Maternal Uteroplacental Lesions Raymond W Redline MD University










- Slides: 58

Maternal- Uteroplacental Lesions Raymond W. Redline, MD University Hospitals Case Medical Center Cleveland, Ohio

Placental Pathology: uses and limitations Very good at identifying 1. Inflammation and vascular/ developmental pathology 2. Accelerating processes beginning > 6 hrs before delivery 3. Tissue-disruptive sentinel events (abruption, fetal hemorrhage) Not very useful for 1. Further elucidating fetal malformations 2. Shedding light on previous adverse outcomes 3. Evaluating very late intrapartum hypoxia

Historical overview Phase 1: Major concepts (1960 -1980) e. g. Amniotic fluid infection, Maternal supply line Benirschke, Gruenwald, Fox, Brosens, Blanc Phase 2: Additional important lesions (1980 -2000) e. g. Villitis of unknown etiology, fetal thrombotic vasculopathy, maternal floor infarction Phase 3: Standardization (Amsterdam Working Group) • • reproducible criteria for grading and staging understanding pathophysiology Phase 4: Utilizing placental data (Human Placenta Project) • • in utero biomarkers and imaging for placental processes therapeutic interventions in future pregnancies

Indications for Placental Exam College of American Pathologists Maternal Indicators • Preterm delivery < 36 weeks • Fever/ suspected infection • Abnormal antenatal testing • Severe oligohydramnios/ polyhydramnios • Hypertension/ diabetes • Vaginal bleeding • Chronic diseases: cardiovascular, renal, autoimmune • Thick meconium with fetal distress Reference: Arch Pathol Lab Med 1997

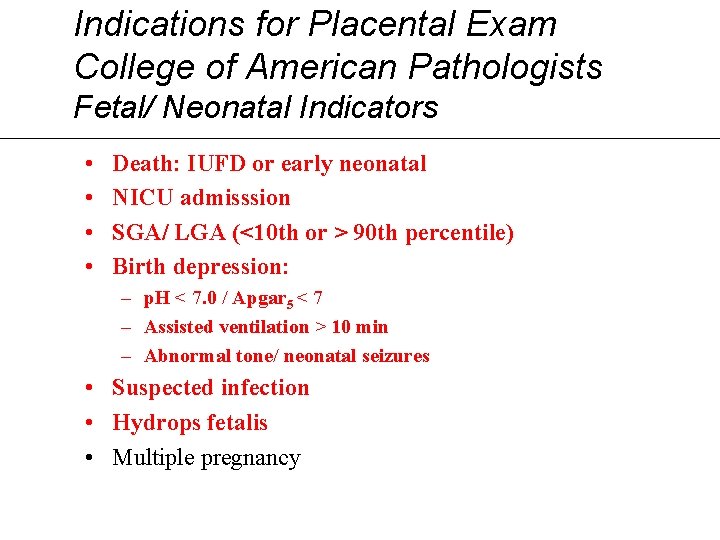
Indications for Placental Exam College of American Pathologists Fetal/ Neonatal Indicators • • Death: IUFD or early neonatal NICU admisssion SGA/ LGA (<10 th or > 90 th percentile) Birth depression: – p. H < 7. 0 / Apgar 5 < 7 – Assisted ventilation > 10 min – Abnormal tone/ neonatal seizures • Suspected infection • Hydrops fetalis • Multiple pregnancy

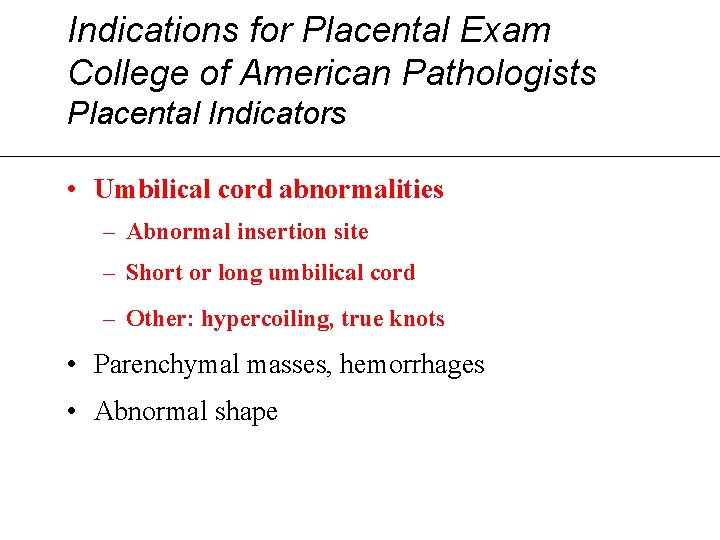
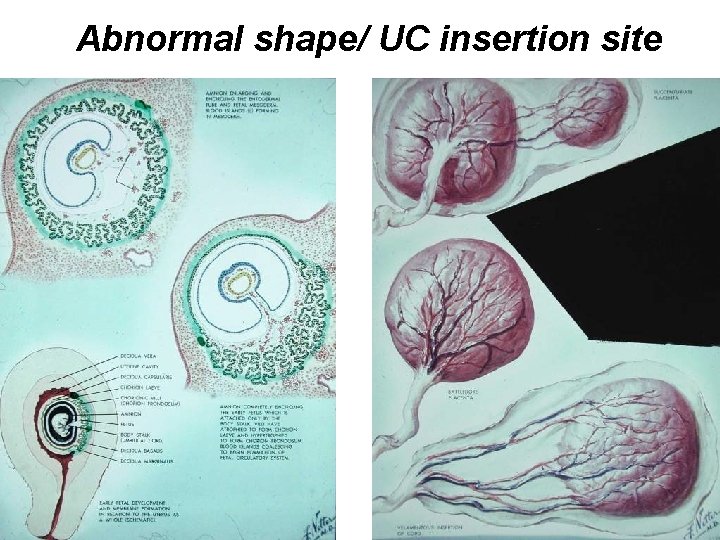
Indications for Placental Exam College of American Pathologists Placental Indicators • Umbilical cord abnormalities – Abnormal insertion site – Short or long umbilical cord – Other: hypercoiling, true knots • Parenchymal masses, hemorrhages • Abnormal shape

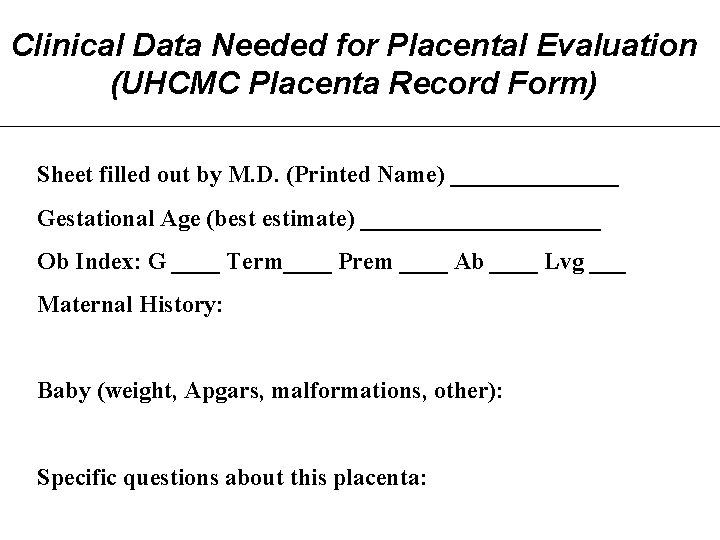
Clinical Data Needed for Placental Evaluation (UHCMC Placenta Record Form) Sheet filled out by M. D. (Printed Name) _______ Gestational Age (best estimate) __________ Ob Index: G ____ Term____ Prem ____ Ab ____ Lvg ___ Maternal History: Baby (weight, Apgars, malformations, other): Specific questions about this placenta:

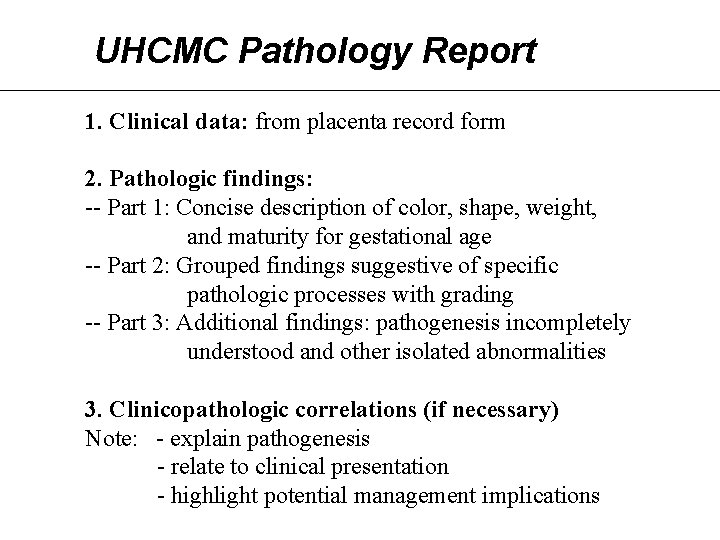
UHCMC Pathology Report 1. Clinical data: from placenta record form 2. Pathologic findings: -- Part 1: Concise description of color, shape, weight, and maturity for gestational age -- Part 2: Grouped findings suggestive of specific pathologic processes with grading -- Part 3: Additional findings: pathogenesis incompletely understood and other isolated abnormalities 3. Clinicopathologic correlations (if necessary) Note: - explain pathogenesis - relate to clinical presentation - highlight potential management implications

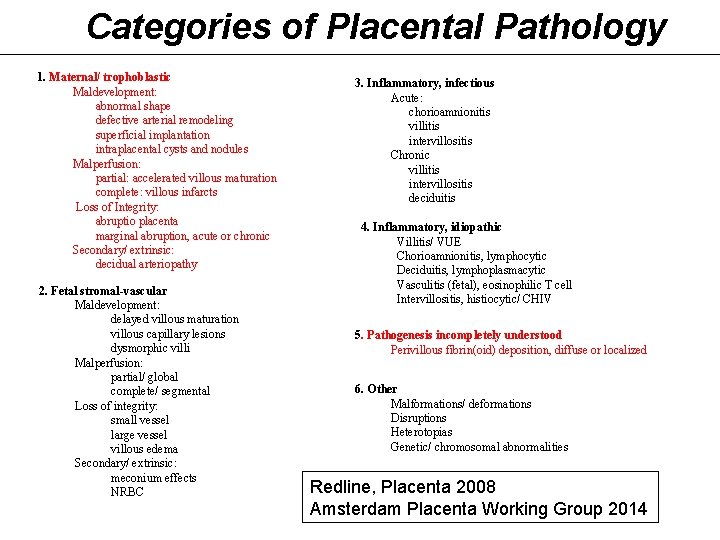
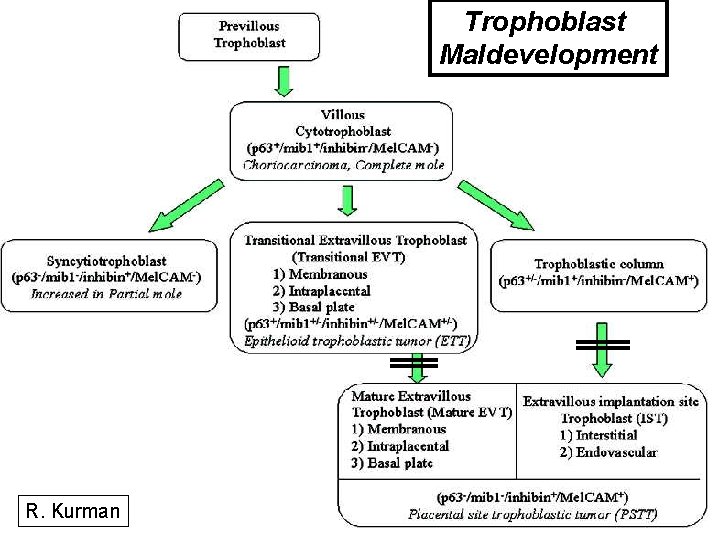
Categories of Placental Pathology 1. Maternal/ trophoblastic Maldevelopment: abnormal shape defective arterial remodeling superficial implantation intraplacental cysts and nodules Malperfusion: partial: accelerated villous maturation complete: villous infarcts Loss of Integrity: abruptio placenta marginal abruption, acute or chronic Secondary/ extrinsic: decidual arteriopathy 2. Fetal stromal-vascular Maldevelopment: delayed villous maturation villous capillary lesions dysmorphic villi Malperfusion: partial/ global complete/ segmental Loss of integrity: small vessel large vessel villous edema Secondary/ extrinsic: meconium effects NRBC 3. Inflammatory, infectious Acute: chorioamnionitis villitis intervillositis Chronic villitis intervillositis deciduitis 4. Inflammatory, idiopathic Villitis/ VUE Chorioamnionitis, lymphocytic Deciduitis, lymphoplasmacytic Vasculitis (fetal), eosinophilic T cell Intervillositis, histiocytic/ CHIV 5. Pathogenesis incompletely understood Perivillous fibrin(oid) deposition, diffuse or localized 6. Other Malformations/ deformations Disruptions Heterotopias Genetic/ chromosomal abnormalities Redline, Placenta 2008 Amsterdam Placenta Working Group 2014

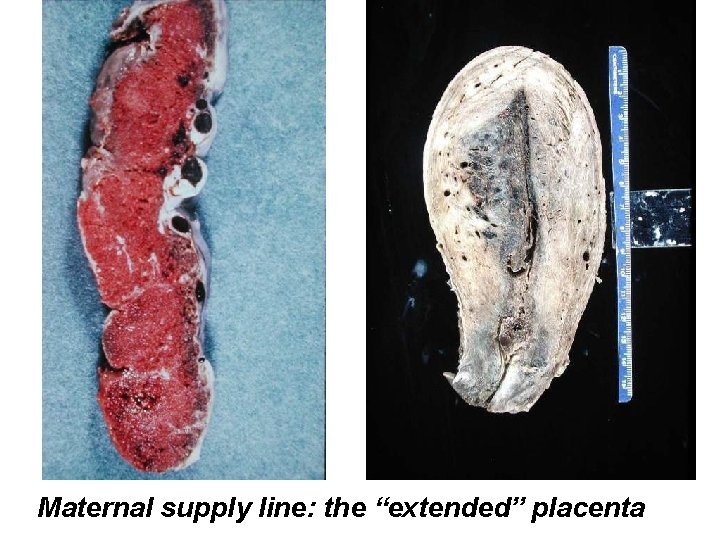
Maternal supply line: the “extended” placenta

SPP Perinatal Section Placental Nosology Project (1998 -2004) Maternal Vascular Underperfusion: Nosology and Reproducibility of Placental Reaction Patterns Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, Prashner HR, Waters BL Pediatr Dev Pathol 2004

Abnormal shape/ UC insertion site

Trophoblast Maldevelopment R. Kurman

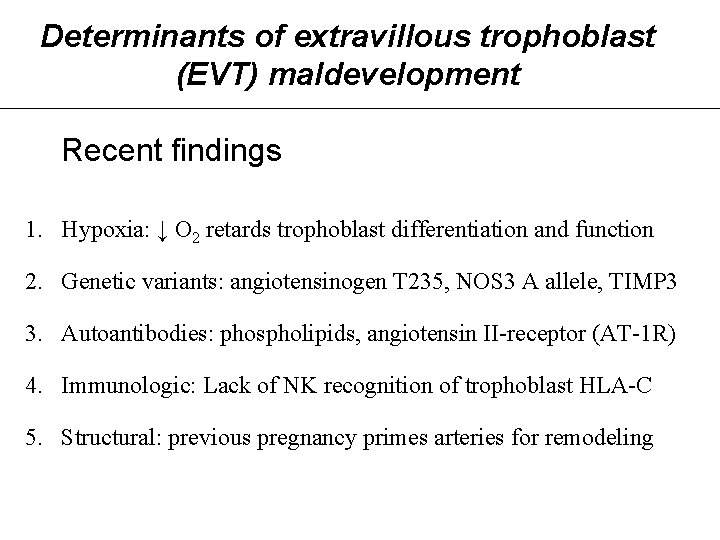
Determinants of extravillous trophoblast (EVT) maldevelopment Recent findings 1. Hypoxia: ↓ O 2 retards trophoblast differentiation and function 2. Genetic variants: angiotensinogen T 235, NOS 3 A allele, TIMP 3 3. Autoantibodies: phospholipids, angiotensin II-receptor (AT-1 R) 4. Immunologic: Lack of NK recognition of trophoblast HLA-C 5. Structural: previous pregnancy primes arteries for remodeling

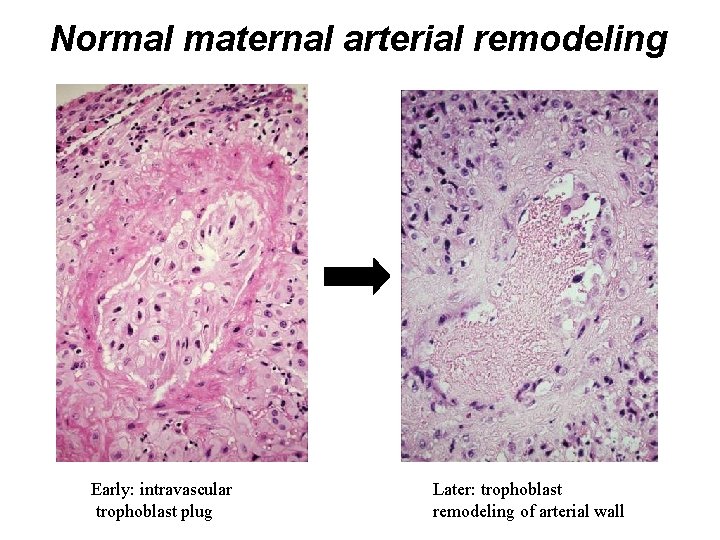
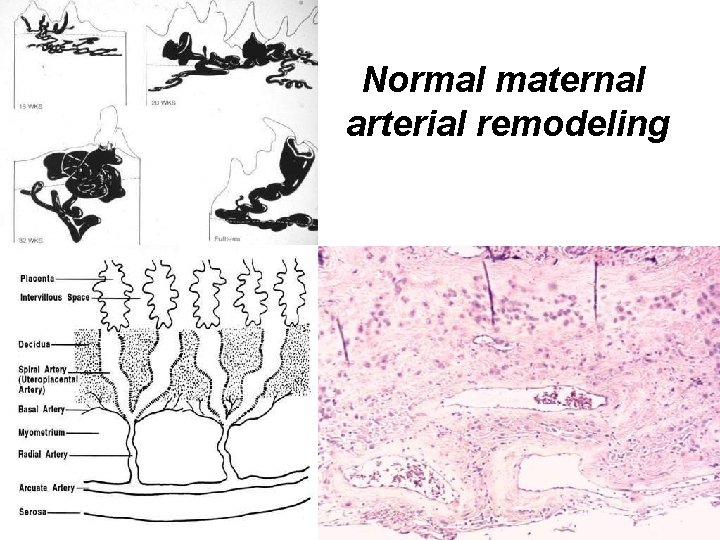
Normal maternal arterial remodeling Early: intravascular trophoblast plug Later: trophoblast remodeling of arterial wall

Normal maternal arterial remodeling

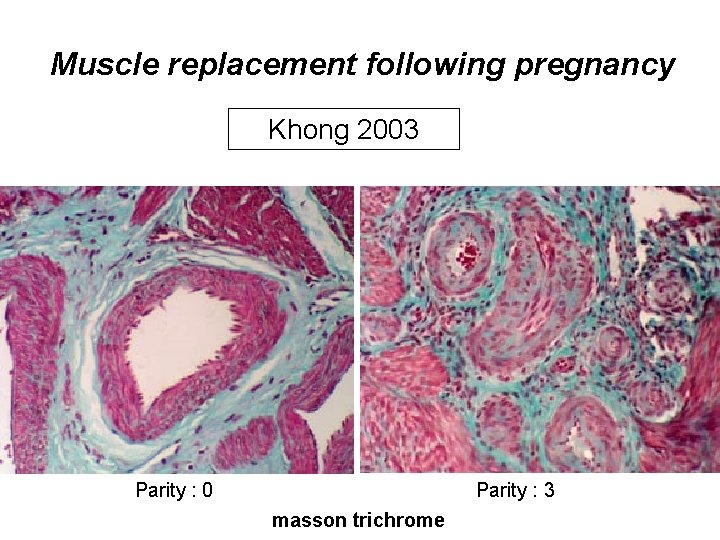
Muscle replacement following pregnancy Khong 2003 Parity : 0 Parity : 3 masson trichrome

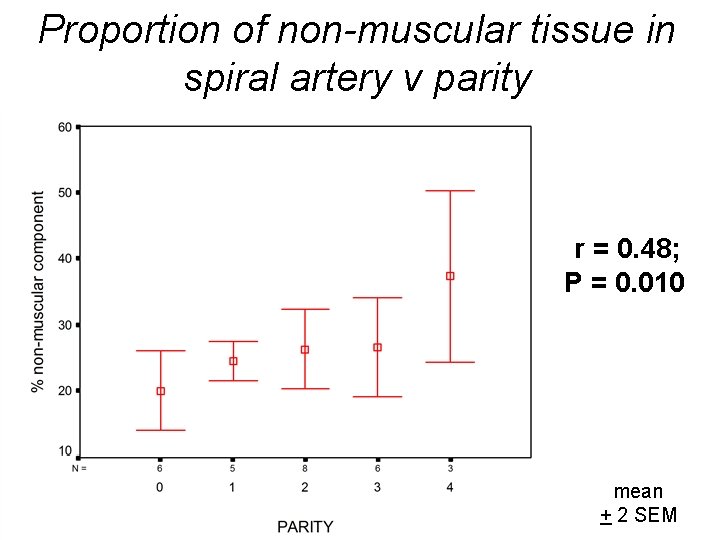
Proportion of non-muscular tissue in spiral artery v parity r = 0. 48; P = 0. 010 mean + 2 SEM

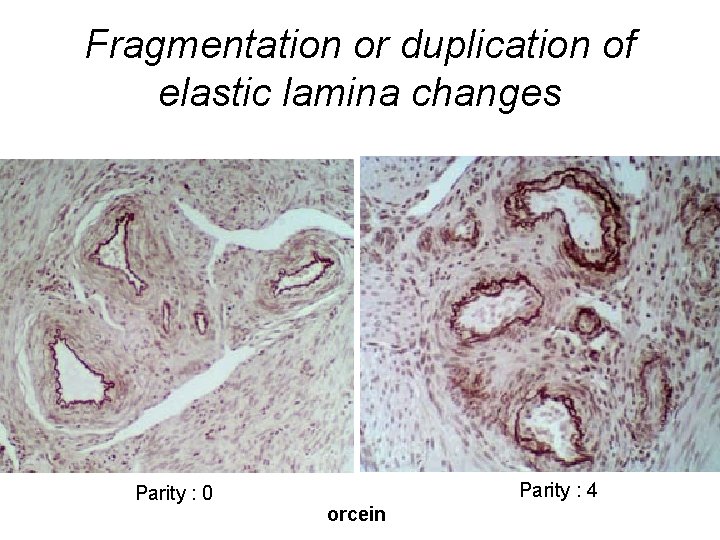
Fragmentation or duplication of elastic lamina changes Parity : 0 Parity : 4 orcein

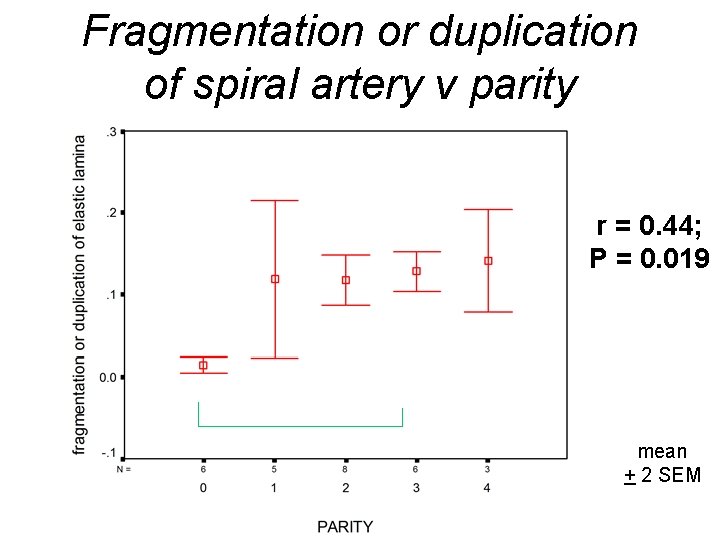
Fragmentation or duplication of spiral artery v parity r = 0. 44; P = 0. 019 P = 0. 002 mean + 2 SEM

• Lower incidence of pre-eclampsia in second than in first pregnancies • Second-born babies have a higher birth weight than first borns – The increase in birth weight is greater between the first and second born than between other subsequent birth order pairs

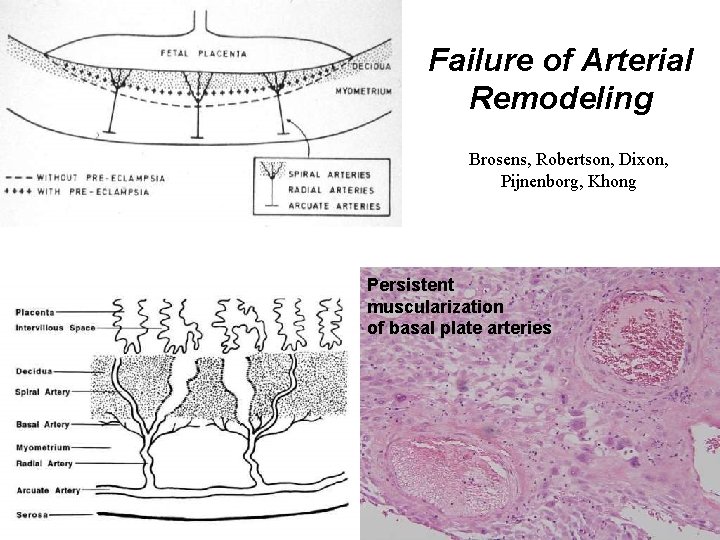
Failure of Arterial Remodeling Brosens, Robertson, Dixon, Pijnenborg, Khong Persistent muscularization of basal plate arteries

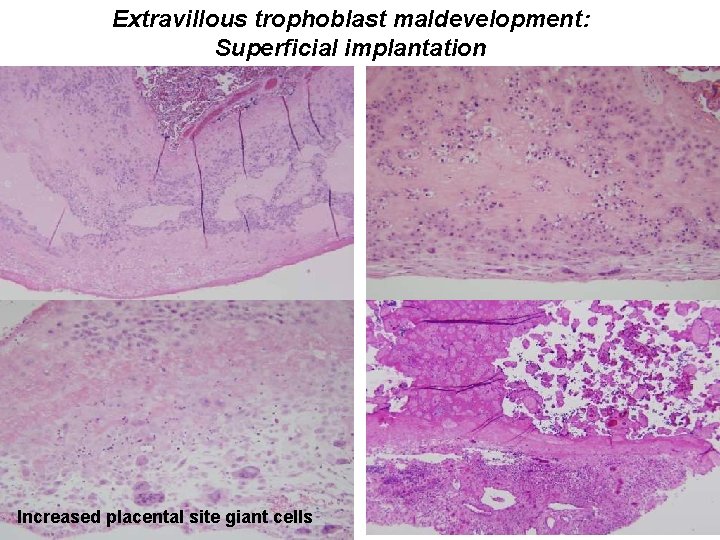
Extravillous trophoblast maldevelopment: Superficial implantation Increased placental site giant cells

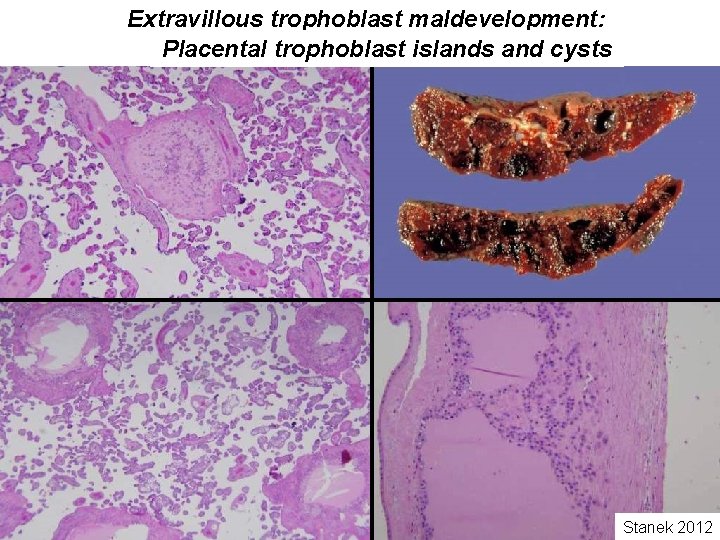
Extravillous trophoblast maldevelopment: Placental trophoblast islands and cysts Stanek 2012

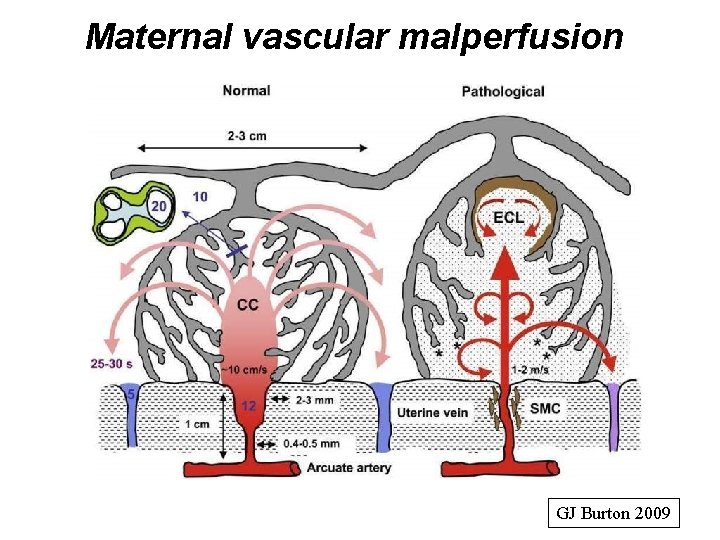
Maternal vascular malperfusion GJ Burton 2009

Findings associated with maternal vascular malperfusion • Partial/ global: - accelerated maturation with alternating areas of - crowding: syncytial knots, villous agglutination, intervillous fibrin - villous paucity, distal villous hypoplasia • Complete/ segmental: - villous infarction • Other: - placental weight, - fetoplacental weight ratio, - thin umbilical cord - decidual arteriopathy/ laminar necrosis

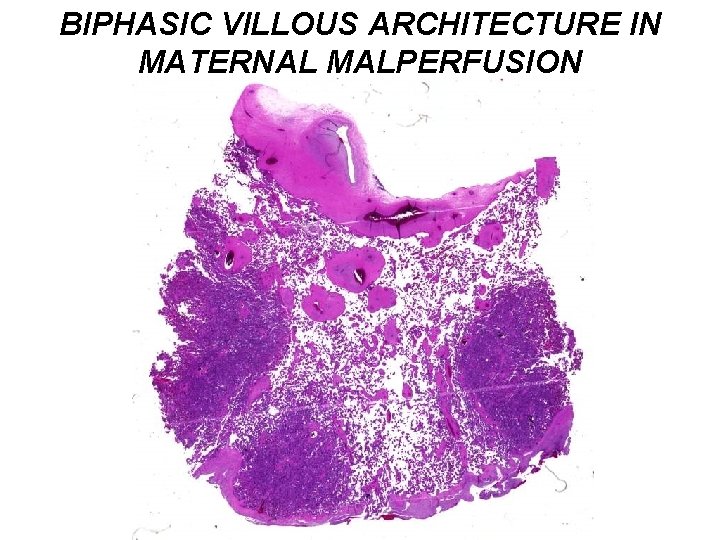
BIPHASIC VILLOUS ARCHITECTURE IN MATERNAL MALPERFUSION

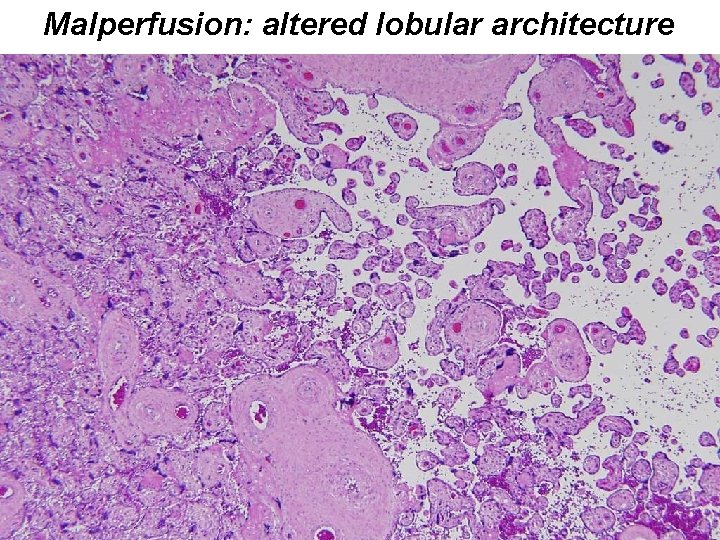
Malperfusion: altered lobular architecture

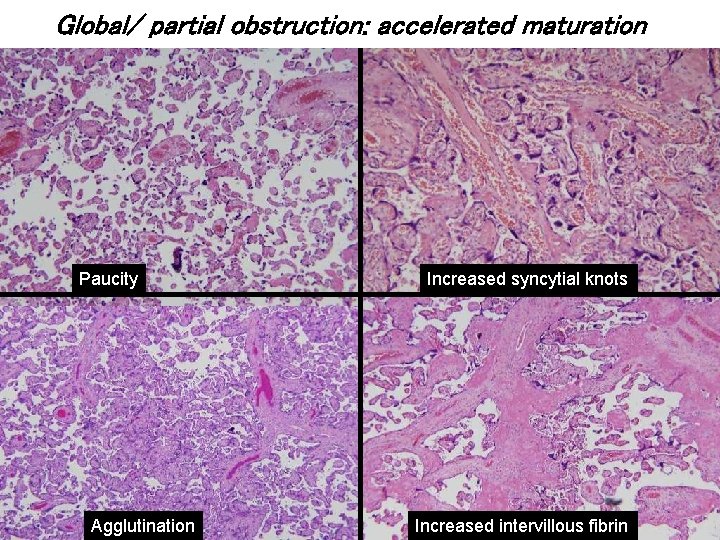
Global/ partial obstruction: accelerated maturation Paucity Agglutination Increased syncytial knots Increased intervillous fibrin

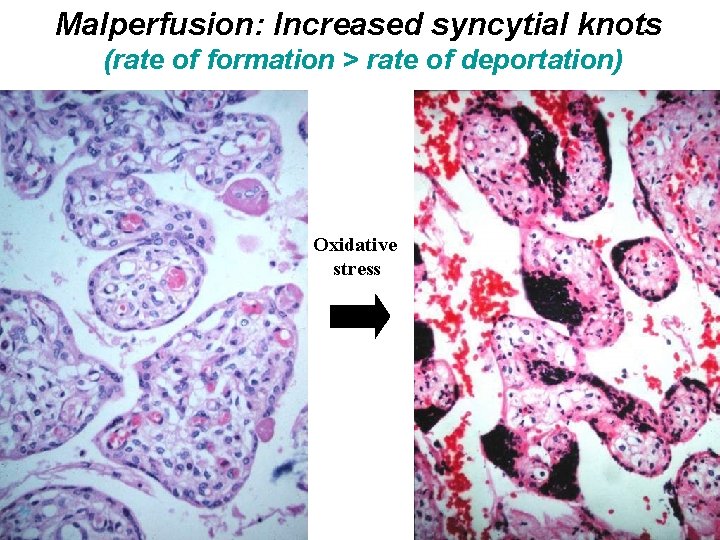
Malperfusion: Increased syncytial knots (rate of formation > rate of deportation) Oxidative stress

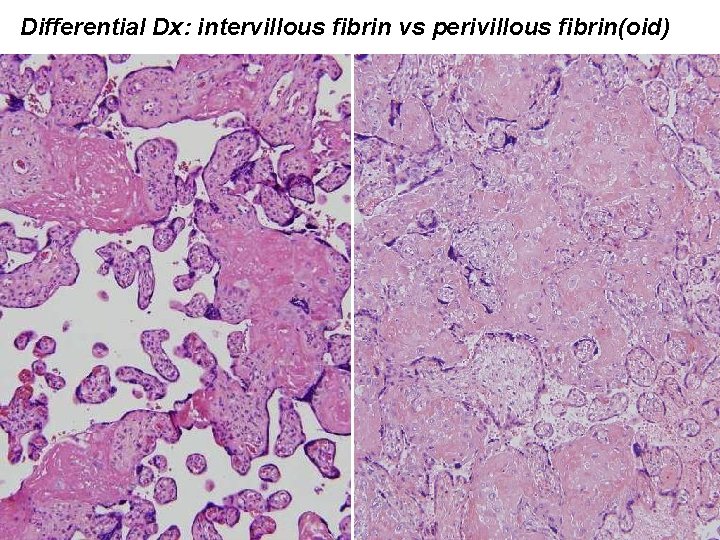
Differential Dx: intervillous fibrin vs perivillous fibrin(oid)

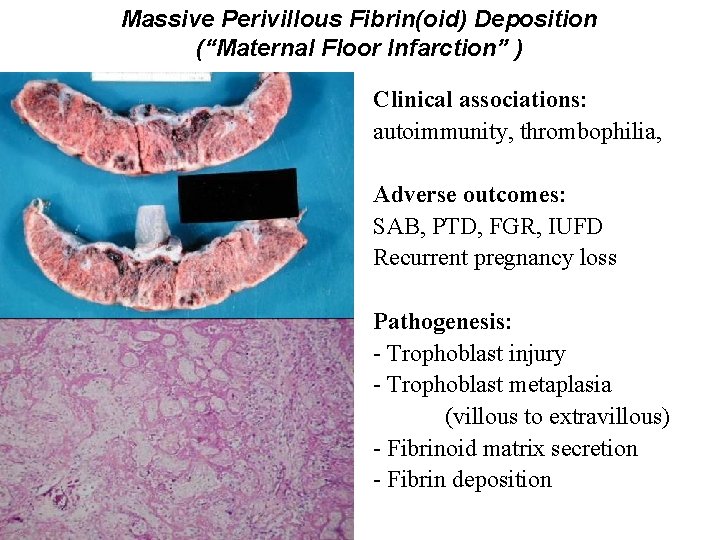
Massive Perivillous Fibrin(oid) Deposition (“Maternal Floor Infarction” ) Clinical associations: autoimmunity, thrombophilia, Adverse outcomes: SAB, PTD, FGR, IUFD Recurrent pregnancy loss Pathogenesis: - Trophoblast injury - Trophoblast metaplasia (villous to extravillous) - Fibrinoid matrix secretion - Fibrin deposition

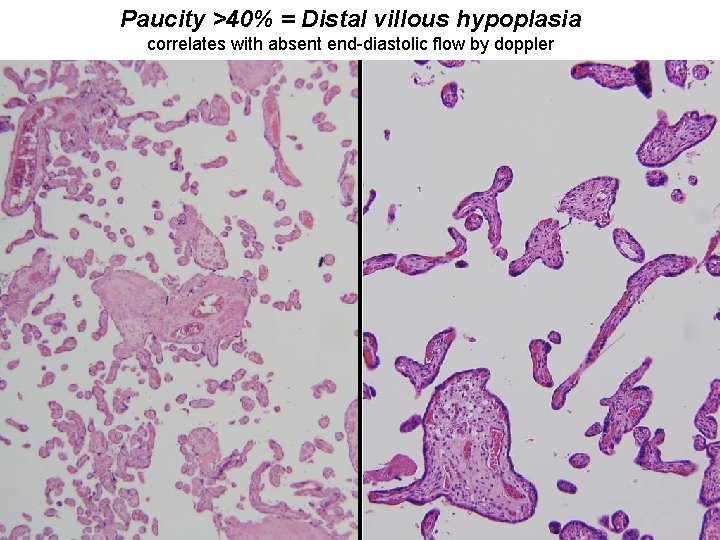
Paucity >40% = Distal villous hypoplasia correlates with absent end-diastolic flow by doppler

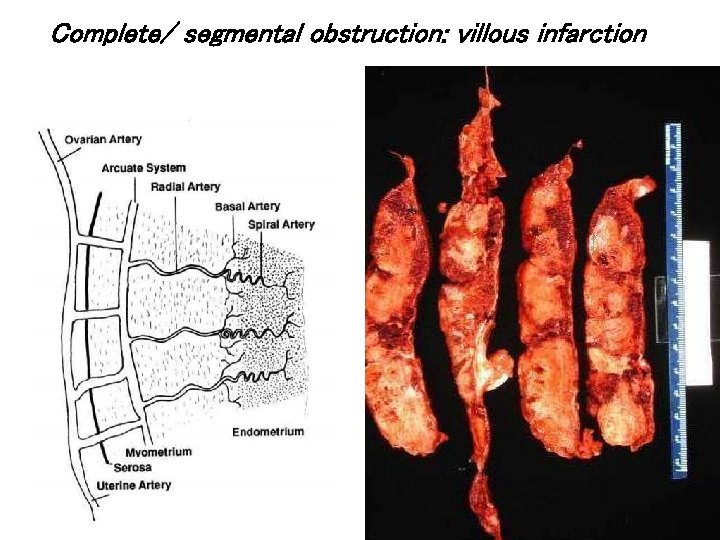
Complete/ segmental obstruction: villous infarction

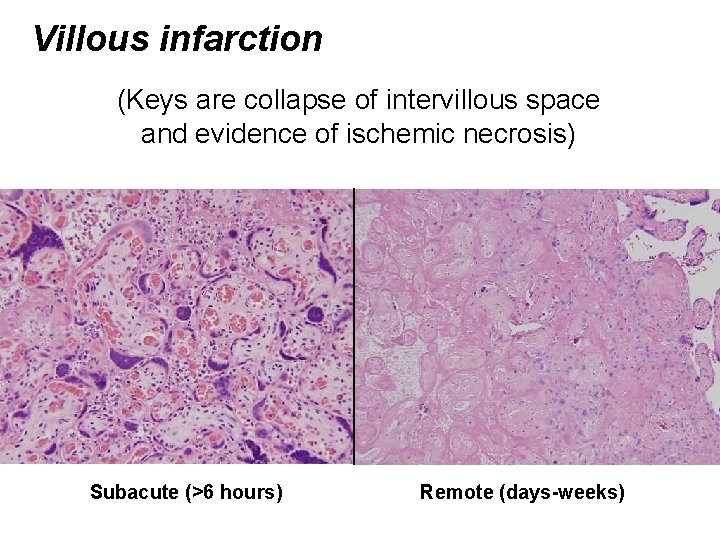
Villous infarction (Keys are collapse of intervillous space and evidence of ischemic necrosis) Subacute (>6 hours) Remote (days-weeks)

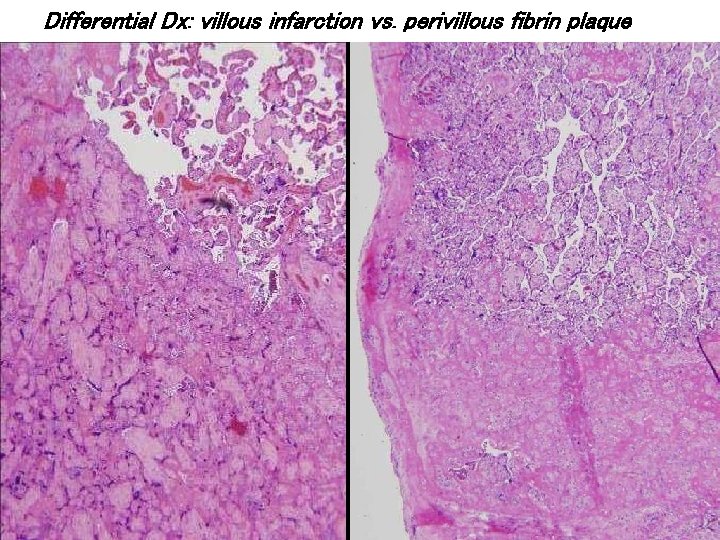
Differential Dx: villous infarction vs. perivillous fibrin plaque

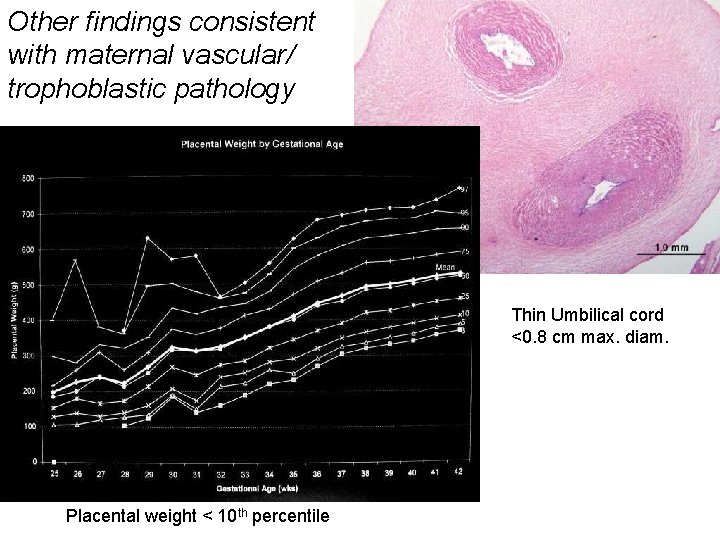
Other findings consistent with maternal vascular/ trophoblastic pathology Thin Umbilical cord <0. 8 cm max. diam. Placental weight < 10 th percentile

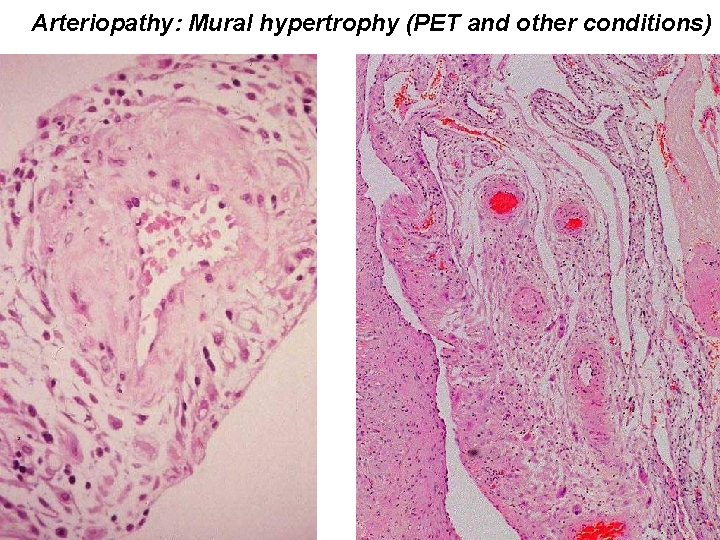
Arteriopathy: Mural hypertrophy (PET and other conditions)

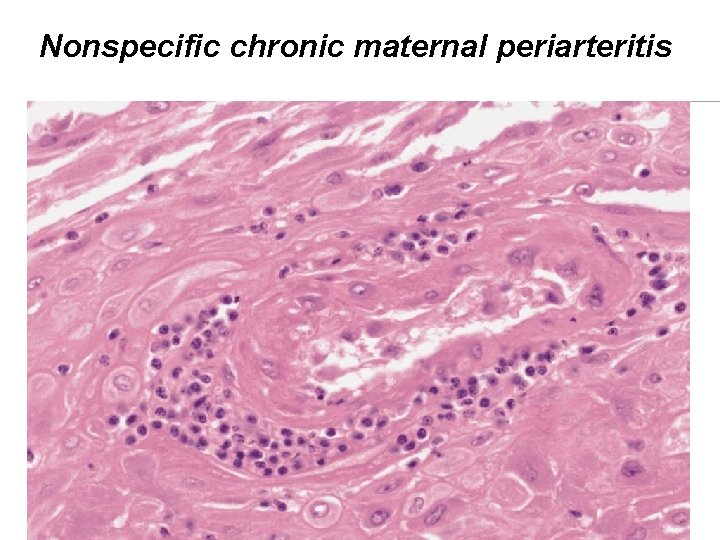
Nonspecific chronic maternal periarteritis

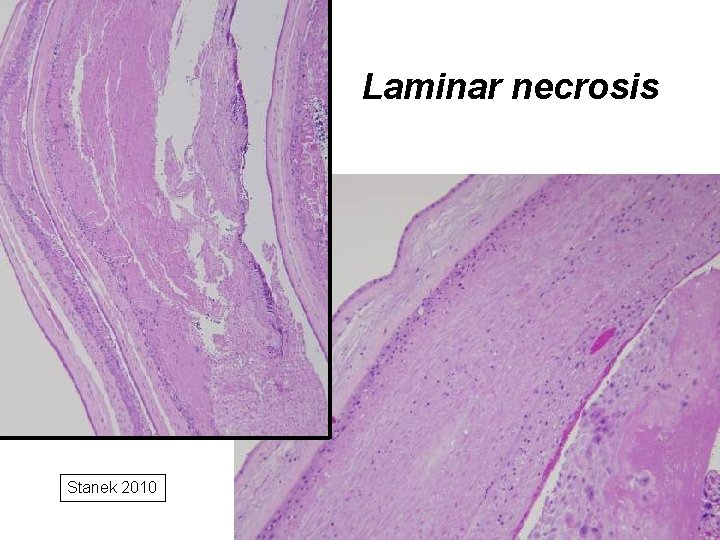
Laminar necrosis Stanek 2010

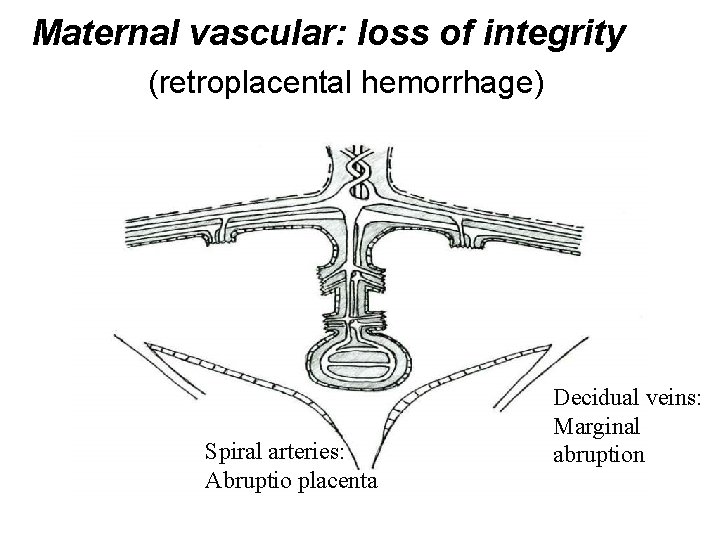
Maternal vascular: loss of integrity (retroplacental hemorrhage) Spiral arteries: Abruptio placenta Decidual veins: Marginal abruption

Retroplacental hemorrhage (clinical syndromes) • Abruptio placenta • Marginal abruption • Chronic abruption

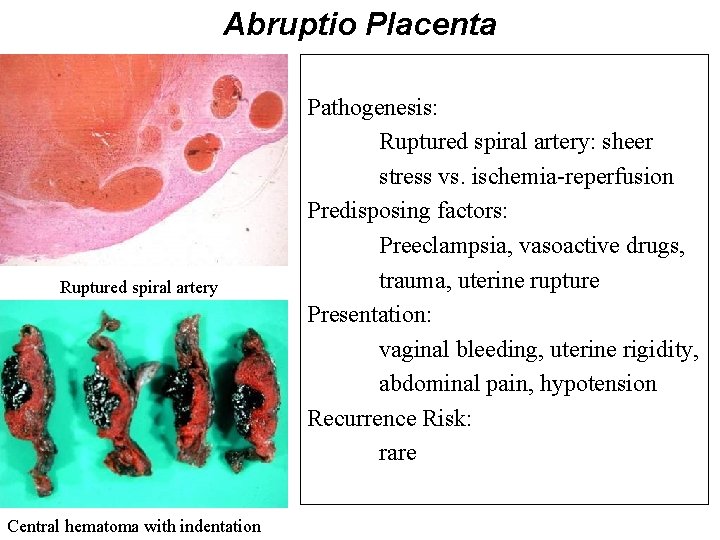
Abruptio Placenta Ruptured spiral artery Central hematoma with indentation Pathogenesis: Ruptured spiral artery: sheer stress vs. ischemia-reperfusion Predisposing factors: Preeclampsia, vasoactive drugs, trauma, uterine rupture Presentation: vaginal bleeding, uterine rigidity, abdominal pain, hypotension Recurrence Risk: rare


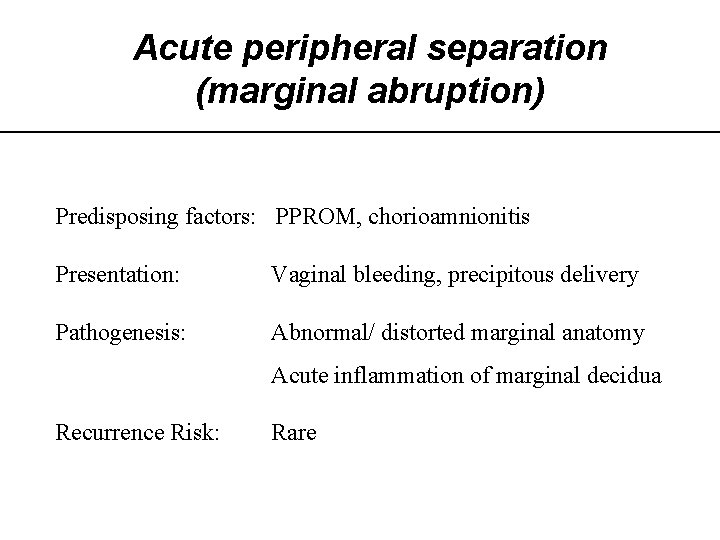
Acute peripheral separation (marginal abruption) Predisposing factors: PPROM, chorioamnionitis Presentation: Vaginal bleeding, precipitous delivery Pathogenesis: Abnormal/ distorted marginal anatomy Acute inflammation of marginal decidua Recurrence Risk: Rare

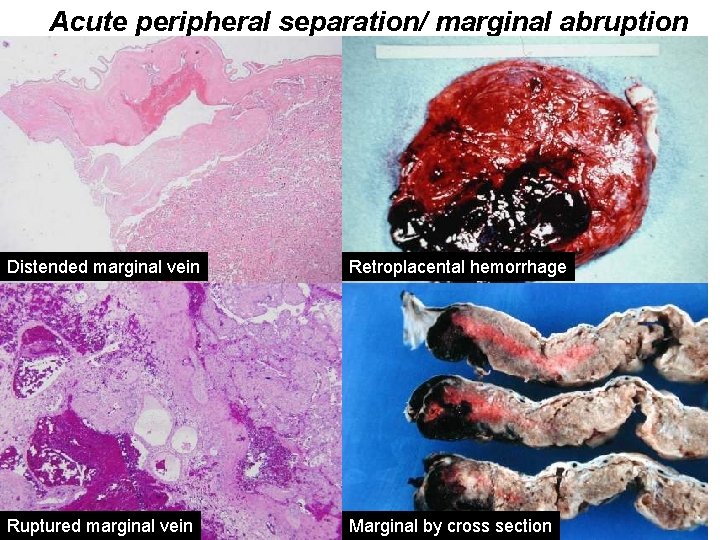
Acute peripheral separation/ marginal abruption Distended marginal vein Retroplacental hemorrhage Ruptured marginal vein Marginal by cross section

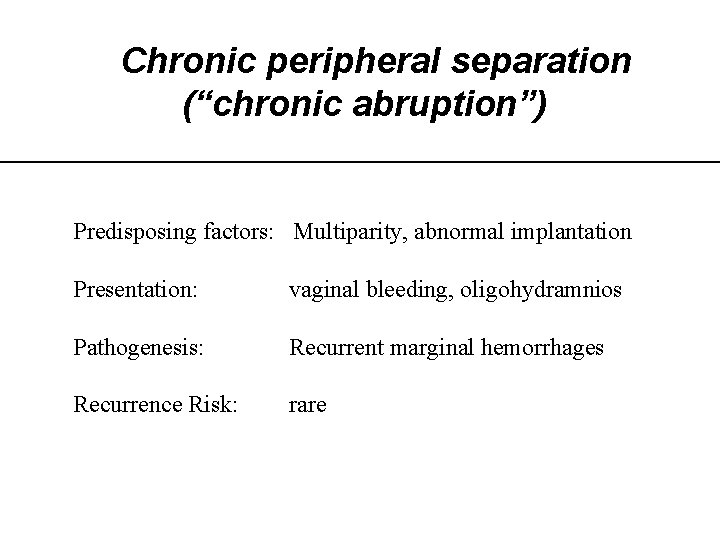
Chronic peripheral separation (“chronic abruption”) Predisposing factors: Multiparity, abnormal implantation Presentation: vaginal bleeding, oligohydramnios Pathogenesis: Recurrent marginal hemorrhages Recurrence Risk: rare

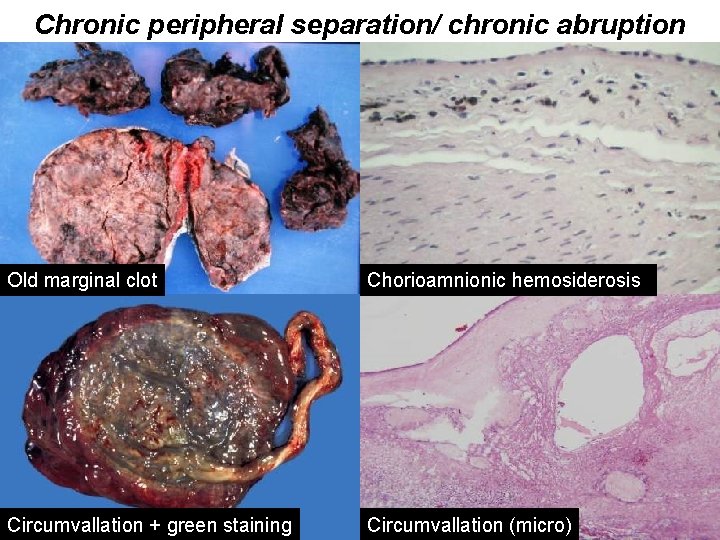
Chronic peripheral separation/ chronic abruption Old marginal clot Chorioamnionic hemosiderosis Circumvallation + green staining Circumvallation (micro)

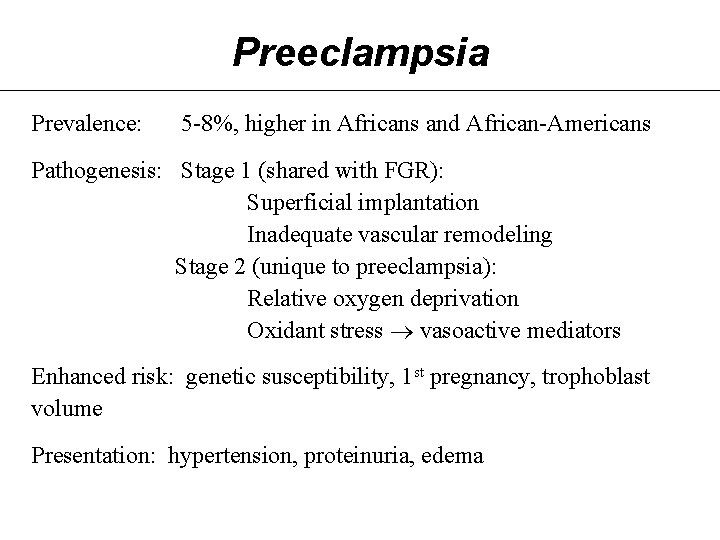
Preeclampsia Prevalence: 5 -8%, higher in Africans and African-Americans Pathogenesis: Stage 1 (shared with FGR): Superficial implantation Inadequate vascular remodeling Stage 2 (unique to preeclampsia): Relative oxygen deprivation Oxidant stress vasoactive mediators Enhanced risk: genetic susceptibility, 1 st pregnancy, trophoblast volume Presentation: hypertension, proteinuria, edema

Underlying vascular disease Early Uterine Hypoxia Poor Placentation (PP 13, ADAM 12, PAPPA) FGR Obesity Diabetes Late Trophoplast Hypoxia Large Placenta Increased Trophoblast antiangiogenic factors (s. Flt-1 s. ENG) Endothelial Dysfunction Preeclampsia (HPL, HGHv) Insulin Resistance

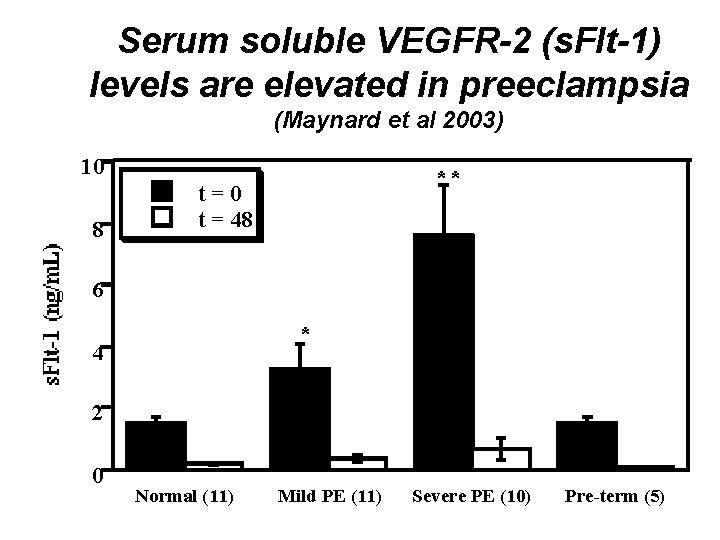
Serum soluble VEGFR-2 (s. Flt-1) levels are elevated in preeclampsia (Maynard et al 2003) 10 8 ** t=0 t = 48 6 * 4 2 0 Normal (11) Mild PE (11) Severe PE (10) Pre-term (5)

Free VEGF is decreased in preeclampsia

Free Pl. GF is decreased in preeclampsia

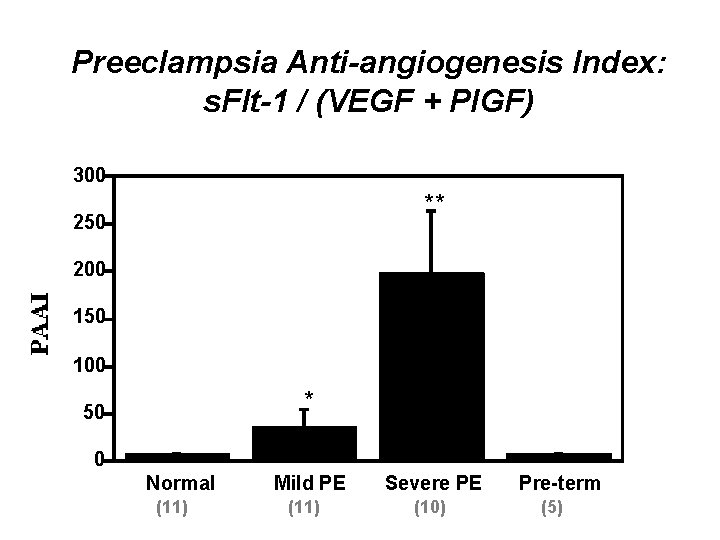
Preeclampsia Anti-angiogenesis Index: s. Flt-1 / (VEGF + Pl. GF) 300 ** 250 200 150 100 * 50 0 Normal (11) Mild PE (11) Severe PE (10) Pre-term (5)

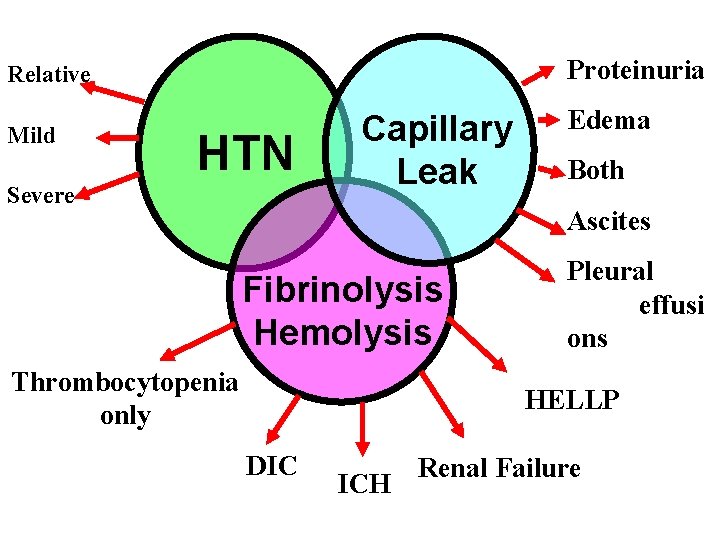
Proteinuria Relative Mild HTN Severe Capillary Leak Edema Both Ascites Fibrinolysis Hemolysis Thrombocytopenia only Pleural effusi ons HELLP DIC ICH Renal Failure

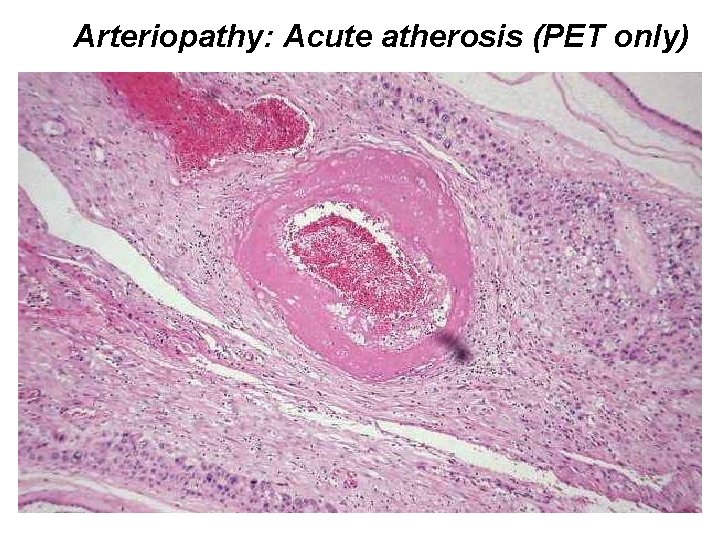
Arteriopathy: Acute atherosis (PET only)

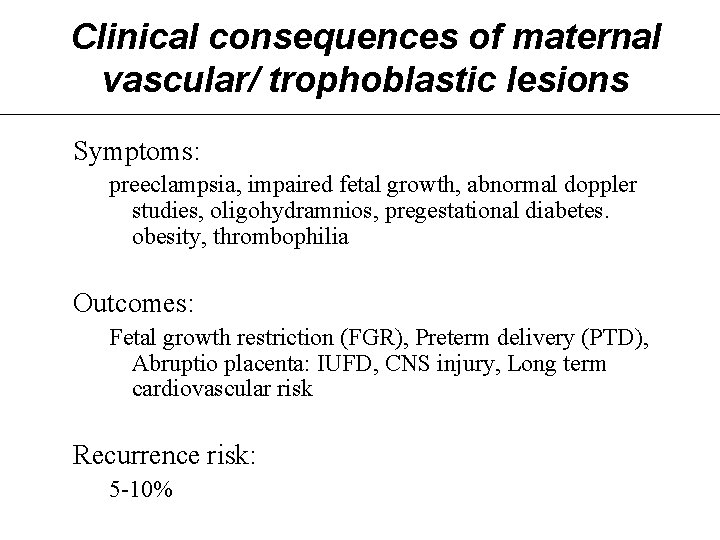
Clinical consequences of maternal vascular/ trophoblastic lesions Symptoms: preeclampsia, impaired fetal growth, abnormal doppler studies, oligohydramnios, pregestational diabetes. obesity, thrombophilia Outcomes: Fetal growth restriction (FGR), Preterm delivery (PTD), Abruptio placenta: IUFD, CNS injury, Long term cardiovascular risk Recurrence risk: 5 -10%

References: 1. Langston C, Kaplan C, Macpherson T, et al. Practice guideline for examination of the placenta. Arch Pathol Lab Med. 1997; 121: 449 -476. 2. Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta. Mar 2008; 29 Suppl A: S 86 -91. 3. Redline RW, Boyd T, Campbell V, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Develop Pathol. 2004; 7: 237 -249. 4. Khong TY, Adema ED, Erwich JJ. On an anatomical basis for the increase in birth weight in second and subsequent born children. Placenta. Apr 2003; 24(4): 348 -353. 5. Stanek J. Utility of diagnosing various histological patterns of diffuse chronic hypoxic placental injury Pediatr Develop Pathol 2012; 15(1): 13 -23. 6. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. Jun 2009; 30(6): 473 -482. 7. Kraus FT, Redline R, Gersell DJ, Nelson DM, Dicke JM. Placental Pathology. Washington, D. C. : American Registry of Pathology; 2004. 8. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (s. Flt 1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003; 111(5): 649 -658.
 Extranuclear inheritance
Extranuclear inheritance Non nuclear inheritance
Non nuclear inheritance White lesions
White lesions Describing skin lesions
Describing skin lesions Vesiculobullous lesions
Vesiculobullous lesions Scattered white matter lesions
Scattered white matter lesions Rubrospinal tract
Rubrospinal tract Eye lesions
Eye lesions No touch lesions bone
No touch lesions bone Site:slidetodoc.com
Site:slidetodoc.com Premalignant lesions of esophagus
Premalignant lesions of esophagus Daughter mother grandmother pancreatic lesions
Daughter mother grandmother pancreatic lesions Gingival third vs cervical third
Gingival third vs cervical third Pie on the floor visual field defect
Pie on the floor visual field defect Site:slidetodoc.com
Site:slidetodoc.com Lesion to vagus nerve
Lesion to vagus nerve Primary and secondary lesions of skin
Primary and secondary lesions of skin Classification of periradicular diseases
Classification of periradicular diseases Amiante
Amiante Pyramidal vs extrapyramidal lesions
Pyramidal vs extrapyramidal lesions Lésions
Lésions It projektov�� mana����r pr��ca
It projektov�� mana����r pr��ca Chapter 17 oral pathology short answer questions
Chapter 17 oral pathology short answer questions Olneys lesions
Olneys lesions Eye lesions
Eye lesions Aakash saw his sister's son's maternal aunts
Aakash saw his sister's son's maternal aunts Factors influencing maternal health
Factors influencing maternal health The passageway consists of the maternal and soft tissues
The passageway consists of the maternal and soft tissues Maternal deprivation
Maternal deprivation Clinical pelvimetry
Clinical pelvimetry Chapter 15 maternal and fetal nutrition
Chapter 15 maternal and fetal nutrition Ramona t mercer
Ramona t mercer Pedigree chart symbols
Pedigree chart symbols National health intervention program for mother and child
National health intervention program for mother and child Maternal effect
Maternal effect Maternal effect
Maternal effect Maternal behavior
Maternal behavior La muda de la flor eduardo kingman
La muda de la flor eduardo kingman Sanford maternal fetal medicine
Sanford maternal fetal medicine Desde o seio maternal sois meu amparo
Desde o seio maternal sois meu amparo Onset of labour
Onset of labour Maternal death reporting format
Maternal death reporting format Ursulinehs
Ursulinehs Cmqcc data center
Cmqcc data center Jama 2017
Jama 2017 Isolated maternal hypothyroxinemia
Isolated maternal hypothyroxinemia Break definition
Break definition Calendario maternal
Calendario maternal An inspector calls act 1 revision
An inspector calls act 1 revision Potter face oligohydramnios
Potter face oligohydramnios Lochia alba smell
Lochia alba smell What is maternal health
What is maternal health Maternal pelvis and fetal skull
Maternal pelvis and fetal skull Maternal and child health services
Maternal and child health services History of maternal and child health
History of maternal and child health Polyhydroamnios
Polyhydroamnios Global causes of maternal death: a who systematic analysis
Global causes of maternal death: a who systematic analysis Sepsis 6 bundle
Sepsis 6 bundle Maternal pelvis and fetal skull
Maternal pelvis and fetal skull