MSE 528 Fall 2010 Atomic Structure and Interatomic









- Slides: 57

MSE 528 Fall 2010










Atomic Structure and Interatomic Bonding ISSUES TO ADDRESS. . . • What promotes bonding? • What types of bonds are there? • What properties are inferred from bonding? 1

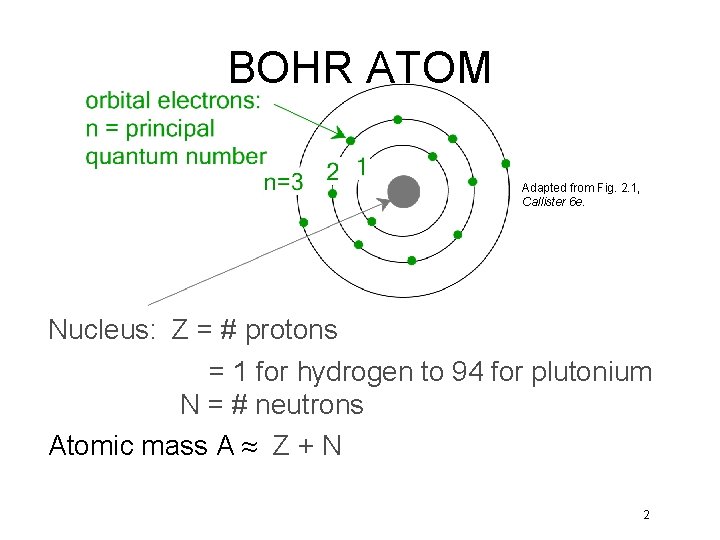
BOHR ATOM Adapted from Fig. 2. 1, Callister 6 e. Nucleus: Z = # protons = 1 for hydrogen to 94 for plutonium N = # neutrons Atomic mass A ≈ Z + N 2

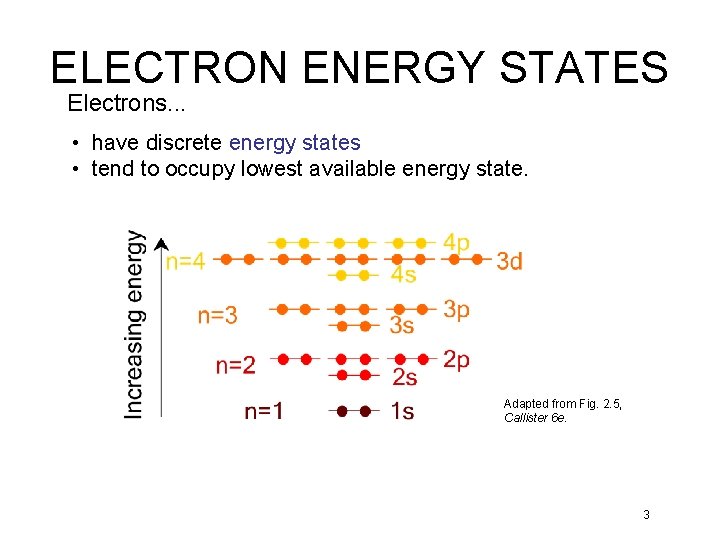
ELECTRON ENERGY STATES Electrons. . . • have discrete energy states • tend to occupy lowest available energy state. Adapted from Fig. 2. 5, Callister 6 e. 3

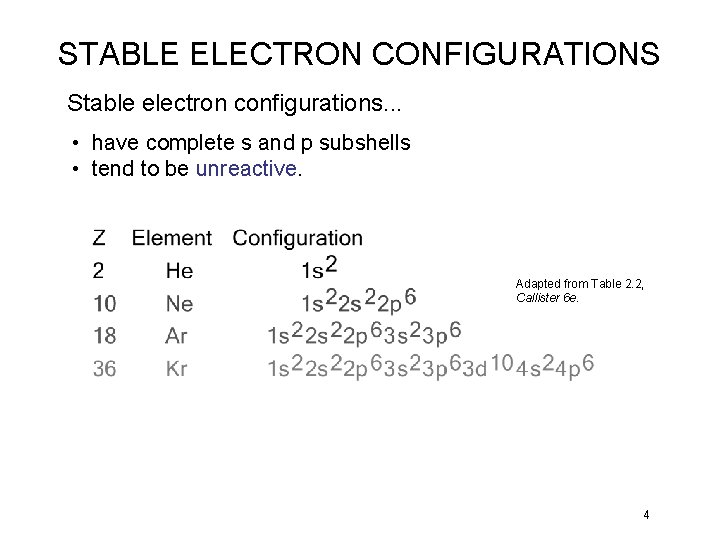
STABLE ELECTRON CONFIGURATIONS Stable electron configurations. . . • have complete s and p subshells • tend to be unreactive. Adapted from Table 2. 2, Callister 6 e. 4

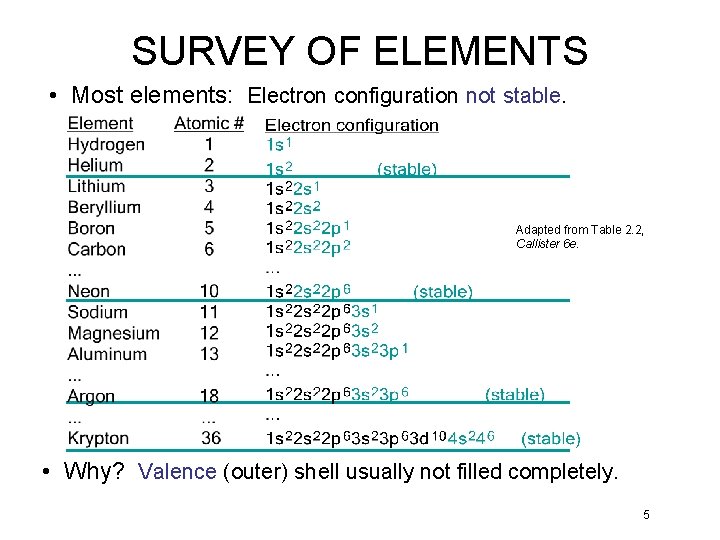
SURVEY OF ELEMENTS • Most elements: Electron configuration not stable. Adapted from Table 2. 2, Callister 6 e. • Why? Valence (outer) shell usually not filled completely. 5

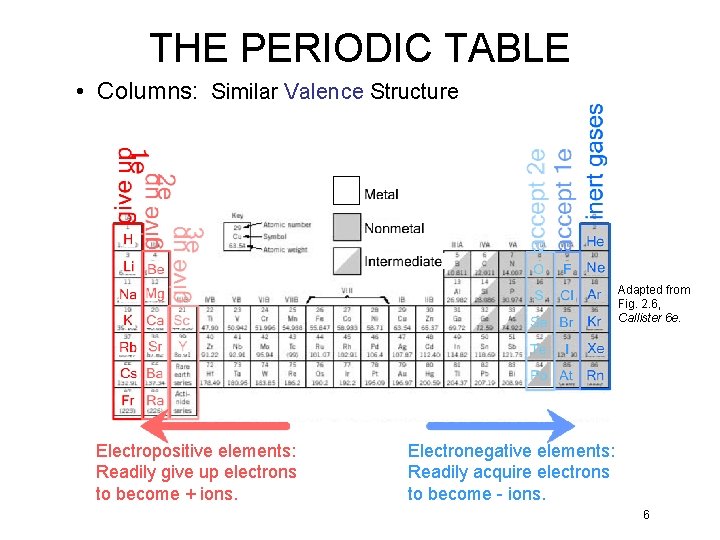
THE PERIODIC TABLE • Columns: Similar Valence Structure Adapted from Fig. 2. 6, Callister 6 e. Electropositive elements: Readily give up electrons to become + ions. Electronegative elements: Readily acquire electrons to become - ions. 6

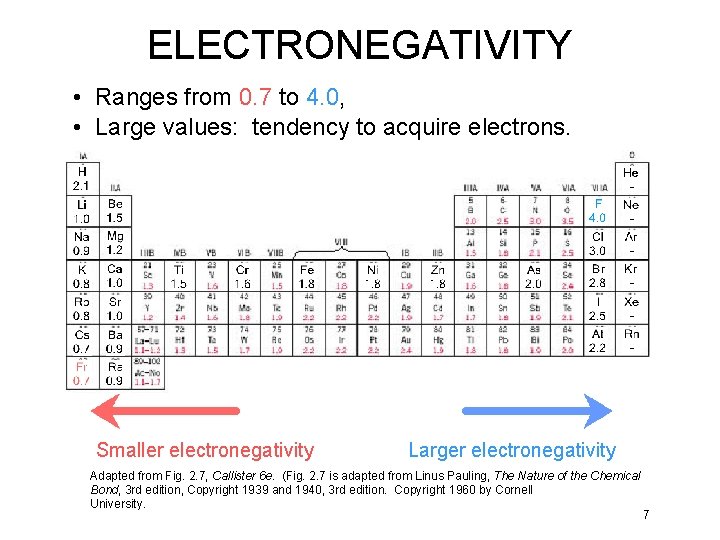
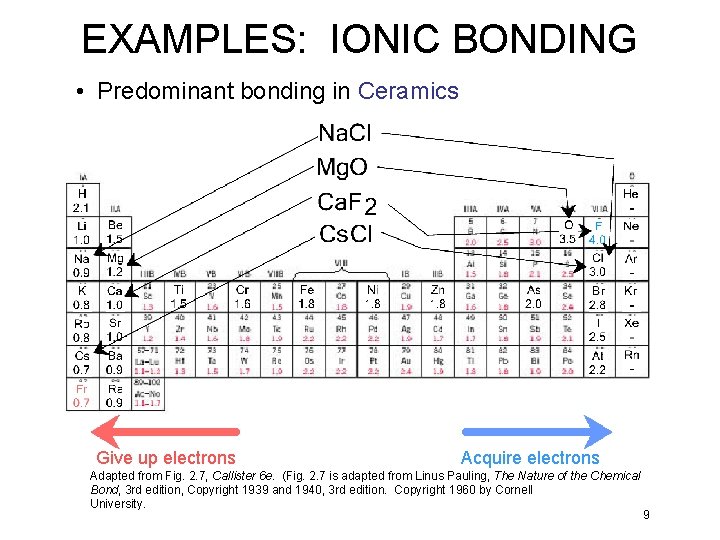
ELECTRONEGATIVITY • Ranges from 0. 7 to 4. 0, • Large values: tendency to acquire electrons. Smaller electronegativity Larger electronegativity Adapted from Fig. 2. 7, Callister 6 e. (Fig. 2. 7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3 rd edition, Copyright 1939 and 1940, 3 rd edition. Copyright 1960 by Cornell University. 7

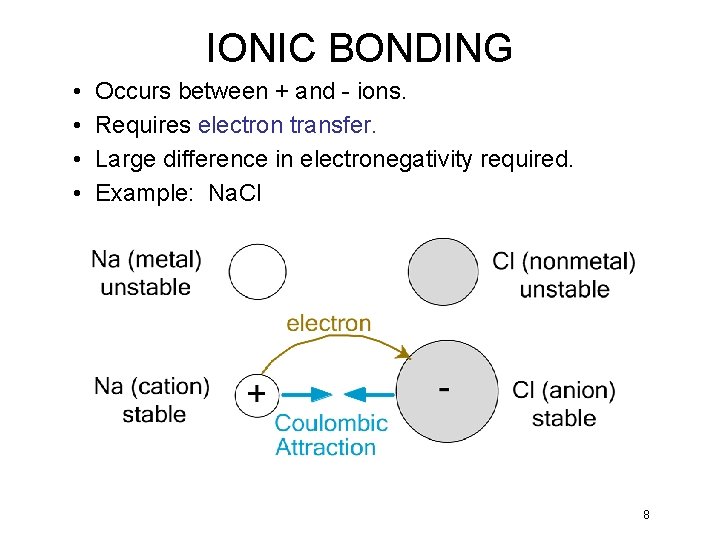
IONIC BONDING • • Occurs between + and - ions. Requires electron transfer. Large difference in electronegativity required. Example: Na. Cl 8

EXAMPLES: IONIC BONDING • Predominant bonding in Ceramics Give up electrons Acquire electrons Adapted from Fig. 2. 7, Callister 6 e. (Fig. 2. 7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3 rd edition, Copyright 1939 and 1940, 3 rd edition. Copyright 1960 by Cornell University. 9

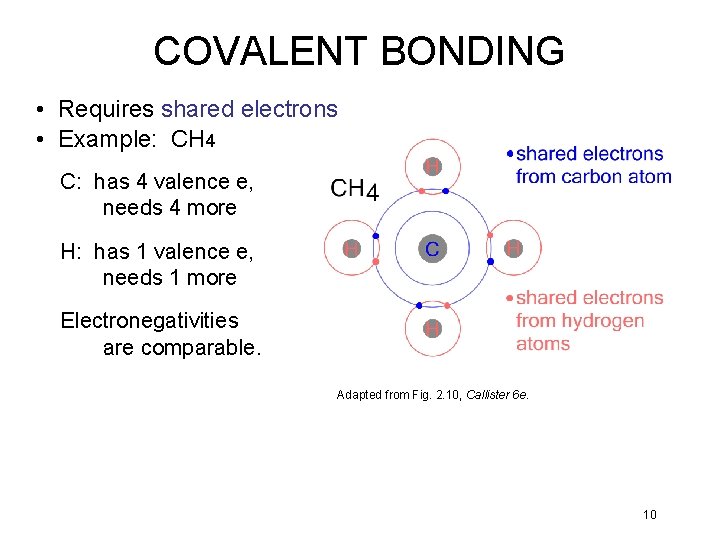
COVALENT BONDING • Requires shared electrons • Example: CH 4 C: has 4 valence e, needs 4 more H: has 1 valence e, needs 1 more Electronegativities are comparable. Adapted from Fig. 2. 10, Callister 6 e. 10

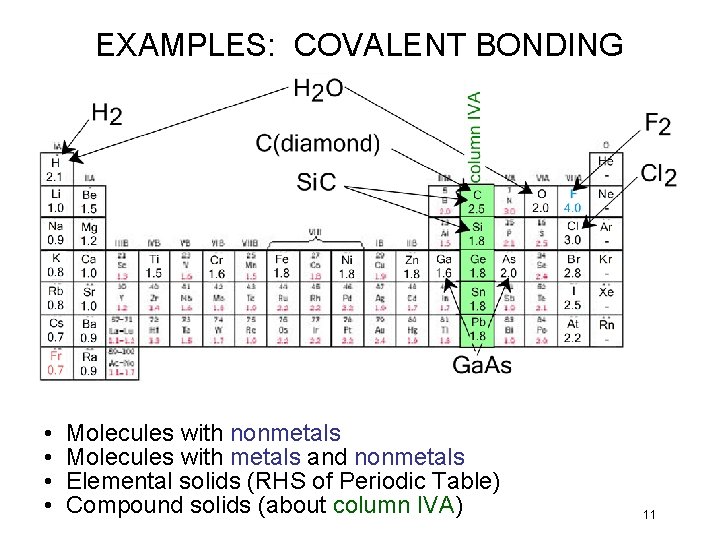
EXAMPLES: COVALENT BONDING • • Molecules with nonmetals Molecules with metals and nonmetals Elemental solids (RHS of Periodic Table) Compound solids (about column IVA) 11

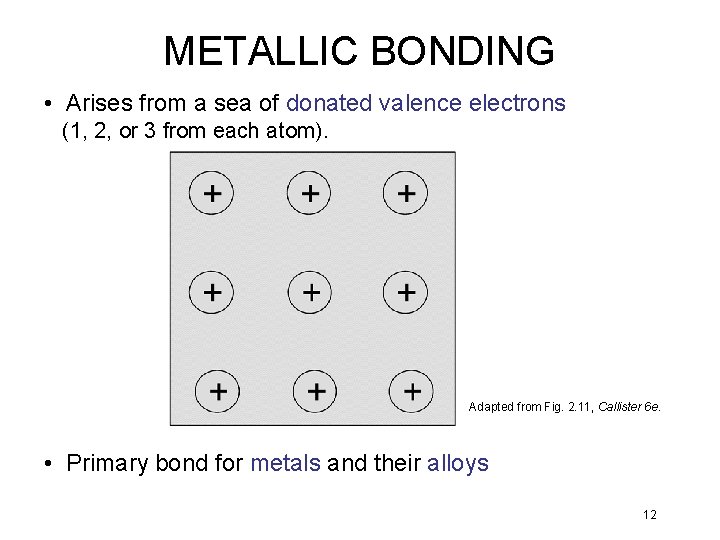
METALLIC BONDING • Arises from a sea of donated valence electrons (1, 2, or 3 from each atom). Adapted from Fig. 2. 11, Callister 6 e. • Primary bond for metals and their alloys 12

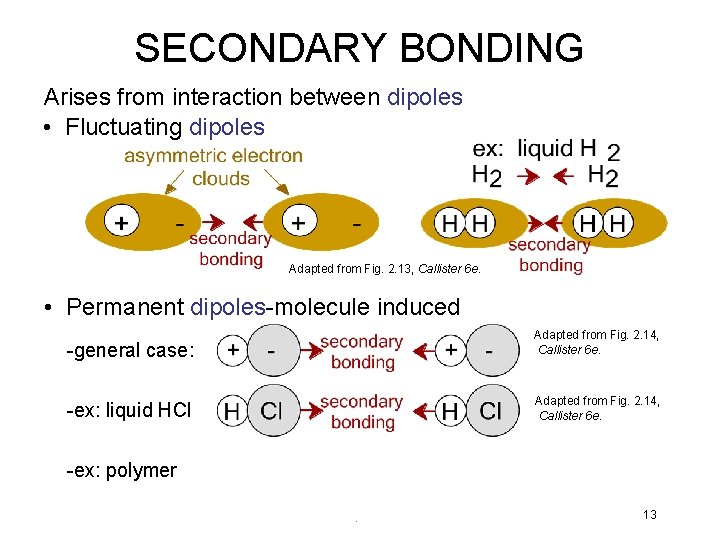
SECONDARY BONDING Arises from interaction between dipoles • Fluctuating dipoles Adapted from Fig. 2. 13, Callister 6 e. • Permanent dipoles-molecule induced -general case: Adapted from Fig. 2. 14, Callister 6 e. -ex: liquid HCl Adapted from Fig. 2. 14, Callister 6 e. -ex: polymer 13

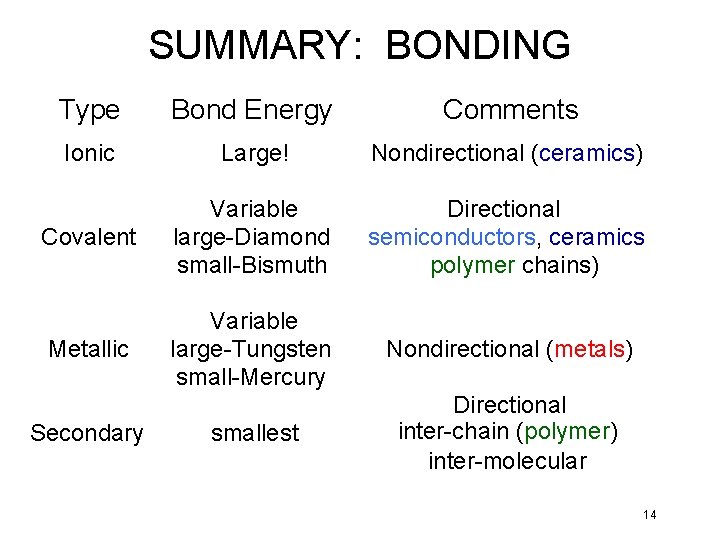
SUMMARY: BONDING Type Bond Energy Comments Ionic Large! Nondirectional (ceramics) Covalent Variable large-Diamond small-Bismuth Directional semiconductors, ceramics polymer chains) Metallic Variable large-Tungsten small-Mercury Nondirectional (metals) smallest Directional inter-chain (polymer) inter-molecular Secondary 14

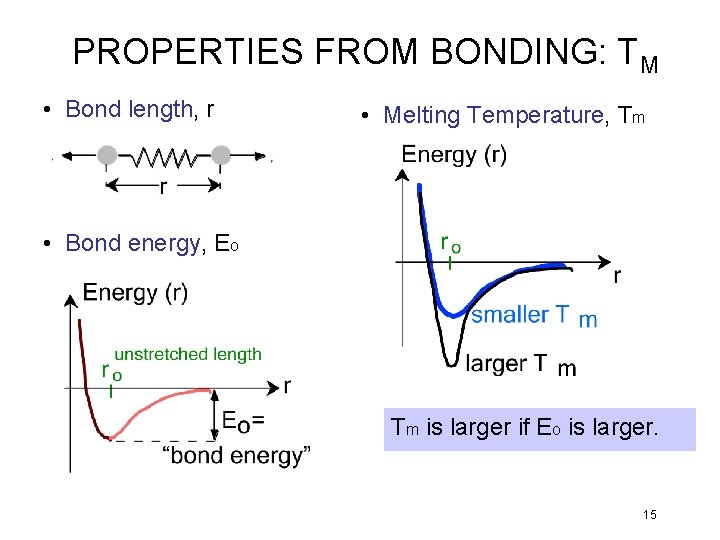
PROPERTIES FROM BONDING: TM • Bond length, r • Melting Temperature, Tm • Bond energy, Eo Tm is larger if Eo is larger. 15

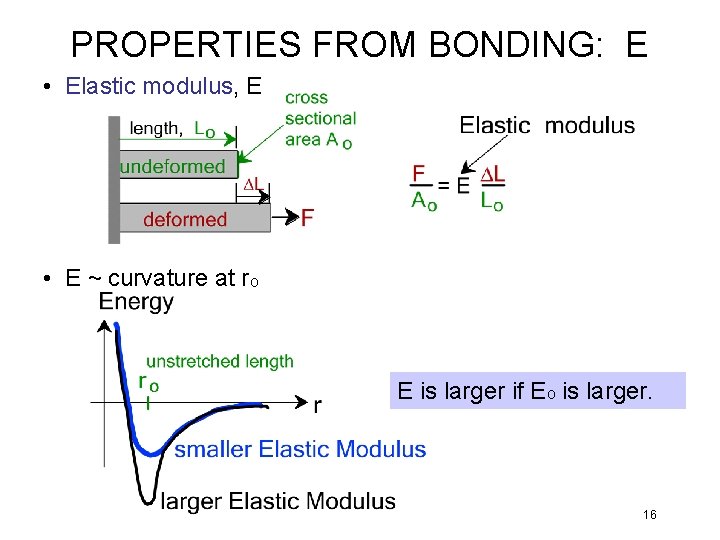
PROPERTIES FROM BONDING: E • Elastic modulus, E • E ~ curvature at ro E is larger if Eo is larger. 16

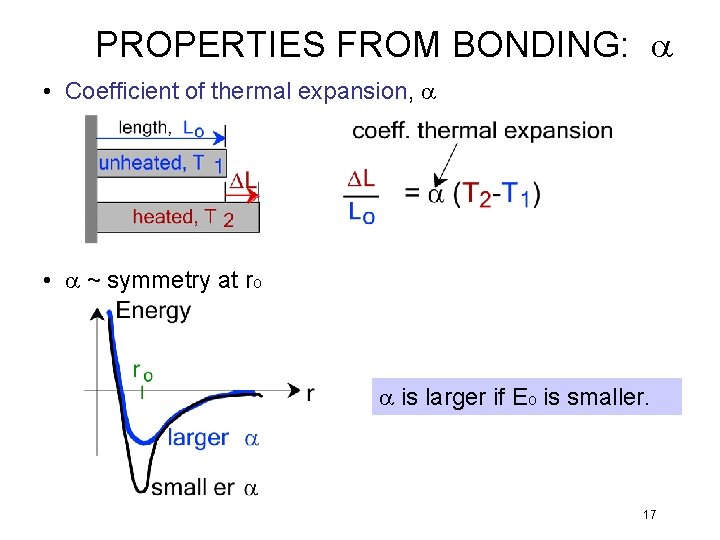
PROPERTIES FROM BONDING: a • Coefficient of thermal expansion, a • a ~ symmetry at ro a is larger if Eo is smaller. 17

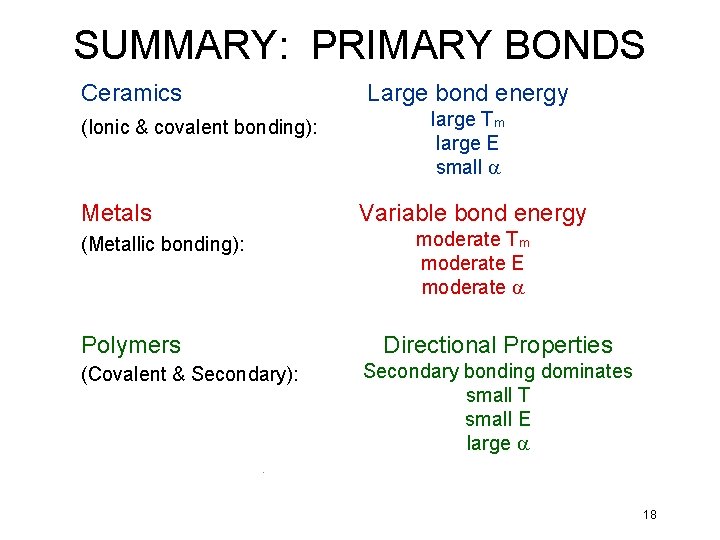
SUMMARY: PRIMARY BONDS Ceramics (Ionic & covalent bonding): Metals (Metallic bonding): Polymers (Covalent & Secondary): Large bond energy large Tm large E small a Variable bond energy moderate Tm moderate E moderate a Directional Properties Secondary bonding dominates small T small E large a 18

Structures of Metals and Ceramics ISSUES TO ADDRESS. . . • How do atoms assemble into solid structures? • How do the structures of ceramic materials differ from those of metals? • How does the density of a material depend on its structure? • When do material properties vary with the sample (i. e. , part) orientation? 1

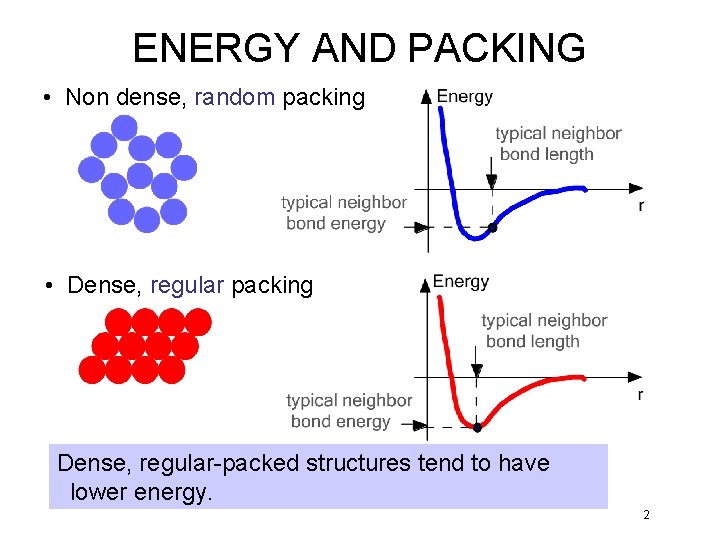
ENERGY AND PACKING • Non dense, random packing • Dense, regular packing Dense, regular-packed structures tend to have lower energy. 2

METALLIC CRYSTALS • tend to be densely packed. • have several reasons for dense packing: -Typically, only one element is present, so all atomic radii are the same. -Metallic bonding is not directional. -Nearest neighbor distances tend to be small in order to lower bond energy. • have the simplest crystal structures. We will look at three such structures. . . 3


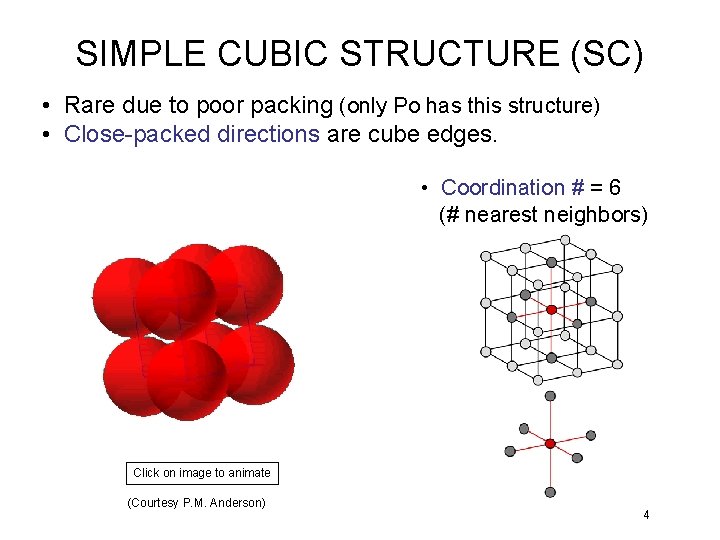
SIMPLE CUBIC STRUCTURE (SC) • Rare due to poor packing (only Po has this structure) • Close-packed directions are cube edges. • Coordination # = 6 (# nearest neighbors) Click on image to animate (Courtesy P. M. Anderson) 4

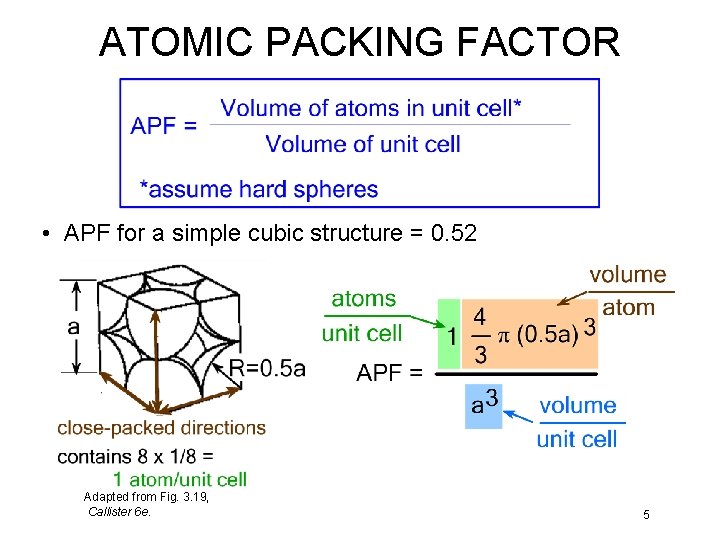
ATOMIC PACKING FACTOR • APF for a simple cubic structure = 0. 52 Adapted from Fig. 3. 19, Callister 6 e. 5

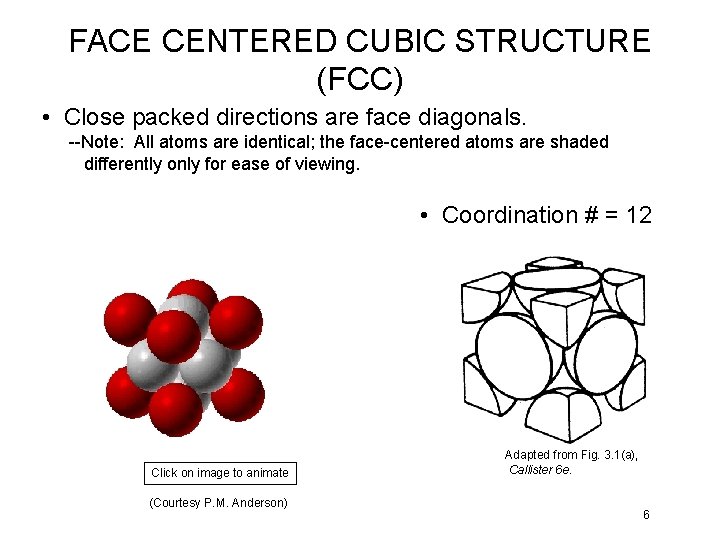
FACE CENTERED CUBIC STRUCTURE (FCC) • Close packed directions are face diagonals. --Note: All atoms are identical; the face-centered atoms are shaded differently only for ease of viewing. • Coordination # = 12 Click on image to animate (Courtesy P. M. Anderson) Adapted from Fig. 3. 1(a), Callister 6 e. 6

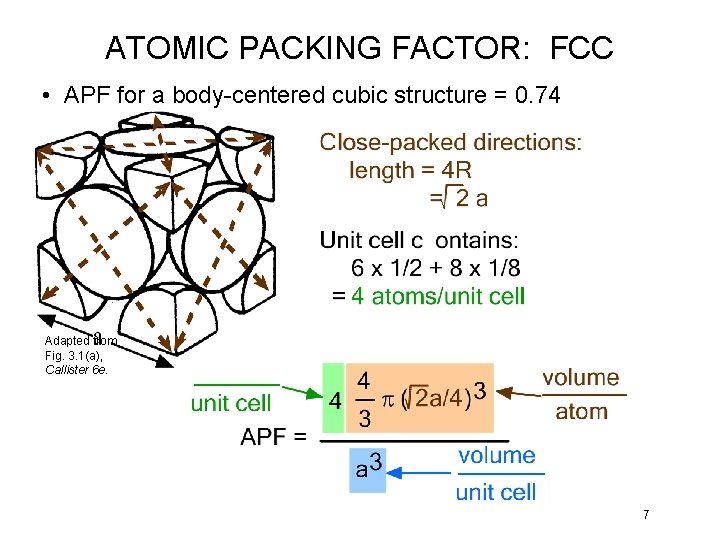
ATOMIC PACKING FACTOR: FCC • APF for a body-centered cubic structure = 0. 74 Adapted from Fig. 3. 1(a), Callister 6 e. 7

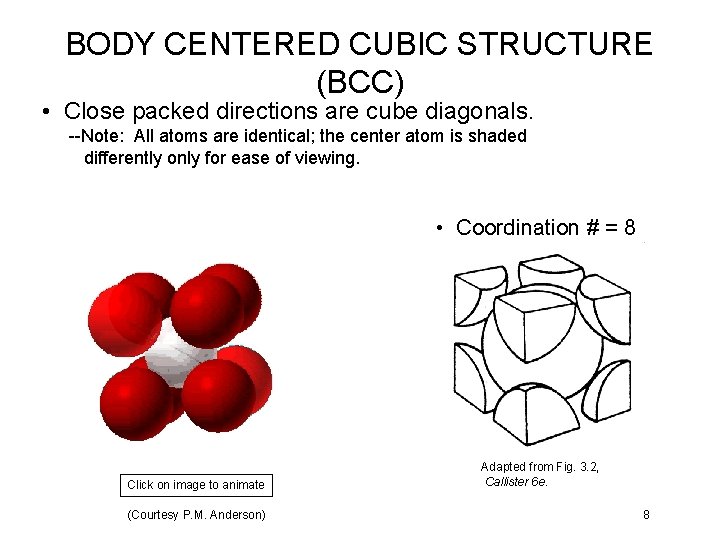
BODY CENTERED CUBIC STRUCTURE (BCC) • Close packed directions are cube diagonals. --Note: All atoms are identical; the center atom is shaded differently only for ease of viewing. • Coordination # = 8 Click on image to animate (Courtesy P. M. Anderson) Adapted from Fig. 3. 2, Callister 6 e. 8

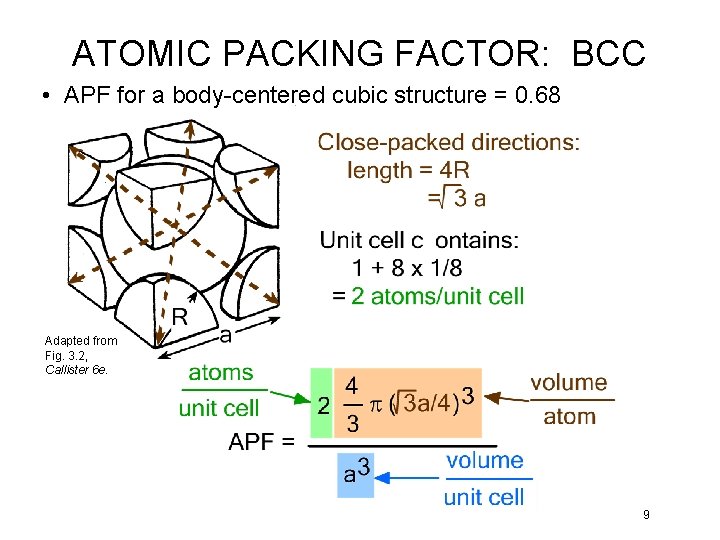
ATOMIC PACKING FACTOR: BCC • APF for a body-centered cubic structure = 0. 68 Adapted from Fig. 3. 2, Callister 6 e. 9

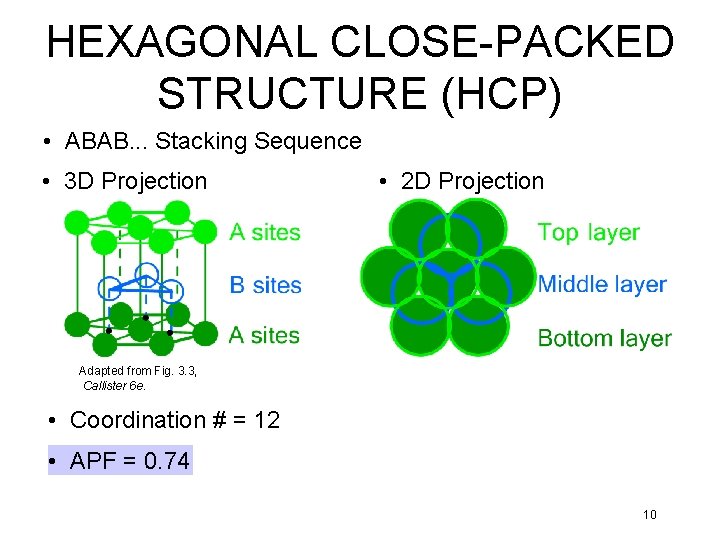
HEXAGONAL CLOSE-PACKED STRUCTURE (HCP) • ABAB. . . Stacking Sequence • 3 D Projection • 2 D Projection Adapted from Fig. 3. 3, Callister 6 e. • Coordination # = 12 • APF = 0. 74 10

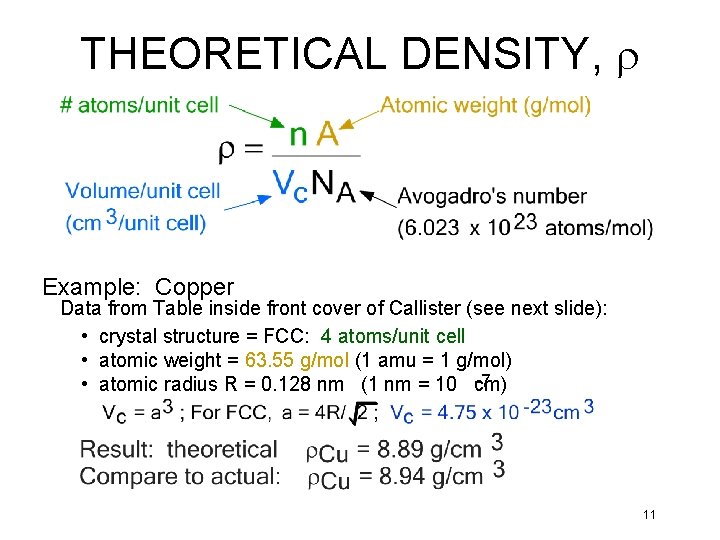
THEORETICAL DENSITY, r Example: Copper Data from Table inside front cover of Callister (see next slide): • crystal structure = FCC: 4 atoms/unit cell • atomic weight = 63. 55 g/mol (1 amu = 1 g/mol) -7 • atomic radius R = 0. 128 nm (1 nm = 10 cm) 11

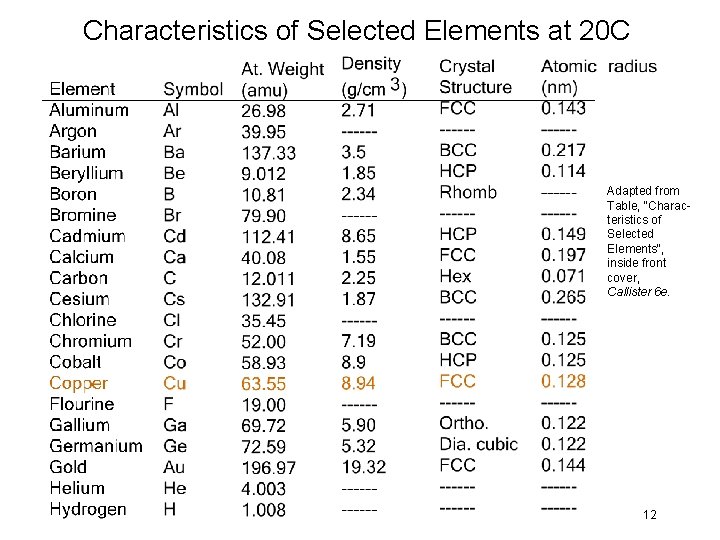
Characteristics of Selected Elements at 20 C Adapted from Table, "Characteristics of Selected Elements", inside front cover, Callister 6 e. 12

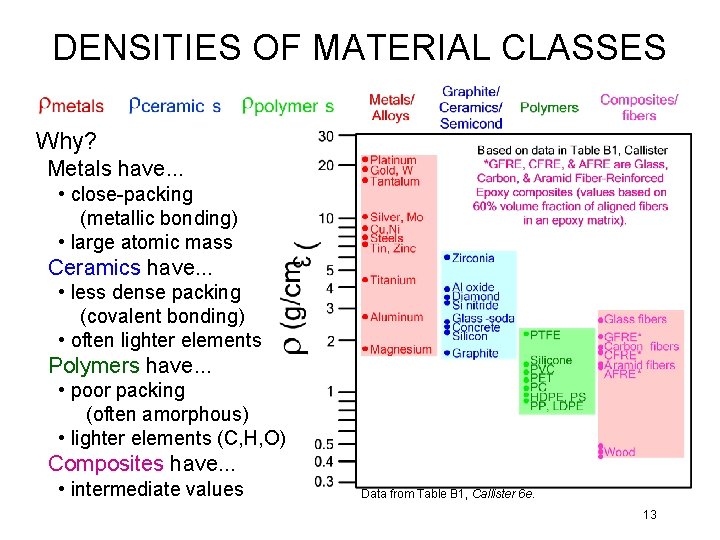
DENSITIES OF MATERIAL CLASSES Why? Metals have. . . • close-packing (metallic bonding) • large atomic mass Ceramics have. . . • less dense packing (covalent bonding) • often lighter elements Polymers have. . . • poor packing (often amorphous) • lighter elements (C, H, O) Composites have. . . • intermediate values Data from Table B 1, Callister 6 e. 13

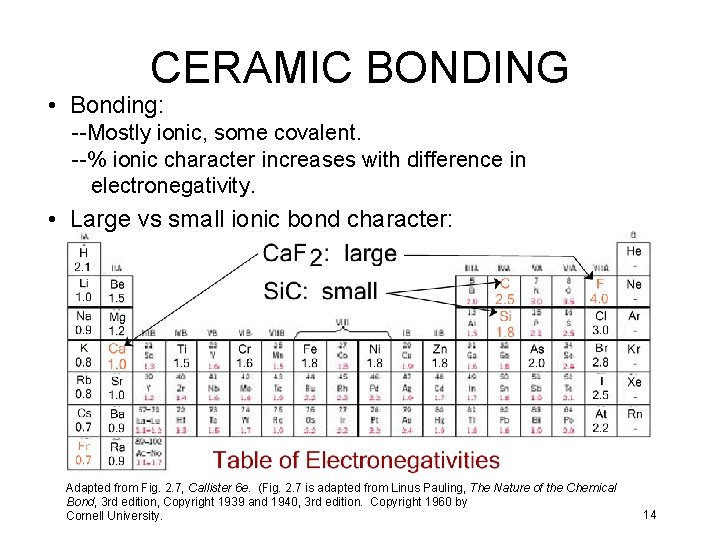
CERAMIC BONDING • Bonding: --Mostly ionic, some covalent. --% ionic character increases with difference in electronegativity. • Large vs small ionic bond character: Adapted from Fig. 2. 7, Callister 6 e. (Fig. 2. 7 is adapted from Linus Pauling, The Nature of the Chemical Bond, 3 rd edition, Copyright 1939 and 1940, 3 rd edition. Copyright 1960 by Cornell University. 14

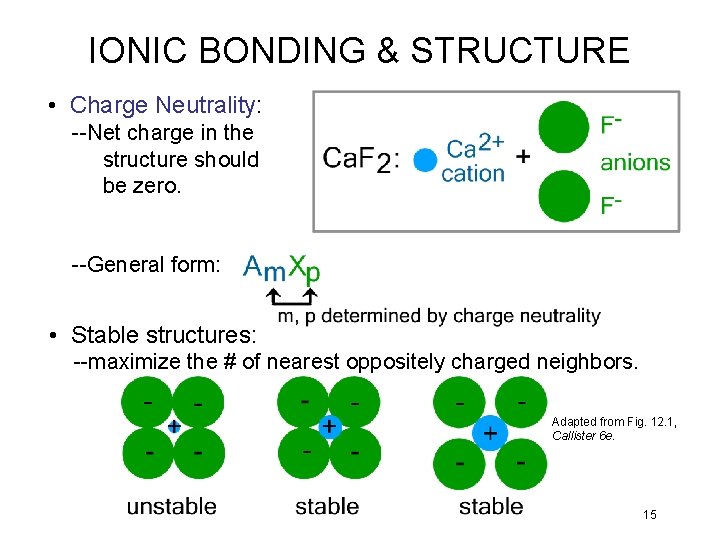
IONIC BONDING & STRUCTURE • Charge Neutrality: --Net charge in the structure should be zero. --General form: • Stable structures: --maximize the # of nearest oppositely charged neighbors. Adapted from Fig. 12. 1, Callister 6 e. 15

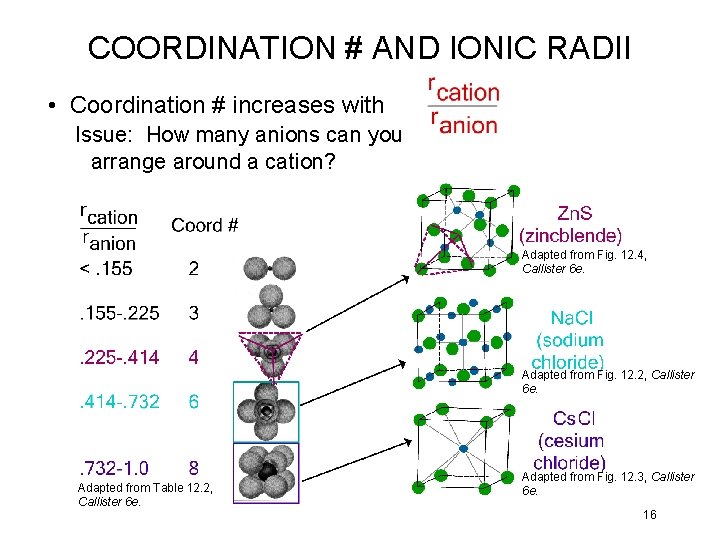
COORDINATION # AND IONIC RADII • Coordination # increases with Issue: How many anions can you arrange around a cation? Adapted from Fig. 12. 4, Callister 6 e. Adapted from Fig. 12. 2, Callister 6 e. Adapted from Table 12. 2, Callister 6 e. Adapted from Fig. 12. 3, Callister 6 e. 16

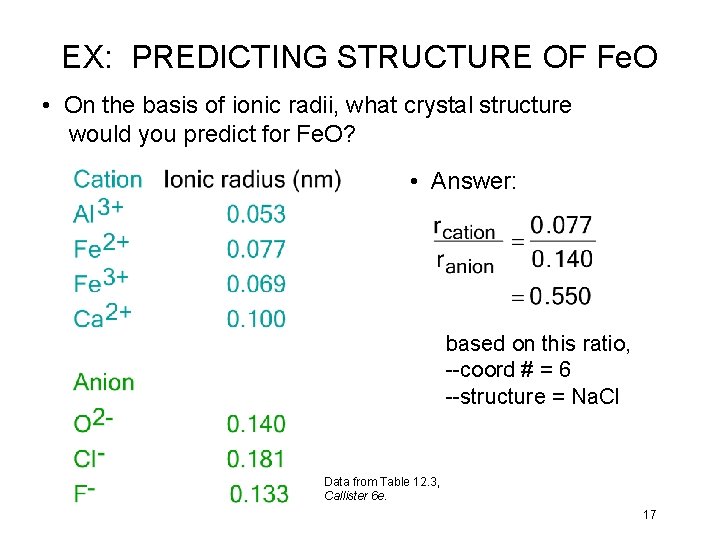
EX: PREDICTING STRUCTURE OF Fe. O • On the basis of ionic radii, what crystal structure would you predict for Fe. O? • Answer: based on this ratio, --coord # = 6 --structure = Na. Cl Data from Table 12. 3, Callister 6 e. 17

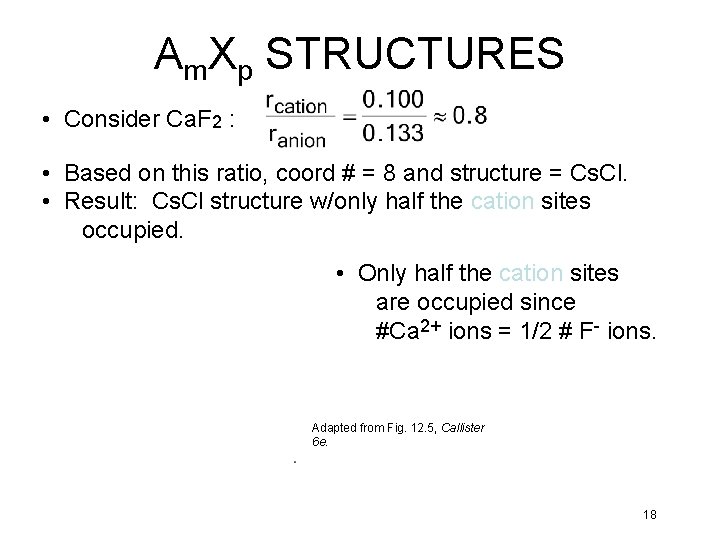
Am. Xp STRUCTURES • Consider Ca. F 2 : • Based on this ratio, coord # = 8 and structure = Cs. Cl. • Result: Cs. Cl structure w/only half the cation sites occupied. • Only half the cation sites are occupied since #Ca 2+ ions = 1/2 # F- ions. Adapted from Fig. 12. 5, Callister 6 e. 18

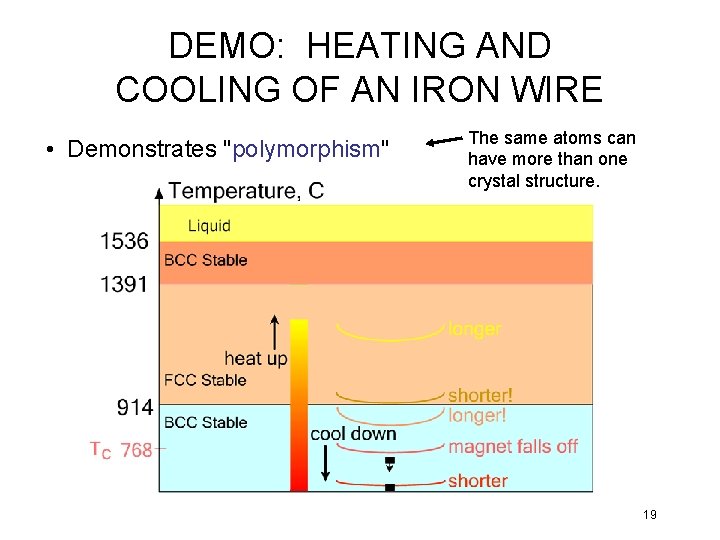
DEMO: HEATING AND COOLING OF AN IRON WIRE • Demonstrates "polymorphism" The same atoms can have more than one crystal structure. 19

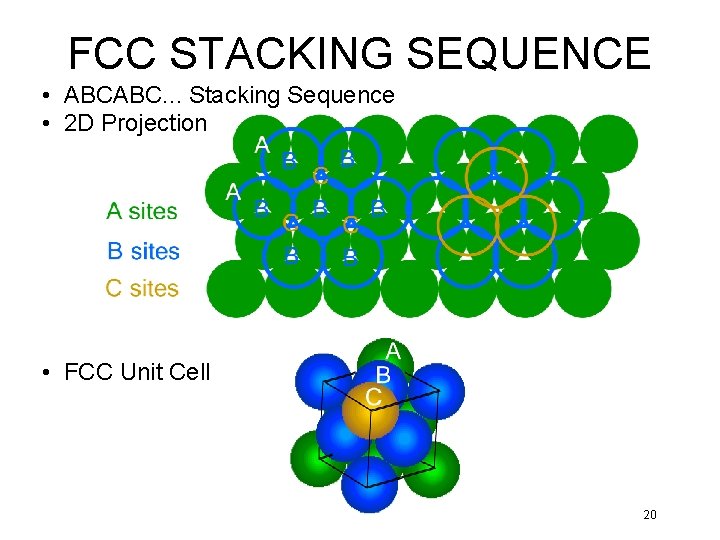
FCC STACKING SEQUENCE • ABCABC. . . Stacking Sequence • 2 D Projection • FCC Unit Cell 20

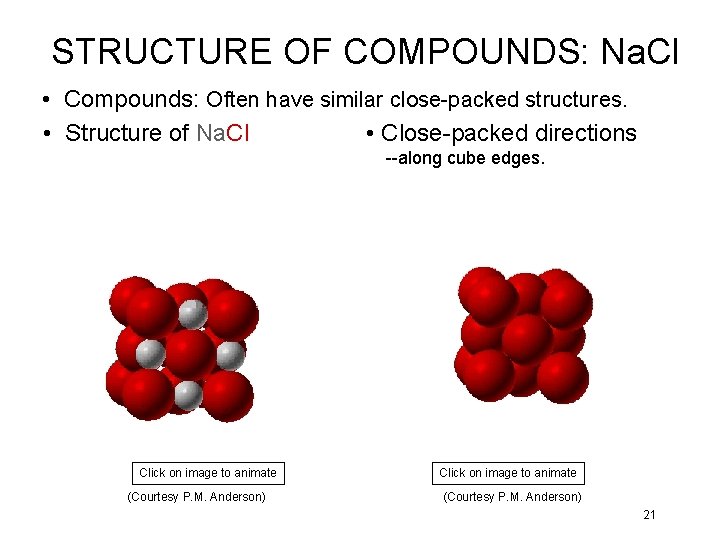
STRUCTURE OF COMPOUNDS: Na. Cl • Compounds: Often have similar close-packed structures. • Structure of Na. Cl • Close-packed directions --along cube edges. Click on image to animate (Courtesy P. M. Anderson) 21

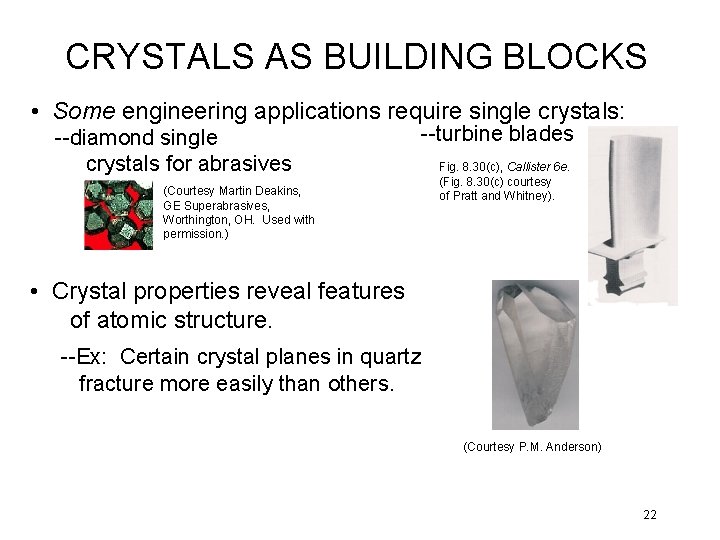
CRYSTALS AS BUILDING BLOCKS • Some engineering applications require single crystals: --diamond single crystals for abrasives --turbine blades (Courtesy Martin Deakins, GE Superabrasives, Worthington, OH. Used with permission. ) Fig. 8. 30(c), Callister 6 e. (Fig. 8. 30(c) courtesy of Pratt and Whitney). • Crystal properties reveal features of atomic structure. --Ex: Certain crystal planes in quartz fracture more easily than others. (Courtesy P. M. Anderson) 22

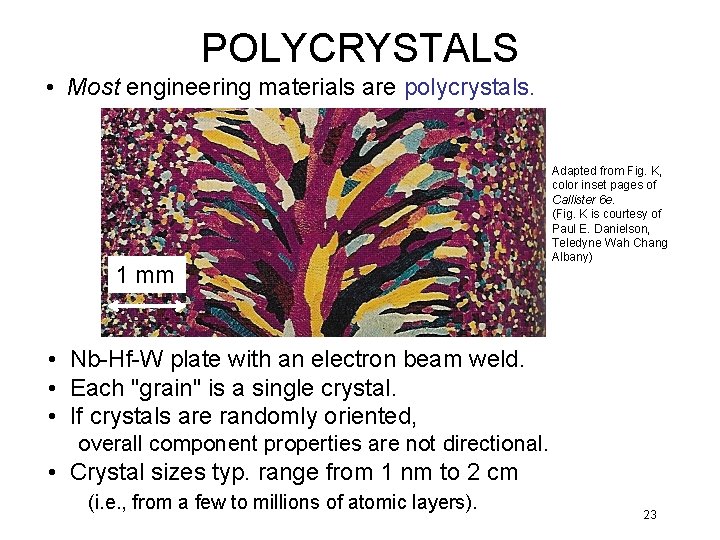
POLYCRYSTALS • Most engineering materials are polycrystals. 1 mm Adapted from Fig. K, color inset pages of Callister 6 e. (Fig. K is courtesy of Paul E. Danielson, Teledyne Wah Chang Albany) • Nb-Hf-W plate with an electron beam weld. • Each "grain" is a single crystal. • If crystals are randomly oriented, overall component properties are not directional. • Crystal sizes typ. range from 1 nm to 2 cm (i. e. , from a few to millions of atomic layers). 23

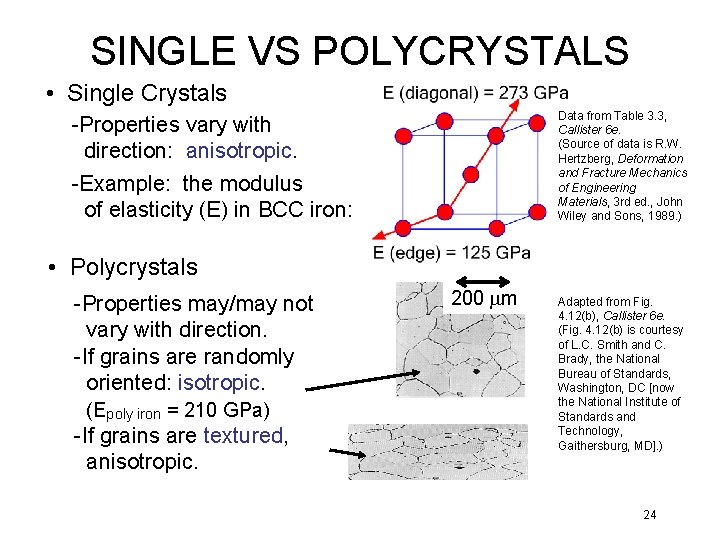
SINGLE VS POLYCRYSTALS • Single Crystals Data from Table 3. 3, Callister 6 e. (Source of data is R. W. Hertzberg, Deformation and Fracture Mechanics of Engineering Materials, 3 rd ed. , John Wiley and Sons, 1989. ) -Properties vary with direction: anisotropic. -Example: the modulus of elasticity (E) in BCC iron: • Polycrystals -Properties may/may not vary with direction. -If grains are randomly oriented: isotropic. (Epoly iron = 210 GPa) -If grains are textured, anisotropic. 200 mm Adapted from Fig. 4. 12(b), Callister 6 e. (Fig. 4. 12(b) is courtesy of L. C. Smith and C. Brady, the National Bureau of Standards, Washington, DC [now the National Institute of Standards and Technology, Gaithersburg, MD]. ) 24

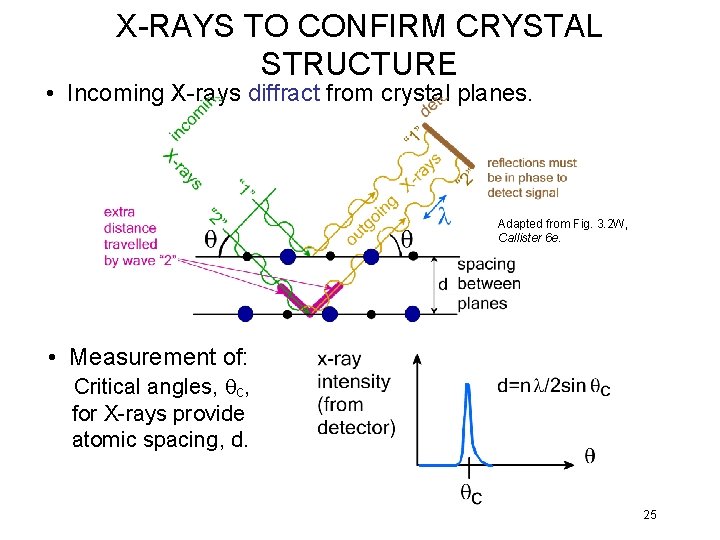
X-RAYS TO CONFIRM CRYSTAL STRUCTURE • Incoming X-rays diffract from crystal planes. Adapted from Fig. 3. 2 W, Callister 6 e. • Measurement of: Critical angles, qc, for X-rays provide atomic spacing, d. 25

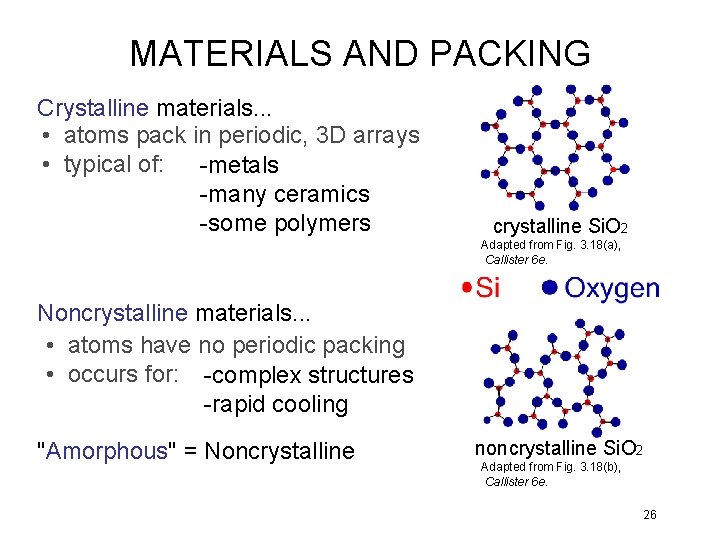
MATERIALS AND PACKING Crystalline materials. . . • atoms pack in periodic, 3 D arrays • typical of: -metals -many ceramics -some polymers crystalline Si. O 2 Adapted from Fig. 3. 18(a), Callister 6 e. Noncrystalline materials. . . • atoms have no periodic packing • occurs for: -complex structures -rapid cooling "Amorphous" = Noncrystalline noncrystalline Si. O 2 Adapted from Fig. 3. 18(b), Callister 6 e. 26

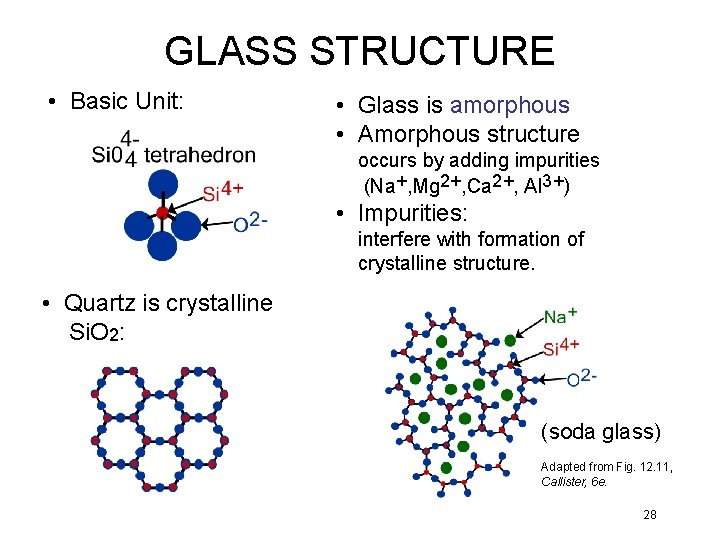
GLASS STRUCTURE • Basic Unit: • Glass is amorphous • Amorphous structure occurs by adding impurities (Na+, Mg 2+, Ca 2+, Al 3+) • Impurities: interfere with formation of crystalline structure. • Quartz is crystalline Si. O 2: (soda glass) Adapted from Fig. 12. 11, Callister, 6 e. 28

SUMMARY • Atoms may assemble into crystalline or amorphous structures. • We can predict the density of a material, provided we know the atomic weight, atomic radius, and crystal geometry (e. g. , FCC, BCC, HCP). • Material properties generally vary with single crystal orientation (i. e. , they are anisotropic), but properties are generally non-directional (i. e. , they are isotropic) in polycrystals with randomly oriented grains. 27