Chemical Nomenclature and Chemical Formula Lesson Outline Writing





- Slides: 12

Chemical Nomenclature and Chemical Formula

Lesson Outline Writing and Naming Ionic Formulas Transition Metals Writing and Naming Molecular Formulas Polyatomic Ions

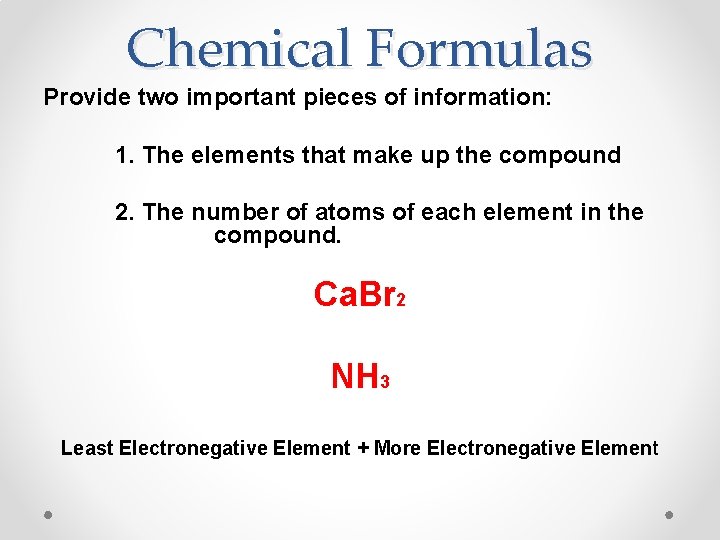
Chemical Formulas Provide two important pieces of information: 1. The elements that make up the compound 2. The number of atoms of each element in the compound. Ca. Br 2 NH 3 Least Electronegative Element + More Electronegative Element

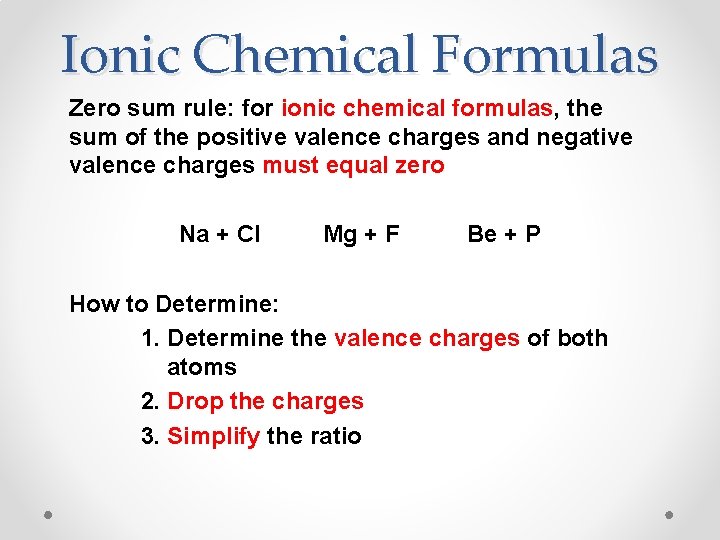
Ionic Chemical Formulas Zero sum rule: for ionic chemical formulas, the sum of the positive valence charges and negative valence charges must equal zero Na + Cl Mg + F Be + P How to Determine: 1. Determine the valence charges of both atoms 2. Drop the charges 3. Simplify the ratio

Naming Ionic Compounds metal + non metal Binary Compounds have two elements Naming Rules: 1. the metal element’s name does not change 2. The non metal’s ending changes to –ide Na. Cl Mg. F 2

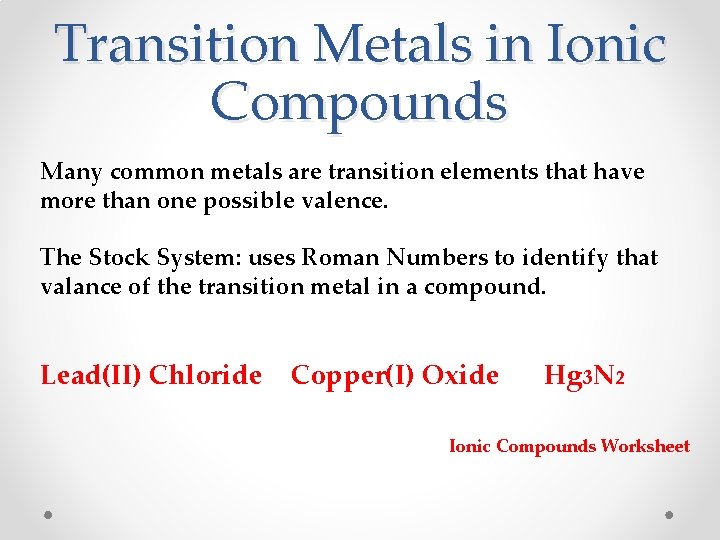
Transition Metals in Ionic Compounds Many common metals are transition elements that have more than one possible valence. The Stock System: uses Roman Numbers to identify that valance of the transition metal in a compound. Lead(II) Chloride Copper(I) Oxide Hg 3 N 2 Ionic Compounds Worksheet

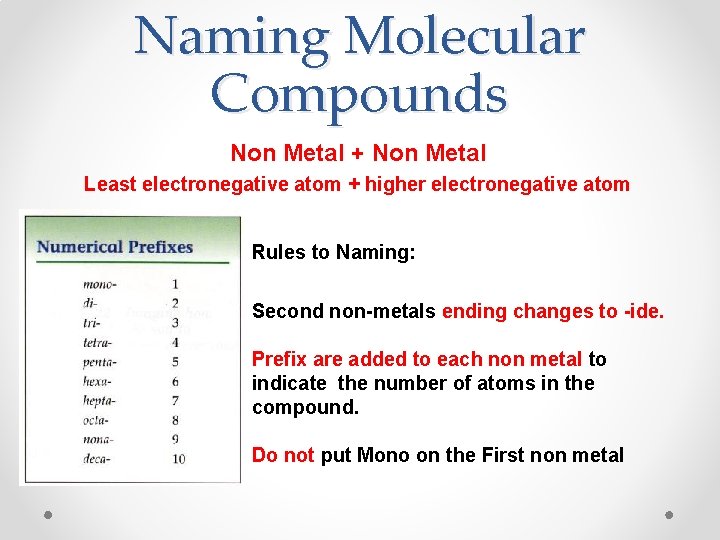
Naming Molecular Compounds Non Metal + Non Metal Least electronegative atom + higher electronegative atom Rules to Naming: Second non-metals ending changes to -ide. Prefix are added to each non metal to indicate the number of atoms in the compound. Do not put Mono on the First non metal

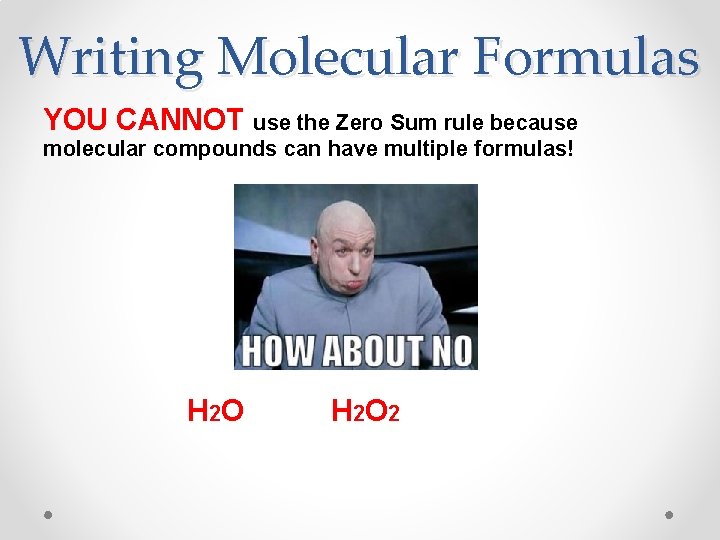
Writing Molecular Formulas YOU CANNOT use the Zero Sum rule because molecular compounds can have multiple formulas! H 2 O 2

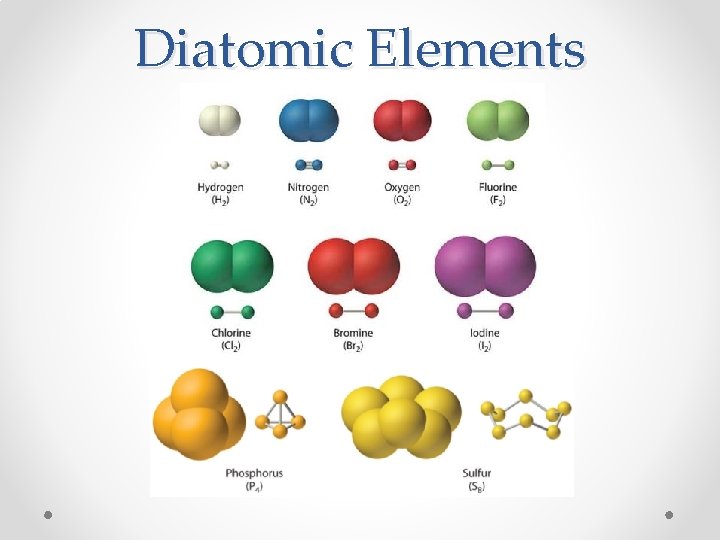
Diatomic Elements

Polyatomic Ions Polyatomic ions are essentially multiple atoms with a charge. Ex. NO 3 Nitrate HSO 3 Hydrogen sulfite SO 42 Sulfate Think of them as just another element! When naming polyatomic ions, the ending does not change to –ide! Na + NO 2 - Be + OH- Mg + PO 43 - Molecular Compounds Worksheet

Need to Know Molecules

Looking Forward Nomenclature of Hydrates and Oxyanions Nomenclature of Acids and Bases
 Outline the binomial system of nomenclature
Outline the binomial system of nomenclature Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Scrap heap magnet diagram
Scrap heap magnet diagram The sun-earth-moon system worksheet answers lesson 1
The sun-earth-moon system worksheet answers lesson 1 Lesson outline lesson 3 describing circuits answers
Lesson outline lesson 3 describing circuits answers Kind of fault
Kind of fault Lesson outline lesson 2 aquatic ecosystems answer key
Lesson outline lesson 2 aquatic ecosystems answer key Weather forecast lesson 3 outline answers
Weather forecast lesson 3 outline answers Lesson outline physical properties lesson 2
Lesson outline physical properties lesson 2 Lesson outline climates of earth
Lesson outline climates of earth Lesson outline lesson 1
Lesson outline lesson 1 Lesson 2 measurement and scientific tools
Lesson 2 measurement and scientific tools Lesson 1 land biomes answer key
Lesson 1 land biomes answer key