Solid Solution MSE 528 solidstate solution of one







































- Slides: 42

Solid Solution MSE 528

• solid-state solution of one or more solutes in a solvent. • Such a mixture is considered a solution rather than a compound when the crystal structure of the solvent remains unchanged by addition of the solutes, and when the mixture remains in a single homogeneous phase. • The solute may incorporate into the solvent crystal lattice substitutionally, by replacing a solvent particle in the lattice, or interstitially, by fitting into the space between solvent particles. • Both of these types of solid solution affect the properties of the material by distorting the crystal lattice and disrupting the physical and electrical homogeneity of the solvent material. Some mixtures will readily form solid solutions over a range of concentrations, while other mixtures will not form solid solutions at all. The propensity for any two substances to form a solid solution is a complicated matter involving the chemical, crystallographic, and quantum properties of the substances in question. • • Solid solutions, in accordance with the Hume-Rothery rules, may form if the solute and solvent have: Similar atomic radii (15% or less difference) Same crystal structure Similar electronegativities Similar valency

• Hume-Rothery (1899 -1968) was a metallurgist who studied the alloying of metals. His research was conducted at Oxford University where in 1958, he was appointed to the first chair in metallurgy. • His research led to some simple and useful rules on the extent to which an element might dissolve in a metal. The rules that he derived are paraphrased here. The rules are still used widely. For example, the miscibility gap in Au-Ni is correlated with the fact that the lattice parameter of Au is 1. 15 times that of Ni, thus acting maximally according to Hume-Rothery. • If a solute differs in its atomic size by more than about 15% from the host, then it is likely to have a low solubility in that metal. The size factor is said to be unfavourable. • If a solute has a large difference in electronegativity (or electropositivity) when compared with the host, then it is more likely to form a compound. Its solibility in the host would therefore be limited. • A metal with a lower valency is more likely to dissolve in one which has a higher valency, than vice versa.

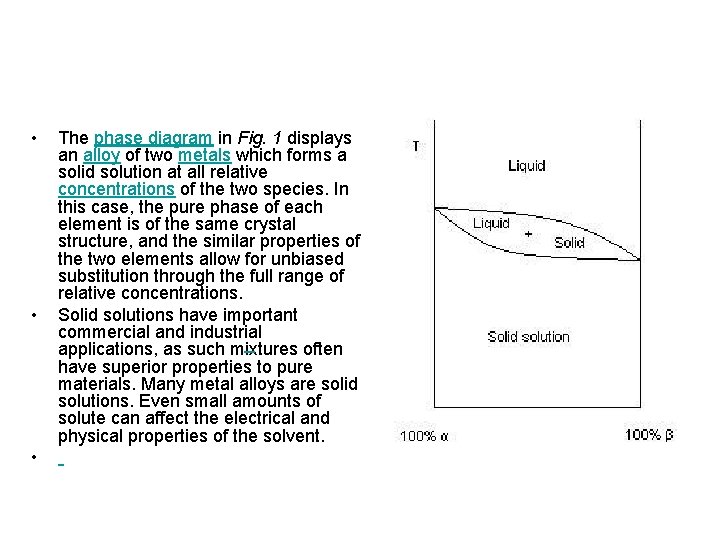
• • • The phase diagram in Fig. 1 displays an alloy of two metals which forms a solid solution at all relative concentrations of the two species. In this case, the pure phase of each element is of the same crystal structure, and the similar properties of the two elements allow for unbiased substitution through the full range of relative concentrations. Solid solutions have important commercial and industrial applications, as such mixtures often have superior properties to pure materials. Many metal alloys are solid solutions. Even small amounts of solute can affect the electrical and physical properties of the solvent.

• The binary phase diagram in Fig. 2 at right shows the phases of a mixture of two substances in varying concentrations, alpha and beta. The region labeled "alpha" is a solid solution, with beta acting as the solute in a matrix of alpha. On the other end of the concentration scale, the region labeled "beta" is also a solid solution, with alpha acting as the solute in a matrix of beta. The large solid region in between the alpha and beta solid solutions, labeled "solid alpha and beta", is not a solid solution. Instead, an examination of the microstructure of a mixture in this range would reveal two phases — solid solution alpha-in -beta and solid solution beta-inalpha

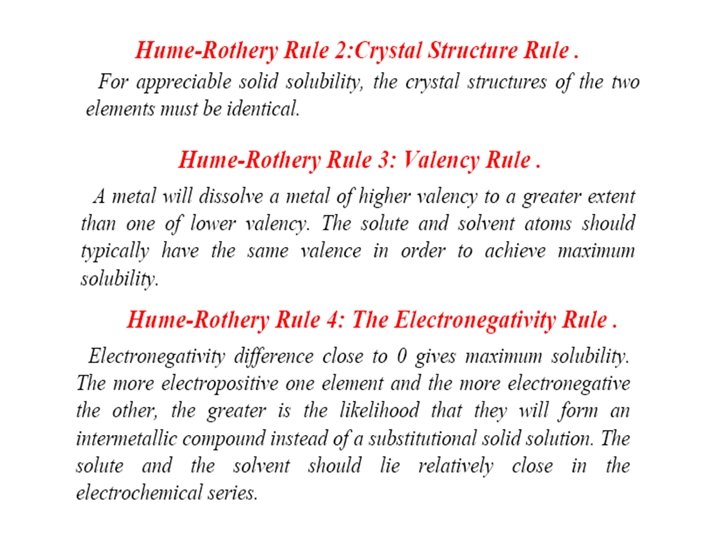
• Hume-Rothery rules • The Hume-Rothery rules are a set of basic rules describing the conditions under which an element could dissolve in a metal, forming a solid solution. There are two sets of rules, one which refers to substitutional solid solutions, and another which refers to interstitial solid solutions. Substitutional Solid Solution Rule for substitutional solid solutions, the Hume-Rothery rules are: – 1. The atomic radii of the solute and solvent atoms must differ by no more than 15%: • – 2. The crystal structures of solute and solvent must match. – 3. Maximum solubility occurs when the solvent and solute have the same valency. Metals with lower valency will tend to dissolve metals with higher valency. – 4. The solute and solvent should have similar electronegativity. If the electronegativity difference is too great, the metals will tend to form intermetallic compounds instead of solid solutions. Interstitial Solid Solution Rules • For interstitial solid solutions, the Hume-Rothery rules are: – 1. Solute atoms must be smaller than the pores in the solvent lattice. – 2. The solute and solvent should have similar electronegativity.



































