Development of chromosome theory of inheritance 1900 rediscovery






- Slides: 30

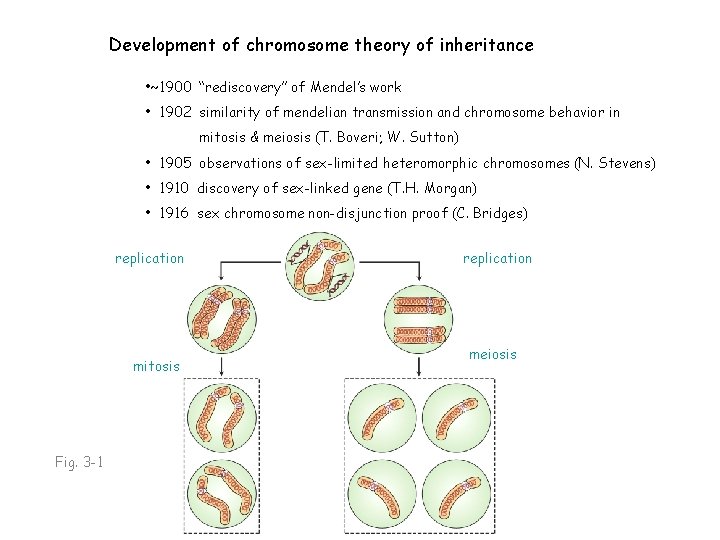
Development of chromosome theory of inheritance • ~1900 “rediscovery” of Mendel’s work • 1902 similarity of mendelian transmission and chromosome behavior in mitosis & meiosis (T. Boveri; W. Sutton) • 1905 observations of sex-limited heteromorphic chromosomes (N. Stevens) • 1910 discovery of sex-linked gene (T. H. Morgan) • 1916 sex chromosome non-disjunction proof (C. Bridges)

Development of chromosome theory of inheritance • ~1900 “rediscovery” of Mendel’s work • 1902 similarity of mendelian transmission and chromosome behavior in mitosis & meiosis (T. Boveri; W. Sutton) • 1905 observations of sex-limited heteromorphic chromosomes (N. Stevens) • 1910 discovery of sex-linked gene (T. H. Morgan) • 1916 sex chromosome non-disjunction proof (C. Bridges) replication mitosis Fig. 3 -1 replication meiosis

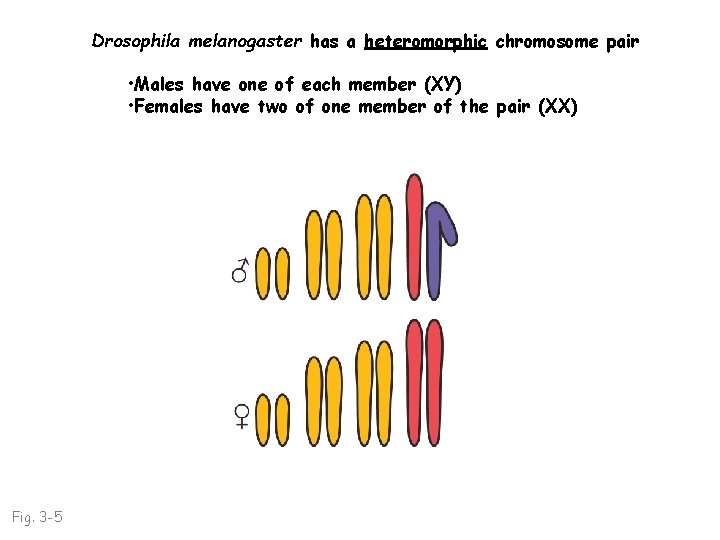
Drosophila melanogaster has a heteromorphic chromosome pair • Males have one of each member (XY) • Females have two of one member of the pair (XX) Fig. 3 -5

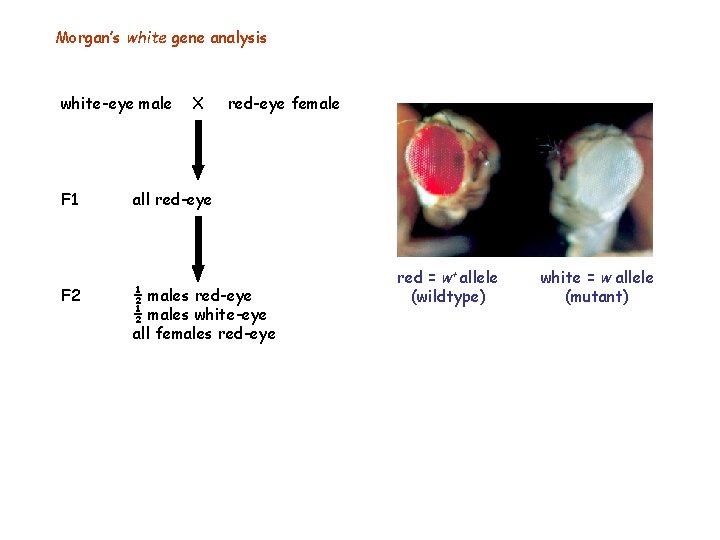
Morgan’s white gene analysis white-eye male F 1 F 2 X red-eye female all red-eye ½ males white-eye all females red-eye red = w+ allele (wildtype) white = w allele (mutant)

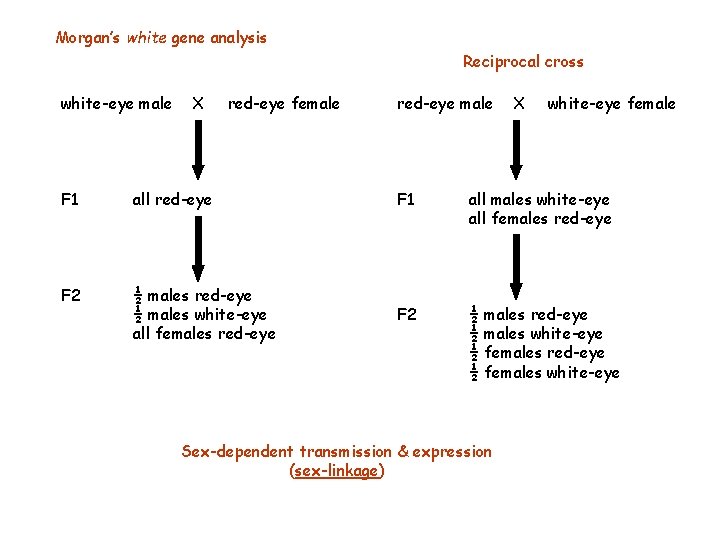
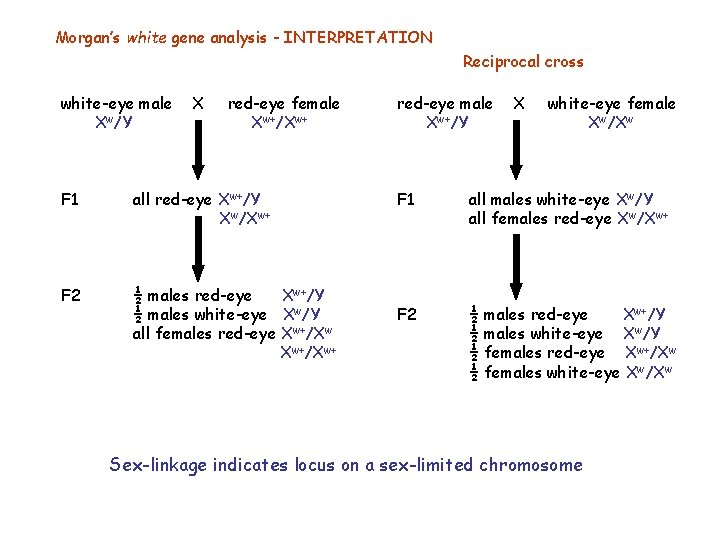
Morgan’s white gene analysis Reciprocal cross white-eye male X red-eye female red-eye male X white-eye female F 1 all red-eye F 1 all males white-eye all females red-eye F 2 ½ males red-eye ½ males white-eye ½ females red-eye ½ females white-eye Sex-dependent transmission & expression (sex-linkage)

Morgan’s white gene analysis - INTERPRETATION Reciprocal cross white-eye male Xw/Y X red-eye female Xw+/Xw+ F 1 all red-eye Xw+/Y Xw/Xw+ F 2 ½ males red-eye Xw+/Y ½ males white-eye Xw/Y all females red-eye Xw+/Xw+ red-eye male Xw+/Y X white-eye female Xw/Xw F 1 all males white-eye Xw/Y all females red-eye Xw/Xw+ F 2 ½ males red-eye Xw+/Y ½ males white-eye Xw/Y ½ females red-eye Xw+/Xw ½ females white-eye Xw/Xw Sex-linkage indicates locus on a sex-limited chromosome

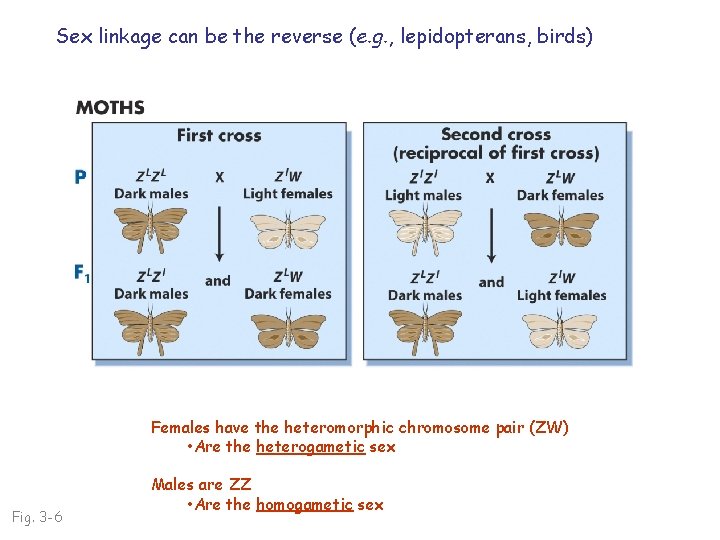
Sex linkage can be the reverse (e. g. , lepidopterans, birds) Females have the heteromorphic chromosome pair (ZW) • Are the heterogametic sex Fig. 3 -6 Males are ZZ • Are the homogametic sex

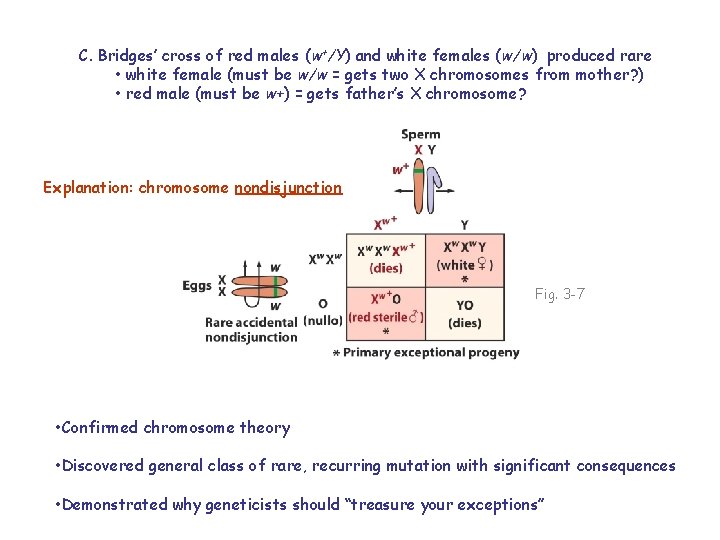
C. Bridges’ cross of red males (w+/Y) and white females (w/w) produced rare • white female (must be w/w = gets two X chromosomes from mother? ) • red male (must be w+) = gets father’s X chromosome?

C. Bridges’ cross of red males (w+/Y) and white females (w/w) produced rare • white female (must be w/w = gets two X chromosomes from mother? ) • red male (must be w+) = gets father’s X chromosome? Explanation: chromosome nondisjunction Fig. 3 -7 • Confirmed chromosome theory • Discovered general class of rare, recurring mutation with significant consequences • Demonstrated why geneticists should “treasure your exceptions”


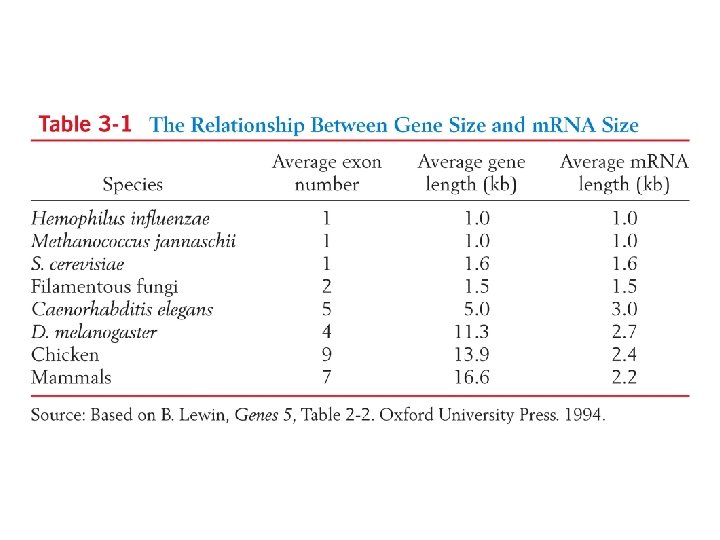
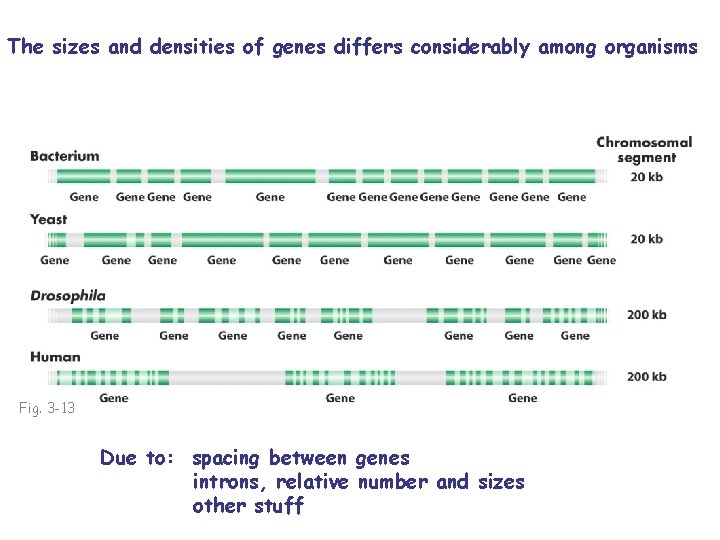
The sizes and densities of genes differs considerably among organisms Fig. 3 -13 Due to: spacing between genes introns, relative number and sizes other stuff

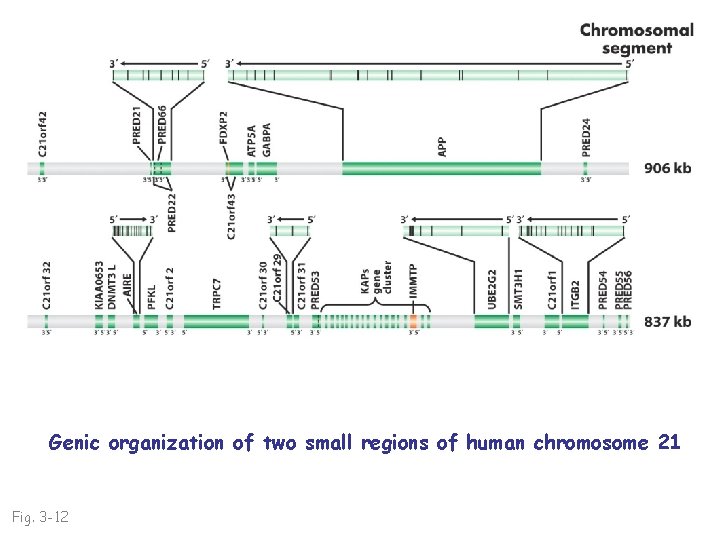
Genic organization of two small regions of human chromosome 21 Fig. 3 -12

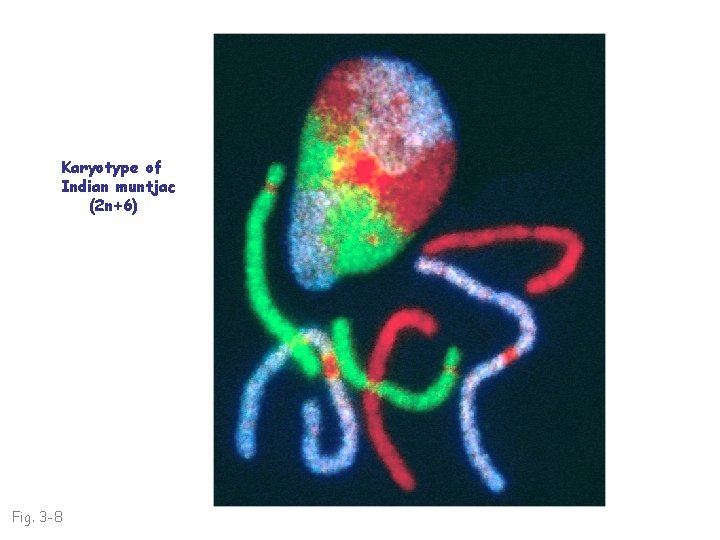
Karyotype of Indian muntjac (2 n+6) Fig. 3 -8

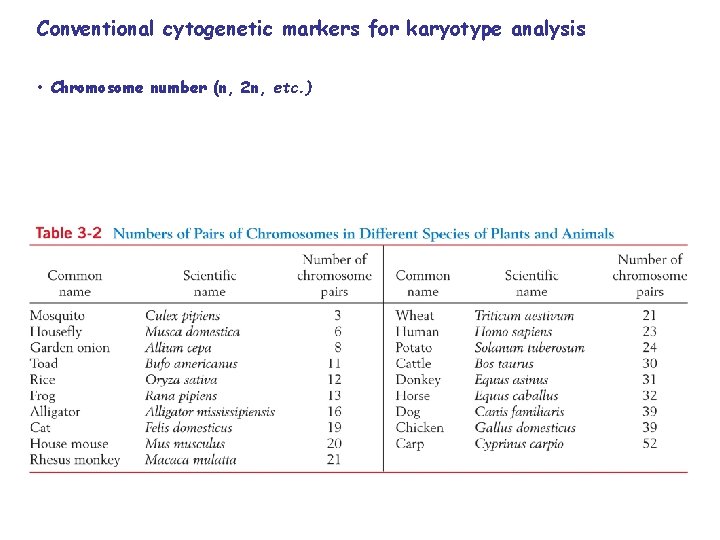
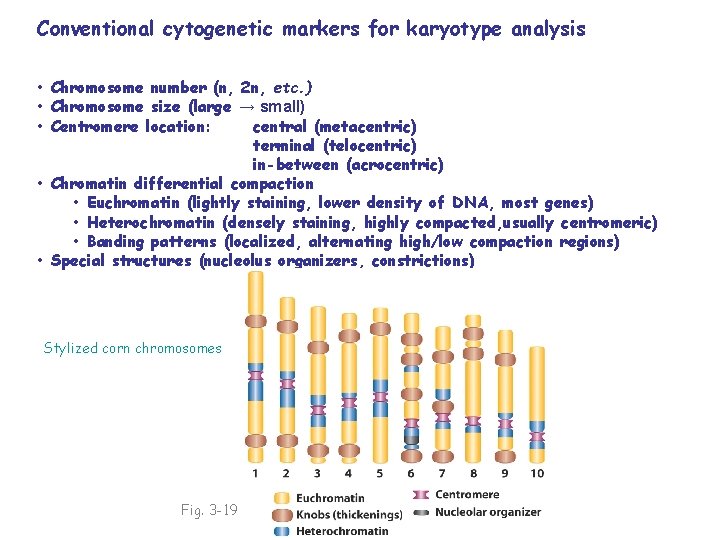
Conventional cytogenetic markers for karyotype analysis • Chromosome number (n, 2 n, etc. )

Conventional cytogenetic markers for karyotype analysis • Chromosome number (n, 2 n, etc. ) • Chromosome size (large → small) • Centromere location: central (metacentric) terminal (telocentric) in-between (acrocentric) • Chromatin differential compaction • Euchromatin (lightly staining, lower density of DNA, most genes) • Heterochromatin (densely staining, highly compacted, usually centromeric) • Banding patterns (localized, alternating high/low compaction regions)

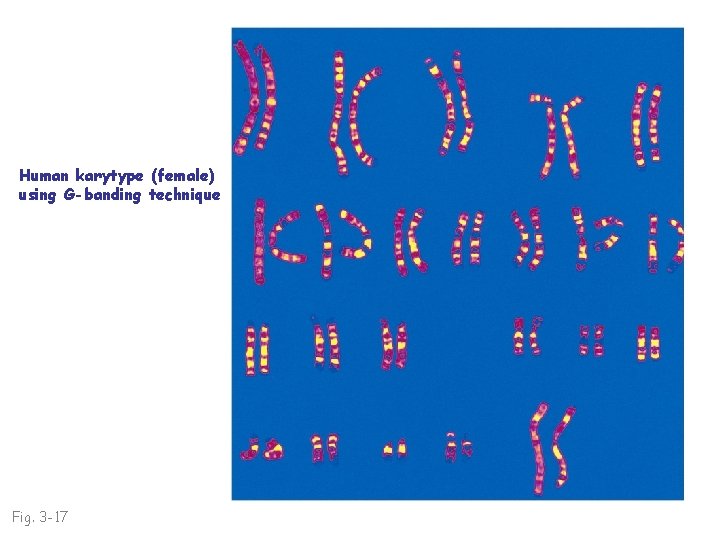
Human karytype (female) using G-banding technique Fig. 3 -17

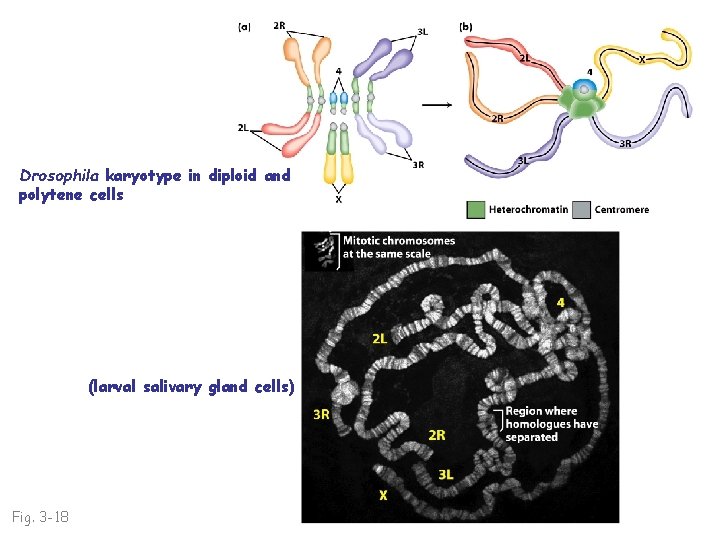
Drosophila karyotype in diploid and polytene cells (larval salivary gland cells) Fig. 3 -18

Conventional cytogenetic markers for karyotype analysis • Chromosome number (n, 2 n, etc. ) • Chromosome size (large → small) • Centromere location: central (metacentric) terminal (telocentric) in-between (acrocentric) • Chromatin differential compaction • Euchromatin (lightly staining, lower density of DNA, most genes) • Heterochromatin (densely staining, highly compacted, usually centromeric) • Banding patterns (localized, alternating high/low compaction regions) • Special structures (nucleolus organizers, constrictions) Stylized corn chromosomes Fig. 3 -19

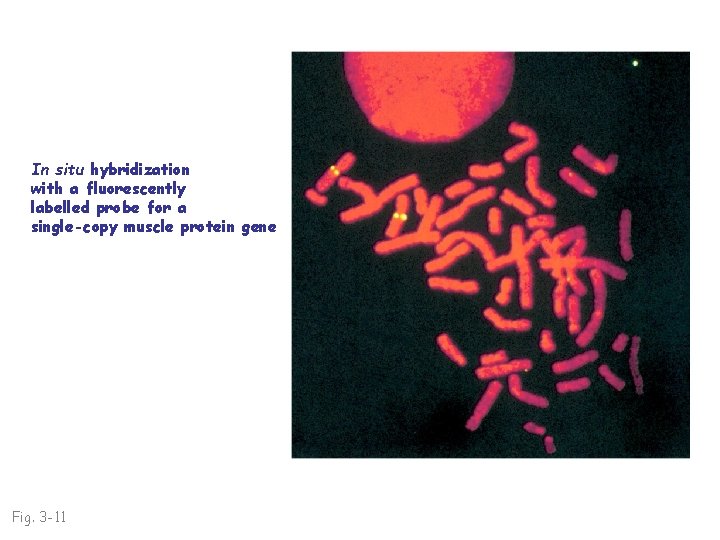
In situ hybridization with a fluorescently labelled probe for a single-copy muscle protein gene Fig. 3 -11

In situ hybridization with a 3 H-labelled probe for a mouse satellite DNA Fig. 3 -14

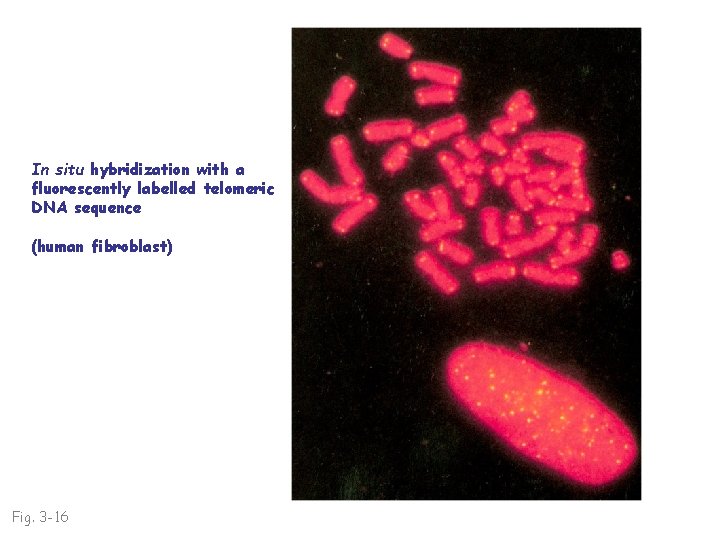
In situ hybridization with a fluorescently labelled telomeric DNA sequence (human fibroblast) Fig. 3 -16

Repeated from Cell Biology (must read/review) Pages 88 -90: chromosome/chromatin structure Pages 90 -95: mitosis & meiosis Pages 96 -97: life cycles of haploid and diploid organisms

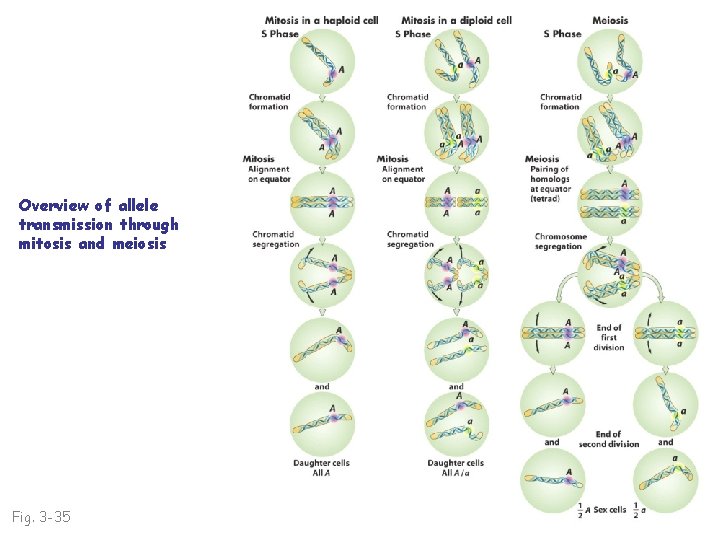
Overview of allele transmission through mitosis and meiosis Fig. 3 -35

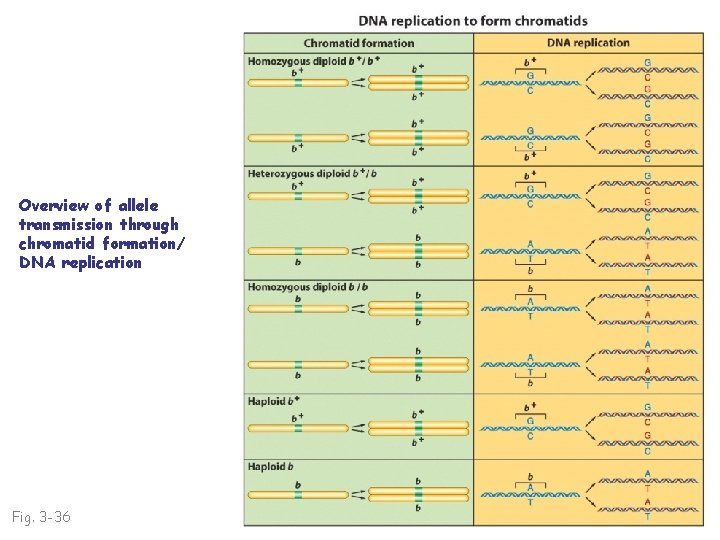
Overview of allele transmission through chromatid formation/ DNA replication Fig. 3 -36

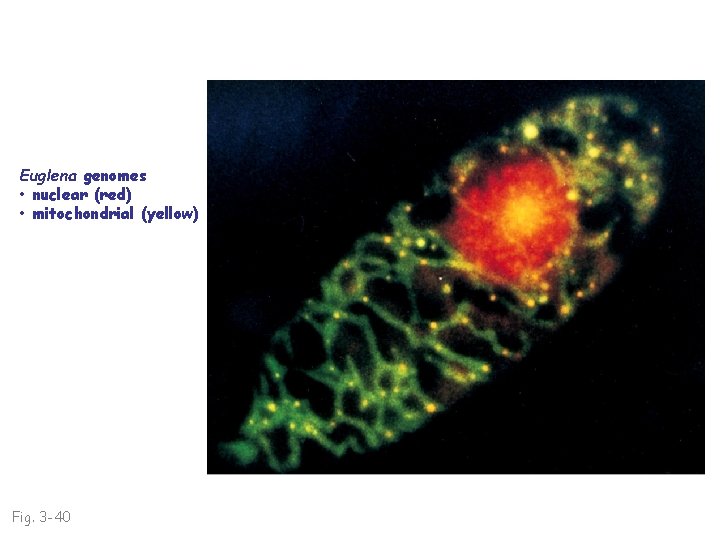
Euglena genomes • nuclear (red) • mitochondrial (yellow) Fig. 3 -40

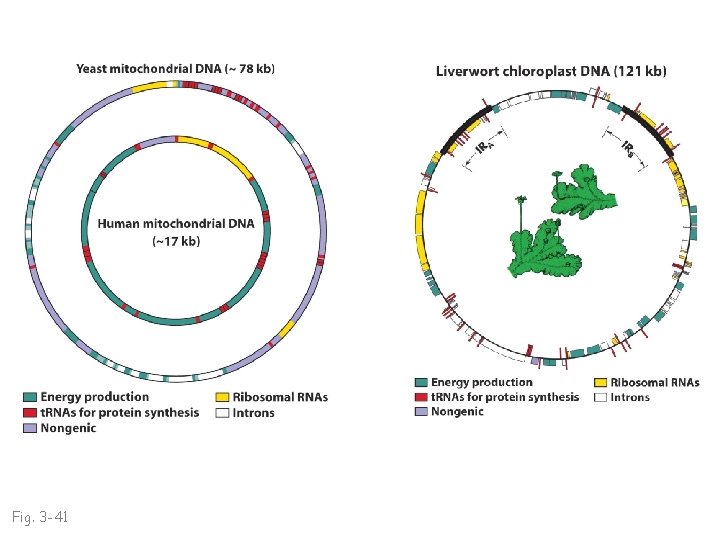
Fig. 3 -41

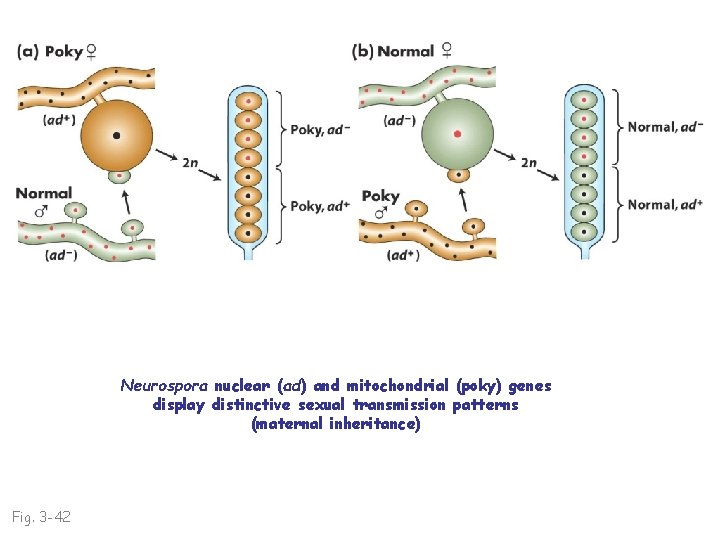
Neurospora nuclear (ad) and mitochondrial (poky) genes display distinctive sexual transmission patterns (maternal inheritance) Fig. 3 -42

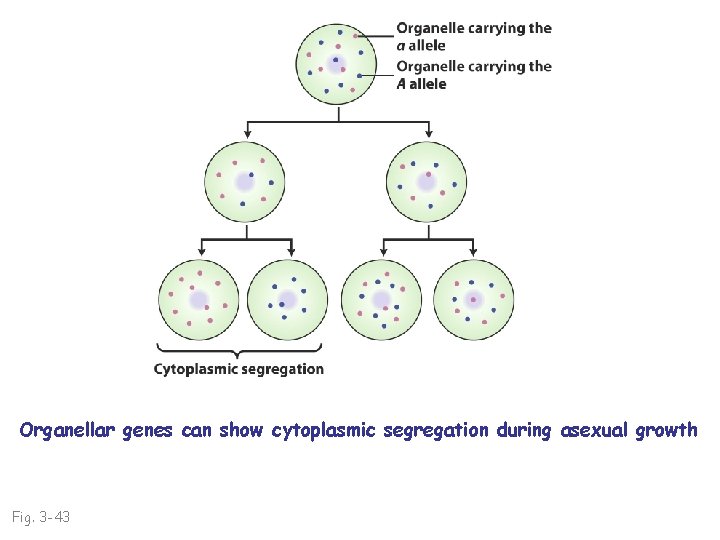
Organellar genes can show cytoplasmic segregation during asexual growth Fig. 3 -43

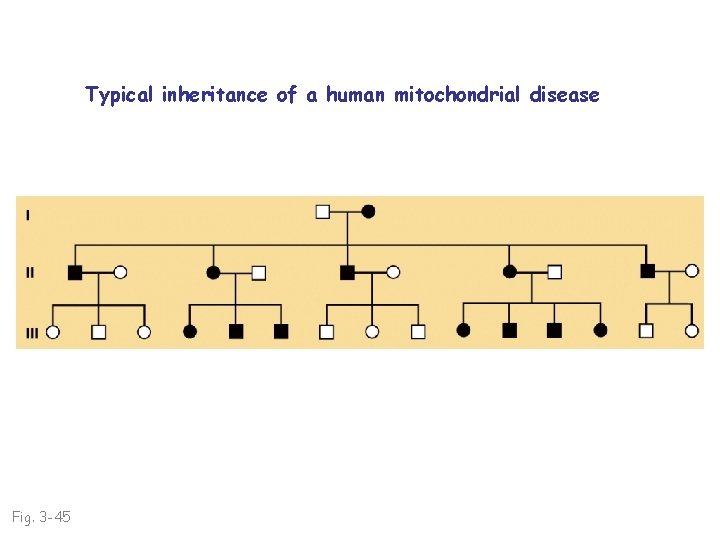
Typical inheritance of a human mitochondrial disease Fig. 3 -45

Fig. 3 -1
 Chromosome theory of inheritance
Chromosome theory of inheritance How to read chromosome
How to read chromosome Chapter 15: the chromosomal basis of inheritance
Chapter 15: the chromosomal basis of inheritance Mendel's experimental design
Mendel's experimental design Purebred vs hybrid
Purebred vs hybrid 1750-1900 portfolio map
1750-1900 portfolio map Bumblebee totem pole
Bumblebee totem pole Characteristics of the romantic period
Characteristics of the romantic period The rise of industrial america 1865-1900
The rise of industrial america 1865-1900 Four features of industrial manufacturing (1865-1900)
Four features of industrial manufacturing (1865-1900) Lacey act 1900
Lacey act 1900 The interpretation of dreams 1900
The interpretation of dreams 1900 Iso1900
Iso1900 1820-1900
1820-1900 Revolutionary leaders 1900-1939
Revolutionary leaders 1900-1939 Moda 1900
Moda 1900 Kapcie emu 1900
Kapcie emu 1900 Moda 1910
Moda 1910 Litteraturen 1900 til 1945
Litteraturen 1900 til 1945 Literatur um die jahrhundertwende
Literatur um die jahrhundertwende 1900 max planck
1900 max planck Språkdebatten 1900
Språkdebatten 1900 John ruskin (1819-1900)
John ruskin (1819-1900) Inf1900
Inf1900 Moderne massasamenleving
Moderne massasamenleving French fashion 1800
French fashion 1800 Edwardian era fashion
Edwardian era fashion Kristallpalast london bauzeit
Kristallpalast london bauzeit Scoperte scientifiche dal 1850 al 1900
Scoperte scientifiche dal 1850 al 1900 Cdmvt navigation
Cdmvt navigation Zurn 1900
Zurn 1900