FORMULA WRITING Say what Formula Writing Ionic Compounds















- Slides: 19

FORMULA WRITING Say what? ?

Formula Writing Ionic Compounds One metal + one non-metal

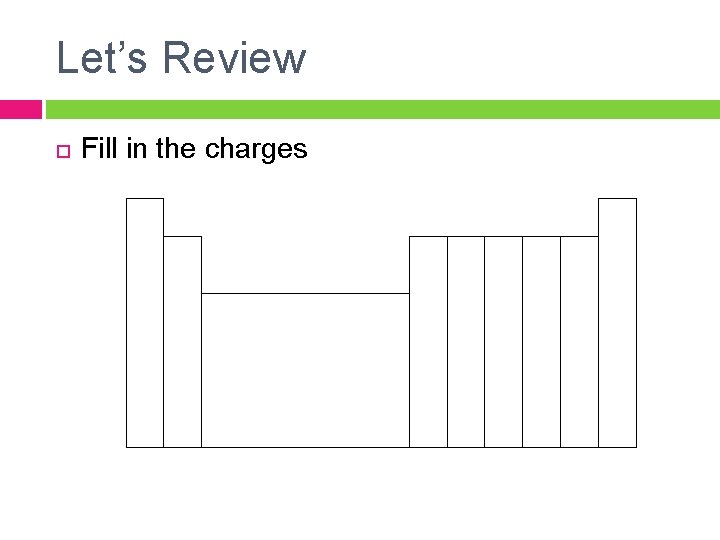
Let’s Review Fill in the charges

More Review How are ions formed? � Positive ions – Aka: � Negative ions – Aka:

Lets Write Some Formulas… Positive ions are ALWAYS written first. � If more than one ion can exist, use parentheses and the charge in roman numeral form Ie: Cu+2 is named copper (II) These are called multivalent ions. Negative ions are ALWAYS written second. So… � Can two positive ions ever be put together? � Can two negative ions ever be put together?

How do you know what numbers to use? Criss-Cross Method � Identify the charge of each ion � “criss-cross” the charges ignore the + and – Numbers become subscripts for other element Let’s do a few examples… pick any cation and anion:

Naming Compounds Cation stays the same Anion ending changes to –ide � Often this simply involves changing the –ine ending Chlorine chloride Fluorine fluoride Bromine bromide � However, there are some you need to remember: Examples: Phosphorus phosphide Sulfur sulfide Oxygen oxide

Let’s do some more practice… Charges Name Ca and Cl Ba and O Cu and I Criss Cross

Formula Writing Polyatomic Ion Compounds

Polyatomic Ions Remember: � Cation (positive ion) is written first � Anion (negative ion) is written second New: � Put parentheses around the polyatomic ion ALWAYS

Naming the Compounds with Polyatomics You need to recognize the Polyatomic Ions If monoatomic ion: cation and anion is the same If polyatomic ion: use names provided

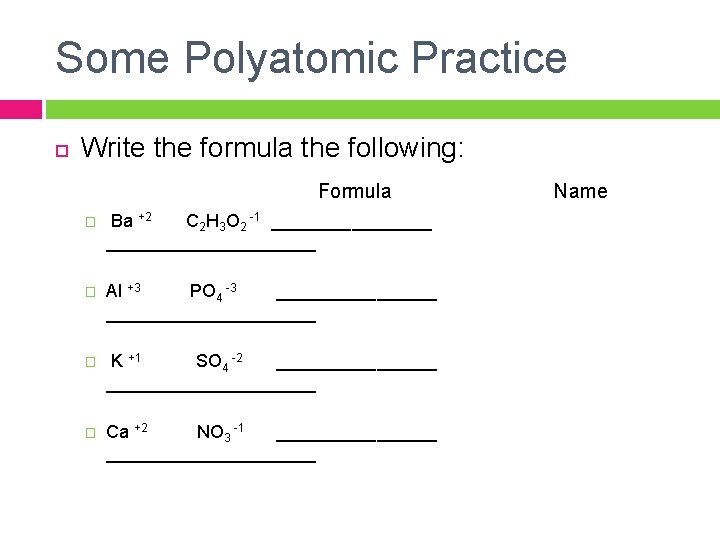
Some Polyatomic Practice Write the formula the following: Formula Name � Ba +2 C 2 H 3 O 2 -1 _____________________ � Al +3 PO 4 -3 _____________________ � K +1 SO 4 -2 _____________________ � Ca +2 NO 3 -1 _____________________

Formula Writing Covalent Molecules 2 non-metals together

Writing Formulas Remember, bonds require 2 electrons � Covalent is the sharing of electrons

Naming Covalent Compounds The less electronegative element is written first. � Remember the trend (increasing up and to the right) � Use a number indicating prefix only if more than one atom is present Ie N 2 is dinitrogen A prefix is always added to the name of the second element � Will change ending of name to –ide (just like ionic bonds)

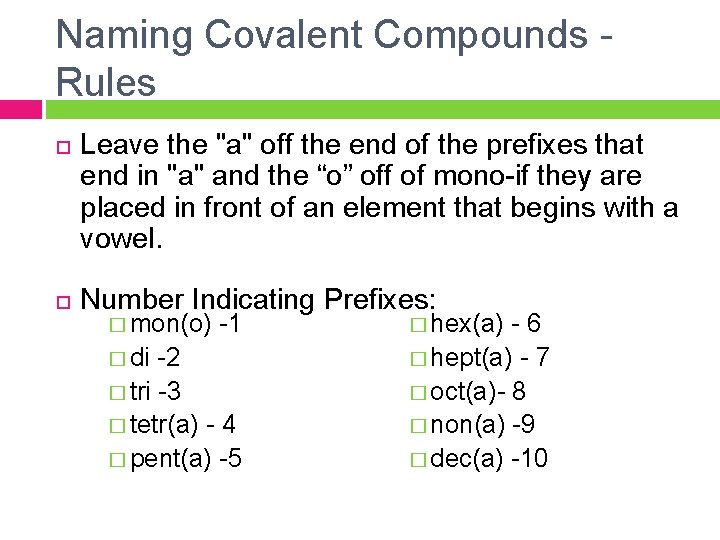
Naming Covalent Compounds - Rules Leave the "a" off the end of the prefixes that end in "a" and the “o” off of mono-if they are placed in front of an element that begins with a vowel. Number Indicating Prefixes: � mon(o) -1 � hex(a) - 6 � di -2 � hept(a) - 7 � tri -3 � oct(a)- 8 � tetr(a) - 4 � non(a) -9 � pent(a) -5 � dec(a) -10

Try Some Practice N 2 S 4 NI 3 CCl 4 P 2 O 5

Try Some Practice ~ ANSWERS N 2 S 4 Dinitrogen tetrasulfide NI 3 Nitrogen triiodide CCl 4 Carbon tetrachloride P 2 O 5 Diphosphorus pentoxide

Common Names H 2 O - water NH 3 - ammonia CH 4 - methane C 2 H 6 - ethane C 3 H 8 - propane