - Nomenclature Cycloalkanes are named all the SAME way, but the prefix cyclo- immediately precedes the name of the parent. 1. Find the parent cycloalkane.

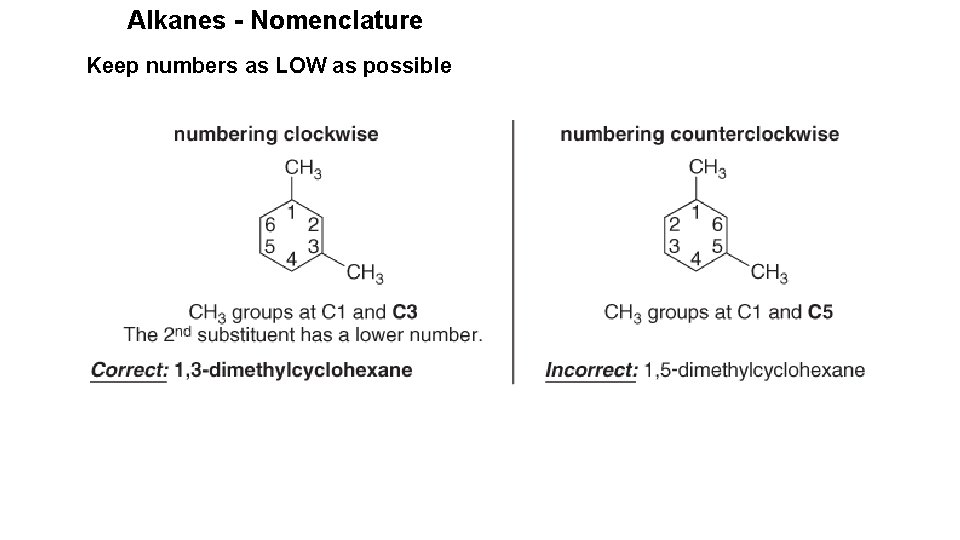
Alkanes - Nomenclature Keep numbers as LOW as possible

ALKENES

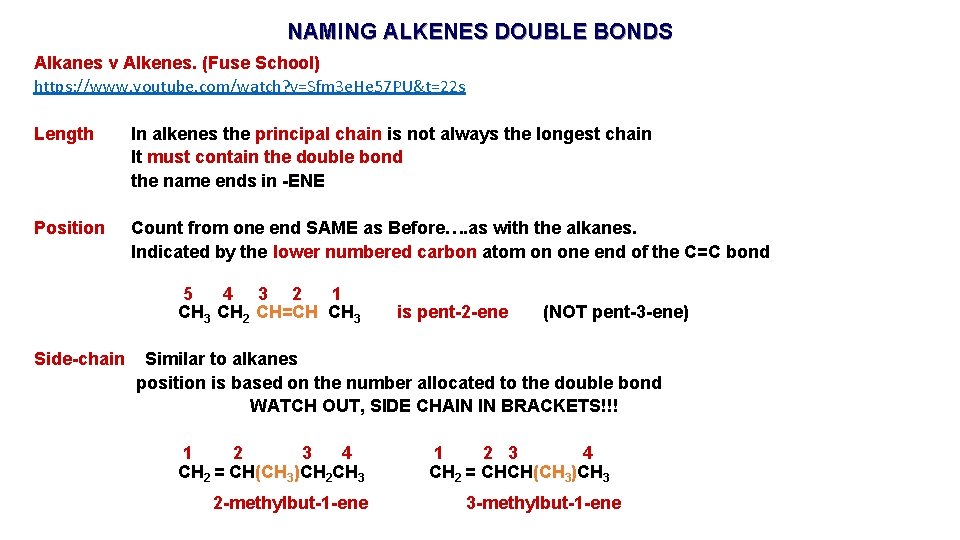
NAMING ALKENES DOUBLE BONDS Alkanes v Alkenes. (Fuse School) https: //www. youtube. com/watch? v=Sfm 3 e. He 57 PU&t=22 s Length In alkenes the principal chain is not always the longest chain It must contain the double bond the name ends in -ENE Position Count from one end SAME as Before…. as with the alkanes. Indicated by the lower numbered carbon atom on one end of the C=C bond 5 4 3 2 1 CH 3 CH 2 CH=CH CH 3 Side-chain is pent-2 -ene (NOT pent-3 -ene) Similar to alkanes position is based on the number allocated to the double bond WATCH OUT, SIDE CHAIN IN BRACKETS!!! 1 2 3 4 CH 2 = CH(CH 3)CH 2 CH 3 2 -methylbut-1 -ene 1 2 3 4 CH 2 = CHCH(CH 3)CH 3 3 -methylbut-1 -ene

Alkynes - Nomenclature • Alkynes are named in the same general way that alkenes are named; just change the suffix to –yne.

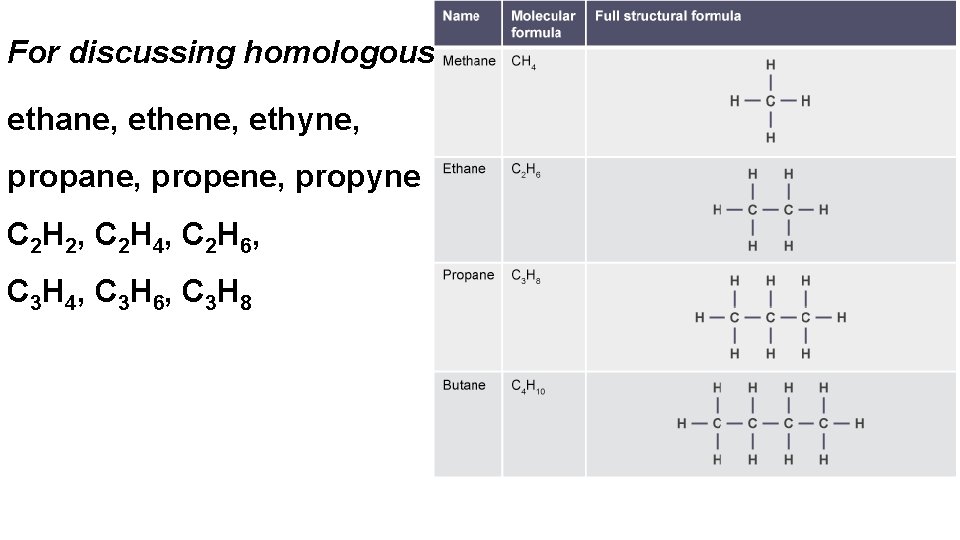
For discussing homologous ethane, ethene, ethyne, propane, propene, propyne C 2 H 2, C 2 H 4, C 2 H 6, C 3 H 4, C 3 H 6, C 3 H 8

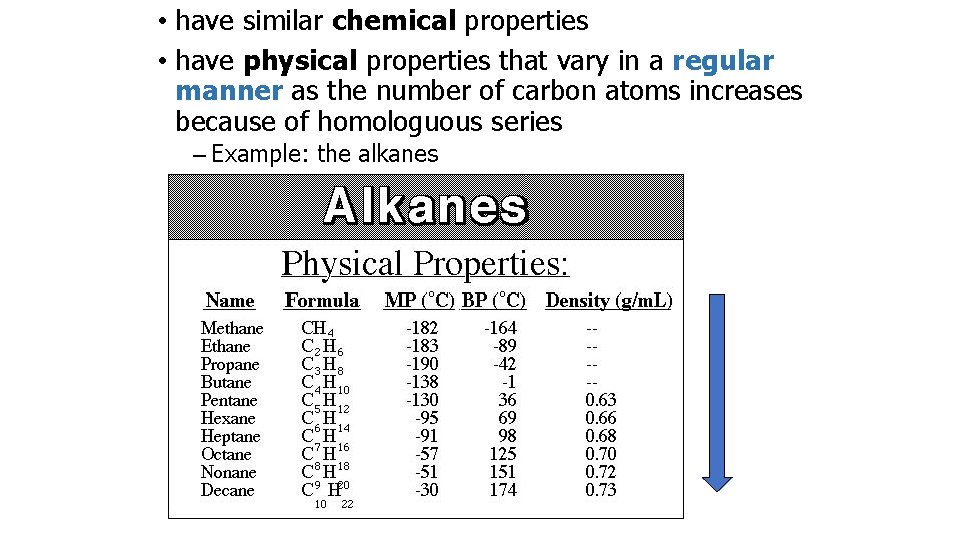
• have similar chemical properties • have physical properties that vary in a regular manner as the number of carbon atoms increases because of homologuous series – Example: the alkanes

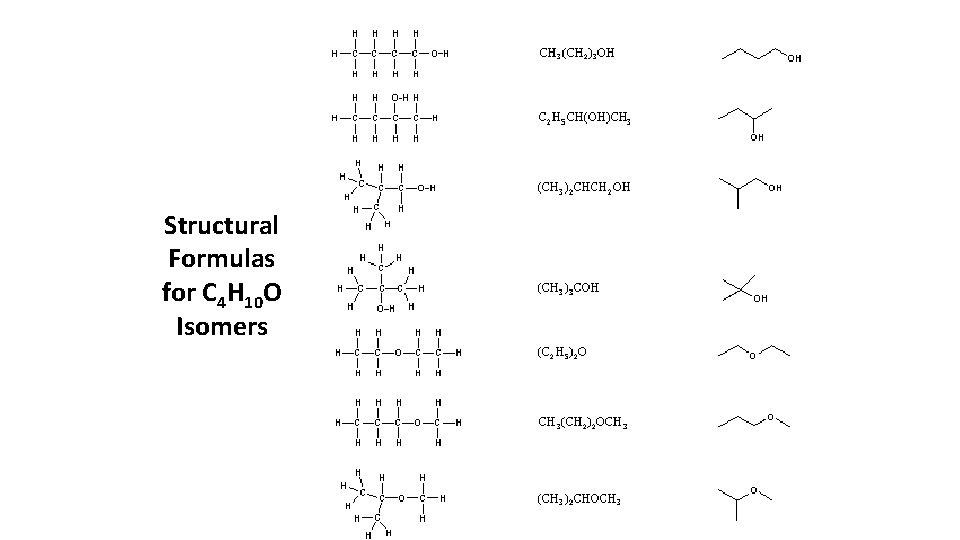
Structural Formulas for C 4 H 10 O Isomers

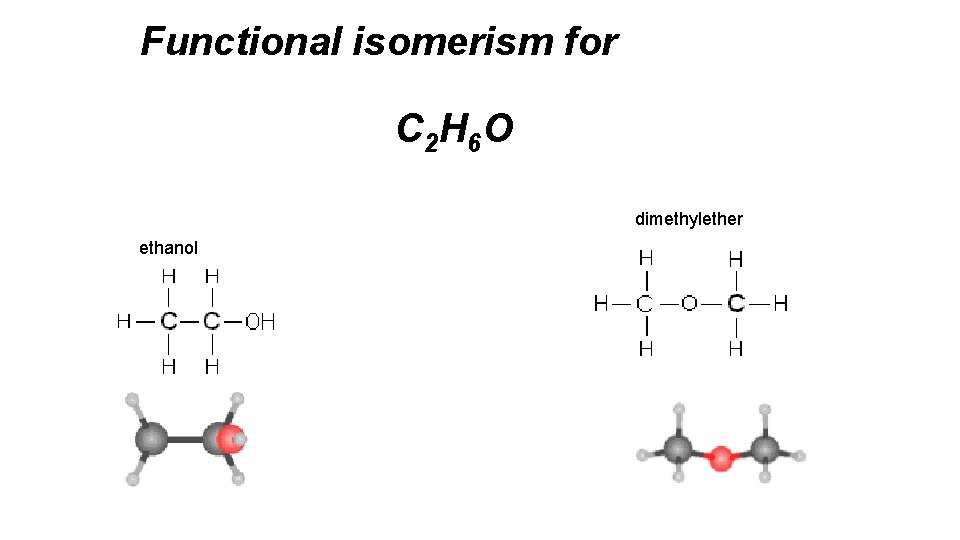
Functional isomerism for C 2 H 6 O dimethylether ethanol

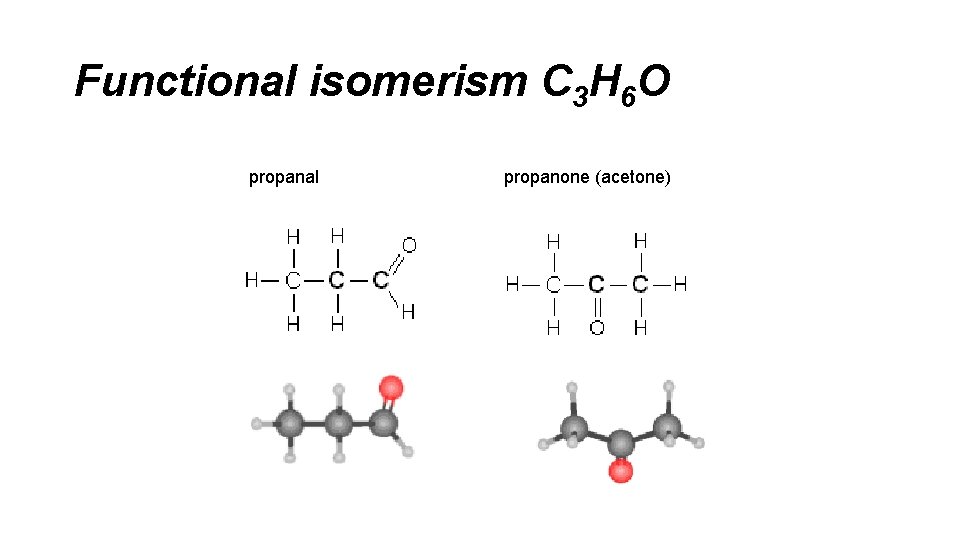
Functional isomerism C 3 H 6 O propanal propanone (acetone)

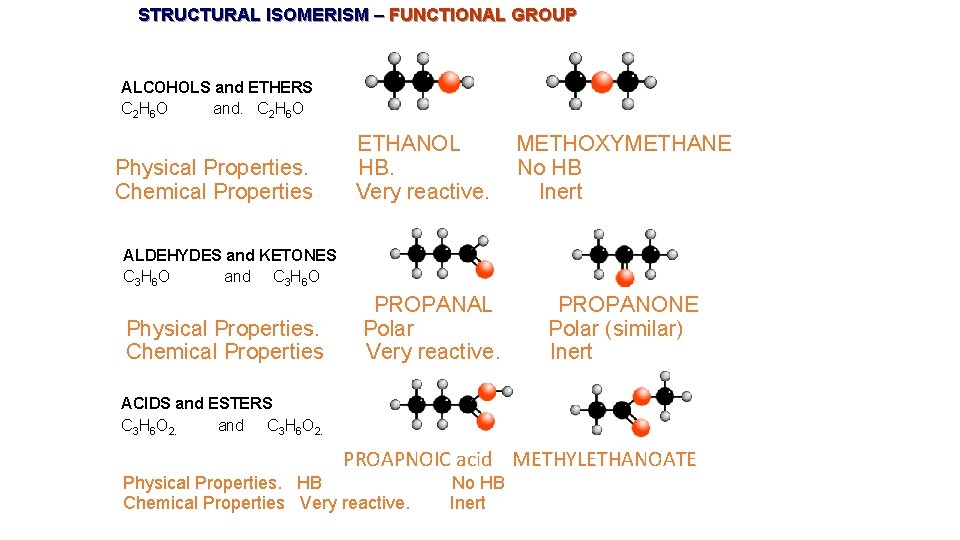
STRUCTURAL ISOMERISM – FUNCTIONAL GROUP ALCOHOLS and ETHERS C 2 H 6 O and. C 2 H 6 O Physical Properties. Chemical Properties ETHANOL HB. Very reactive. METHOXYMETHANE No HB Inert ALDEHYDES and KETONES C 3 H 6 O and C 3 H 6 O Physical Properties. Chemical Properties PROPANAL Polar Very reactive. PROPANONE Polar (similar) Inert ACIDS and ESTERS C 3 H 6 O 2. and C 3 H 6 O 2. PROAPNOIC acid METHYLETHANOATE Physical Properties. HB Chemical Properties Very reactive. No HB Inert

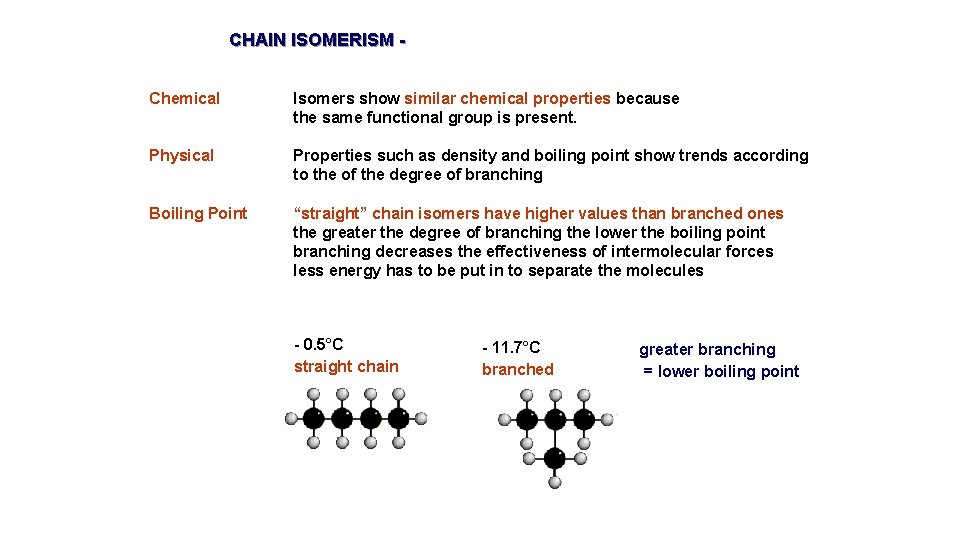
CHAIN ISOMERISM Chemical Isomers show similar chemical properties because the same functional group is present. Physical Properties such as density and boiling point show trends according to the of the degree of branching Boiling Point “straight” chain isomers have higher values than branched ones the greater the degree of branching the lower the boiling point branching decreases the effectiveness of intermolecular forces less energy has to be put in to separate the molecules - 0. 5°C straight chain - 11. 7°C branched greater branching = lower boiling point

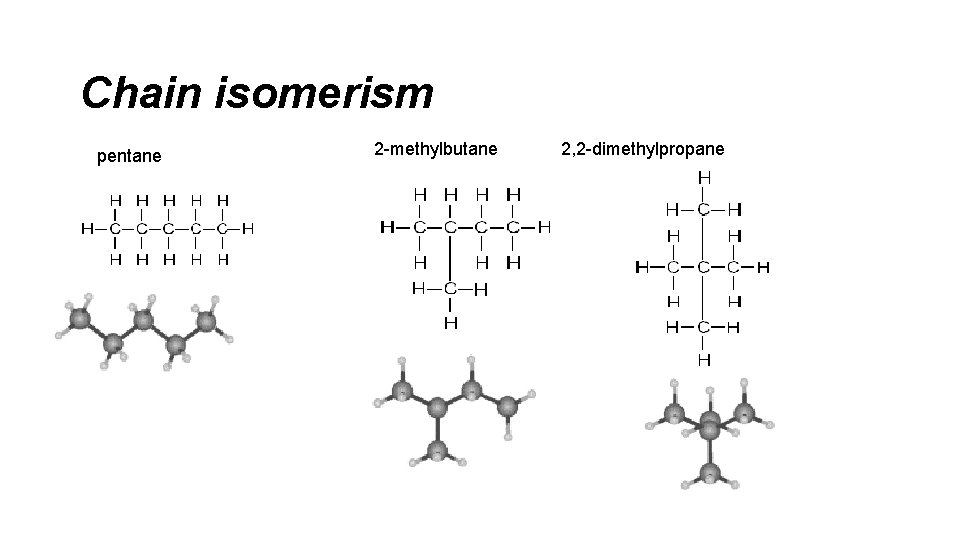
Chain isomerism pentane 2 -methylbutane 2, 2 -dimethylpropane

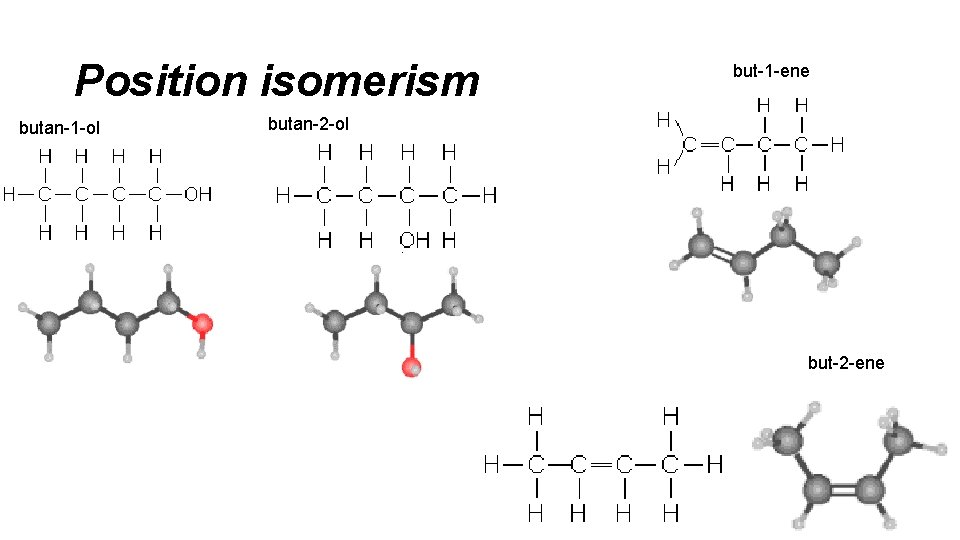
Position isomerism butan-1 -ol but-1 -ene butan-2 -ol but-2 -ene

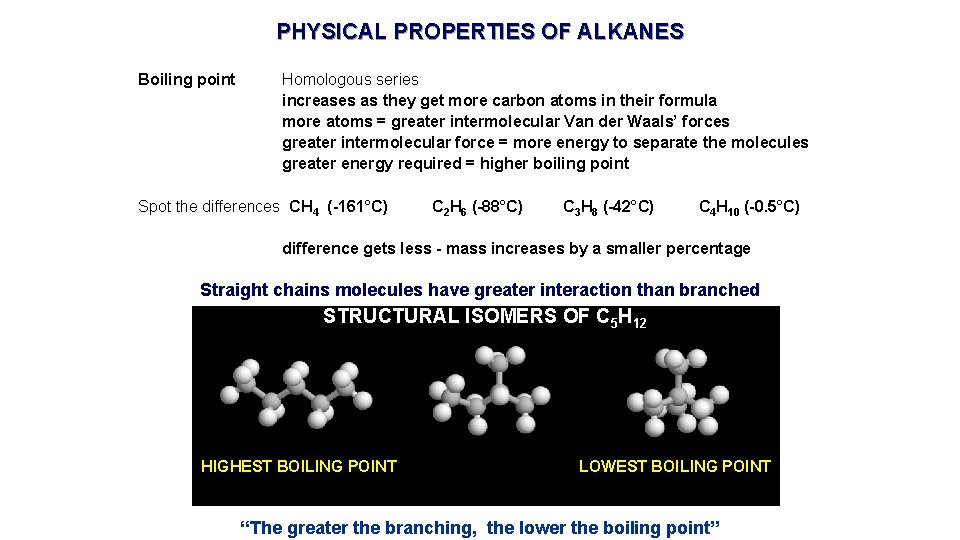
PHYSICAL PROPERTIES OF ALKANES Boiling point Homologous series increases as they get more carbon atoms in their formula more atoms = greater intermolecular Van der Waals’ forces greater intermolecular force = more energy to separate the molecules greater energy required = higher boiling point Spot the differences CH 4 (-161°C) C 2 H 6 (-88°C) C 3 H 8 (-42°C) C 4 H 10 (-0. 5°C) difference gets less - mass increases by a smaller percentage Straight chains molecules have greater interaction than branched STRUCTURAL ISOMERS OF C 5 H 12 HIGHEST BOILING POINT LOWEST BOILING POINT “The greater the branching, the lower the boiling point”

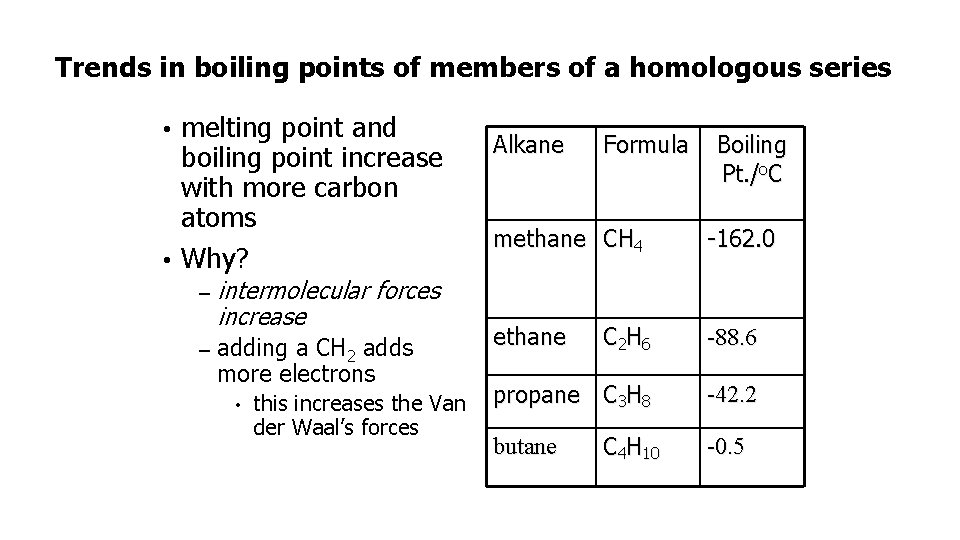
Trends in boiling points of members of a homologous series melting point and boiling point increase with more carbon atoms • Why? • – intermolecular forces increase – adding a CH 2 adds more electrons • this increases the Van der Waal’s forces Alkane Formula Boiling Pt. /o. C methane CH 4 -162. 0 ethane C 2 H 6 -88. 6 propane C 3 H 8 -42. 2 butane C 4 H 10 -0. 5

Functional Groups (Fuse Schol) https: //www. youtube. com/watch? v=n. MTQKBn 2 Iss&t=140 s

• Alcohols -OH hydroxyl • Organic acids -COOH carboxyl • Aldehydes -CHO • Ethers O • Ketones -C-O- • Esters • Amines • Amides carbonyl O -C-O -N- O -C-NH
 Preference order of functional groups
Preference order of functional groups Isomers of pentane
Isomers of pentane Aldehydes and ketones structure
Aldehydes and ketones structure Nomenclature
Nomenclature Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Arrhenius theory
Arrhenius theory Zero sum rule
Zero sum rule Organic chemistry prefix
Organic chemistry prefix Soda-lime test
Soda-lime test Organic chemistry chapter 9
Organic chemistry chapter 9 Functional groups
Functional groups Extraction of caffeine from vivarin tablets lab report
Extraction of caffeine from vivarin tablets lab report Organic chemistry
Organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Which allotrope of carbon feels greasy and crumbles easily?
Which allotrope of carbon feels greasy and crumbles easily? Wiley
Wiley Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Chemistry mind map
Chemistry mind map Organic chemistry introduction
Organic chemistry introduction Analytical chemistry chapter 1
Analytical chemistry chapter 1 Brooklyn college organic chemistry
Brooklyn college organic chemistry David klein organic chemistry
David klein organic chemistry Organic chemistry
Organic chemistry Iodine test for starch
Iodine test for starch Nbs chemistry
Nbs chemistry Neon organic or inorganic
Neon organic or inorganic Rhodopsin
Rhodopsin Organic chemistry myanmar
Organic chemistry myanmar Hono organic chemistry
Hono organic chemistry Organic chemistry
Organic chemistry Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Resonance in benzyl carbocation
Resonance in benzyl carbocation Soap organic chemistry
Soap organic chemistry Cis-2 3-dimethyloxirane
Cis-2 3-dimethyloxirane Chemistry-ethics case studies
Chemistry-ethics case studies Organic chemistry class 11 notes
Organic chemistry class 11 notes A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis How to calculate percentage yield in organic chemistry
How to calculate percentage yield in organic chemistry David klein
David klein Organic chemistry reaction pathways
Organic chemistry reaction pathways Organic chemistry (3rd) edition chapter 2 problem 17s
Organic chemistry (3rd) edition chapter 2 problem 17s Kiliani fischer synthesis
Kiliani fischer synthesis Ee organic chemistry
Ee organic chemistry