MATERIAL SCIENCE PHASE DIAGRAM TRANSFORMATIONS AND THERMAL PROCESSING

















- Slides: 63

MATERIAL SCIENCE PHASE DIAGRAM, TRANSFORMATIONS AND THERMAL PROCESSING DEFINITIONS AND BASIC CONCEPTS PHASE DIAGRAM MICROSTRUCTURE TTT DIAGRAM HEAT TREATMENT

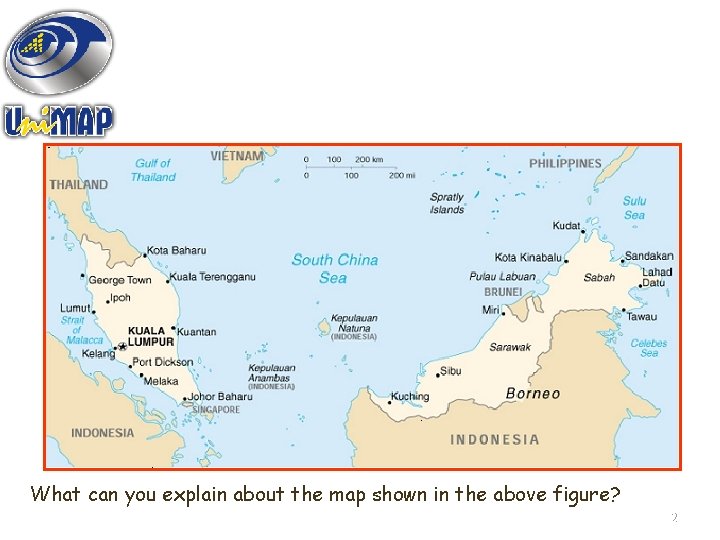
What can you explain about the map shown in the above figure? 2

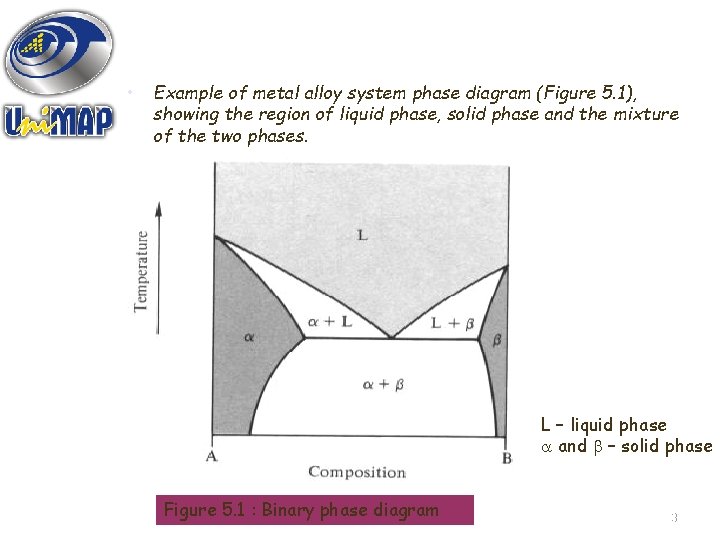
• Example of metal alloy system phase diagram (Figure 5. 1), showing the region of liquid phase, solid phase and the mixture of the two phases. L – liquid phase and – solid phase Figure 5. 1 : Binary phase diagram 3

DEFINITIONS AND BASIC CONCEPTS ð It is fundamental to materials science and engineering that properties of materials reflects their microstructure. ð Microstructures are controlled by the composition of the materials and how it is processed. ð Manufacturing → shaping + assembling engineering product & devices + provides the materials properties required. ð Most materials processing involves thermal history. ð Important to understand how processing history (particularly temperature) control microstructure and properties. 4

SOME ESSENTIAL DEFINITIONS : * Phase Diagram – a diagram with temperature and composition as axes provide some fundamental knowledge of what the equilibrium structure of a metallic (or ceramic) alloy. * Metallic Alloy – a mixture of metals with other metals or nonmetals. Example : Brass → a mixture of copper (Cu) and zinc (Zn) * Components – the chemical elements (or compounds) which make up alloys. Example : in brass the main components are Cu and Zn. 5

* Binary alloy – contains two (2) components. Ternary alloy – contains three (3) components. Quarternary alloy – contains four (4) components. * Phase – all parts of an alloy microstructure with the same physical properties, and the same composition. For example, in a binary alloy (solid state) the microstructure can take one of four forms; (a) a single solid solution; (b) two separated pure components; (c) two separated solid solutions; (d) chemical compounds, together with solid solution. 6

Solid solution – a solid in which an element is “dissolves” in another so that it is homogeneously dispersed, at an atomic scale. Alloy are described by stating the components and their concentrations, in weight or atom %. * The weight % of component A : * The atom (or mol) % of component A : No. of mols = (weight in grams)/(atomic or molecular wt in grams/mol) Wt in grams = (No. of mols) x (atomic or molecular wt in grams/mol) 7

* Constitution – the constitution of an alloy is described by : (a) the phases present (b) the weight fraction of each phase (c) the composition of each phase * Equilibrium constitution – there is no further tendency for the constitution of a sample of given composition to change with time at a constant temperature (T) and pressure (p). 8

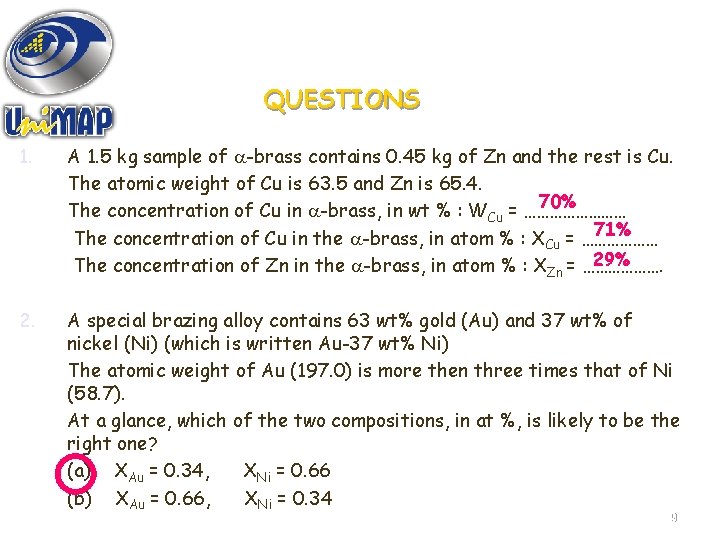
QUESTIONS 1. A 1. 5 kg sample of -brass contains 0. 45 kg of Zn and the rest is Cu. The atomic weight of Cu is 63. 5 and Zn is 65. 4. 70% The concentration of Cu in -brass, in wt % : WCu = ………… 71% The concentration of Cu in the -brass, in atom % : XCu = ……………… 29% The concentration of Zn in the -brass, in atom % : XZn = ………………. 2. A special brazing alloy contains 63 wt% gold (Au) and 37 wt% of nickel (Ni) (which is written Au-37 wt% Ni) The atomic weight of Au (197. 0) is more then three times that of Ni (58. 7). At a glance, which of the two compositions, in at %, is likely to be the right one? (a) XAu = 0. 34, XNi = 0. 66 (b) XAu = 0. 66, XNi = 0. 34 9

3. An alloy consists of XA atom% of A with an atomic weight of a. A, and XB atom% of B with an atomic weight of a. B. Derive an equation for the concentration of A in wt%. By symmetry, write down the equation for the concentration of B in wt%. 4. On heating, pure copper starts to melt at 1083 C. While it is melting, solid and liquid copper co-exist. Using the definitions of a phase, are one or two phases present? Why? Ans ; 5. 2 phases : solid and liquid. Although they have the same chemical composition, they differ in physical properties. Three components A, B and C of an alloy dissolve completely when liquid but have no mutual solubility when solid. They do not form any chemical compounds. How many phases, and of what compositions, do you think would appear in the solid state? Ans ; 3 phases : pure A, pure B and pure C. 10

PHASE DIAGRAM • Processing of engineering materials always involves materials in liquid or solid state. • In these states, pressure (p) only has a small influence on the equilibrium constitution, and can be neglected. • In phase diagram, we will only be concerned with material states which are controlled by the remaining two state variables : temperature and composition. • The phase diagram of the components A and B is determined (at a standard pressure of 1 atm) by the temperature T and the composition XB (or WB) or XA (or WA). 11

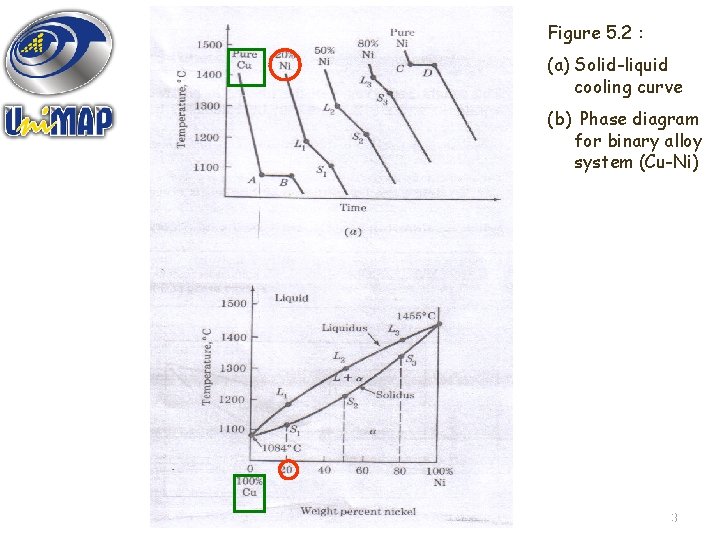
BUILDING A PHASE DIAGRAM; • Example : Water-Salt System Consisting of two (2) components (each is a compound with a definite chemical composition) • Equilibrium phase diagram for binary alloy system (Figure 5. 2 b)– can be built from solid-liquid cooling curve (Figure 5. 2 a). 12

Figure 5. 2 : (a) Solid-liquid cooling curve (b) Phase diagram for binary alloy system (Cu-Ni) 13

Some of the important information obtainable from phase diagrams is : 1. 2. 3. 4. To show what phases are present at different compositions and temperatures under slow cooling (equilibrium) conditions. To indicate the equilibrium solid solubility of one element (or compound) in another. To indicate the temperature at which an alloy cooled under equilibrium conditions starts to solidify and the temperature range over which solidification occurs. To indicate the temperature at which different phases start to melt. 14

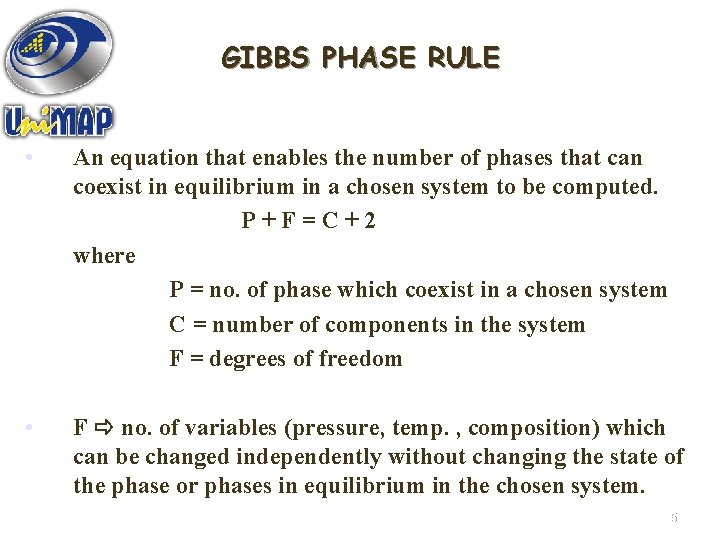
GIBBS PHASE RULE • An equation that enables the number of phases that can coexist in equilibrium in a chosen system to be computed. P+F=C+2 where P = no. of phase which coexist in a chosen system C = number of components in the system F = degrees of freedom • F no. of variables (pressure, temp. , composition) which can be changed independently without changing the state of the phase or phases in equilibrium in the chosen system. 15

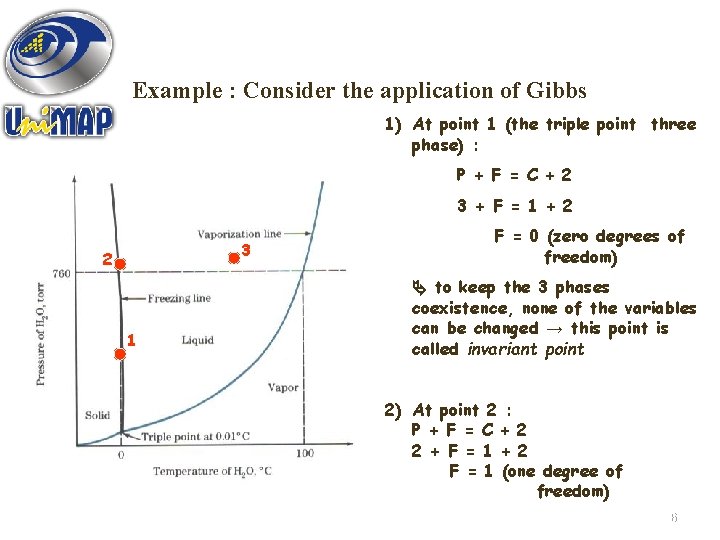
Example : Consider the application of Gibbs 1) At point 1 (the triple point three phase) : P + F = C + 2 3 + F = 1 + 2 3 2 1 F = 0 (zero degrees of freedom) to keep the 3 phases coexistence, none of the variables can be changed → this point is called invariant point 2) At point 2 : P + F = C + 2 2 + F = 1 + 2 Figure 5. 3 : Approximate pressure-temperature F = 1 (one degree of (PT) equilibrium phase diagram for pure water. freedom) 16

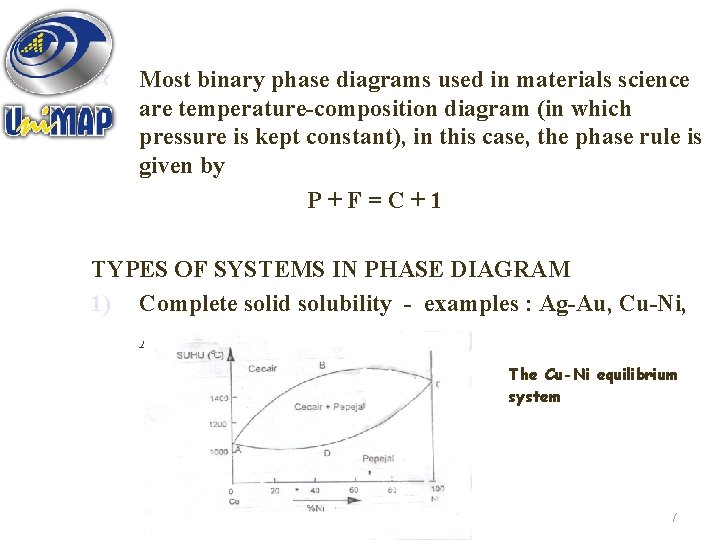
¶ Most binary phase diagrams used in materials science are temperature-composition diagram (in which pressure is kept constant), in this case, the phase rule is given by P+F=C+1 TYPES OF SYSTEMS IN PHASE DIAGRAM 1) Complete solid solubility - examples : Ag-Au, Cu-Ni, Ag-Pt Figure 5. 4 : The Cu-Ni equilibrium system 17

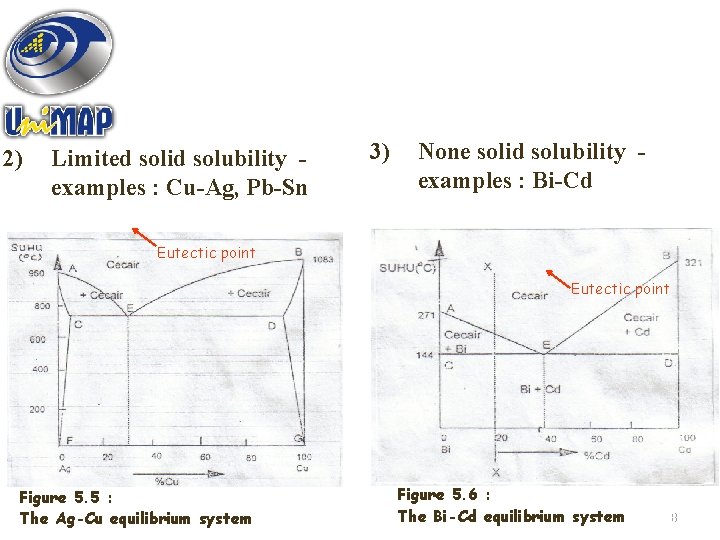
2) Limited solid solubility examples : Cu-Ag, Pb-Sn 3) None solid solubility examples : Bi-Cd Eutectic point Figure 5. 5 : The Ag-Cu equilibrium system Figure 5. 6 : The Bi-Cd equilibrium system 18

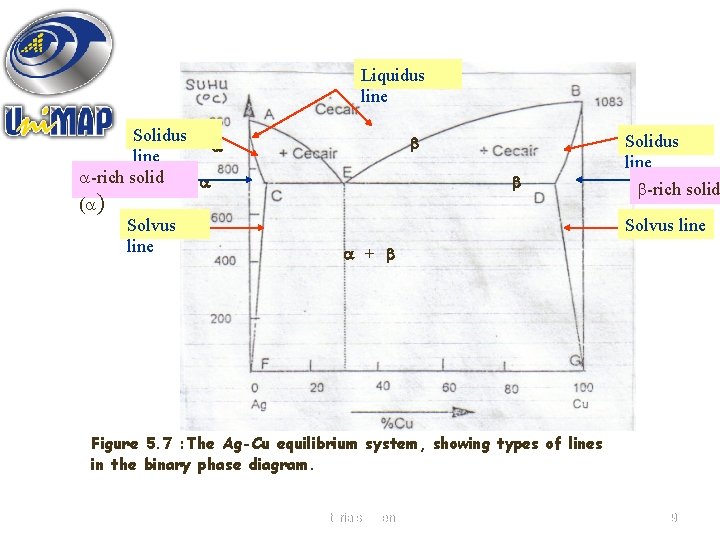
Liquidus line Solidus line -rich solid ( ) Solvus line Solidus line -rich solid Solvus line + Figure 5. 7 : The Ag-Cu equilibrium system, showing types of lines in the binary phase diagram. Materials Science 19

Figure 5. 7 shows the types of line in the phase diagram : • Liquidus line – the phase boundary which limits the bottom of the liquid field • Solidus line – the line giving the upper limit of the single phase solid field. • Solvus line – the line which separates the two phase field with the single phase solid field. 20

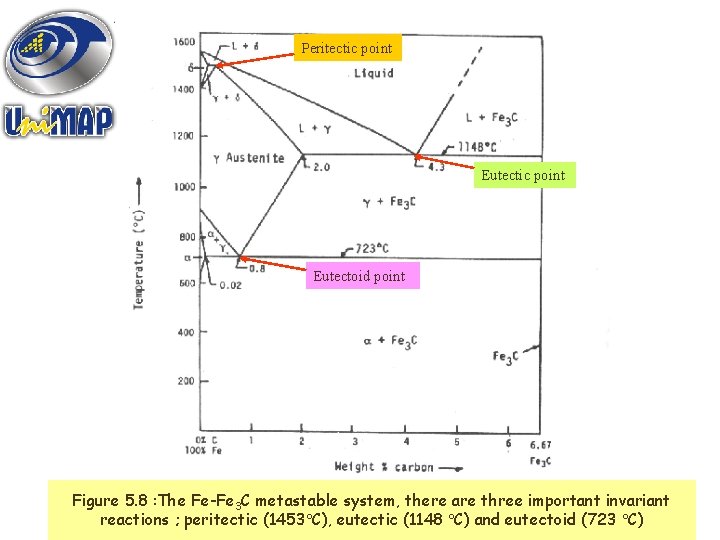
Peritectic point Eutectoid point Figure 5. 8 : The Fe-Fe 3 C metastable system, there are three important invariant Materials Science(1148 C) and eutectoid (723 C) 21 reactions ; peritectic (1453 C), eutectic

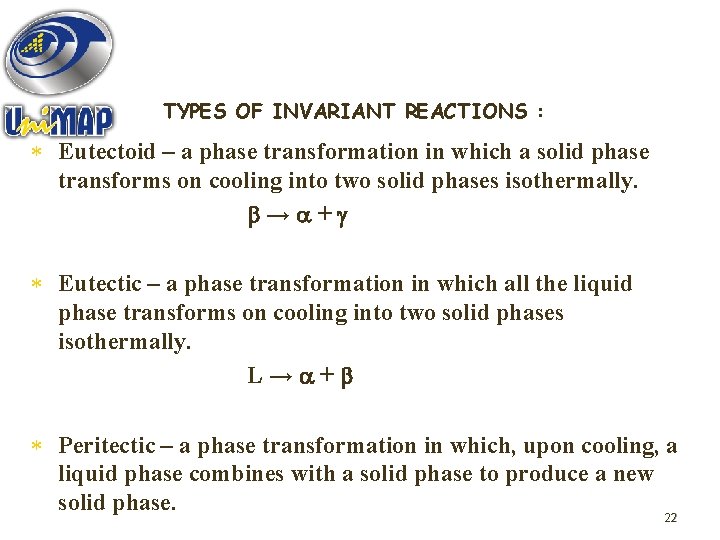
TYPES OF INVARIANT REACTIONS : * Eutectoid – a phase transformation in which a solid phase transforms on cooling into two solid phases isothermally. → + * Eutectic – a phase transformation in which all the liquid phase transforms on cooling into two solid phases isothermally. L→ + * Peritectic – a phase transformation in which, upon cooling, a liquid phase combines with a solid phase to produce a new solid phase. 22

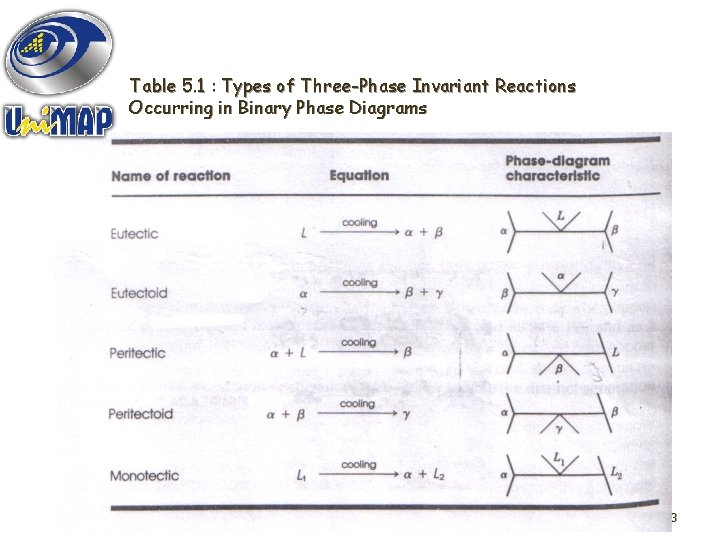
Table 5. 1 : Types of Three-Phase Invariant Reactions Occurring in Binary Phase Diagrams 23

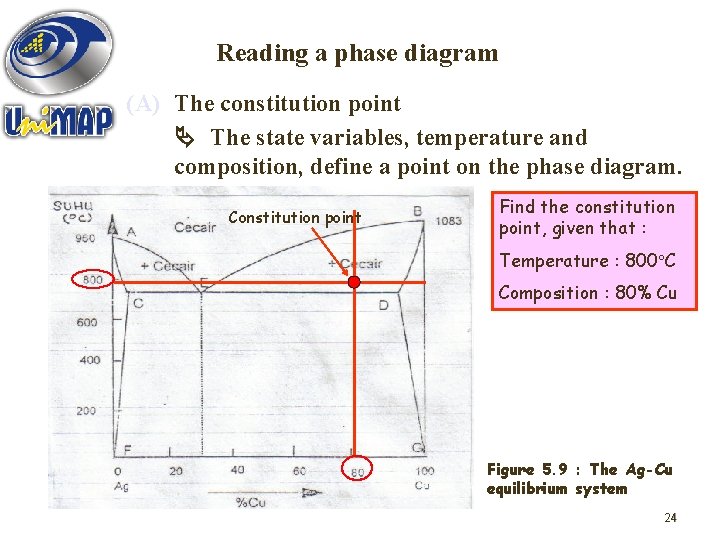
Reading a phase diagram (A) The constitution point The state variables, temperature and composition, define a point on the phase diagram. Constitution point Find the constitution point, given that : Temperature : 800 C Composition : 80% Cu Figure 5. 9 : The Ag-Cu equilibrium system 24

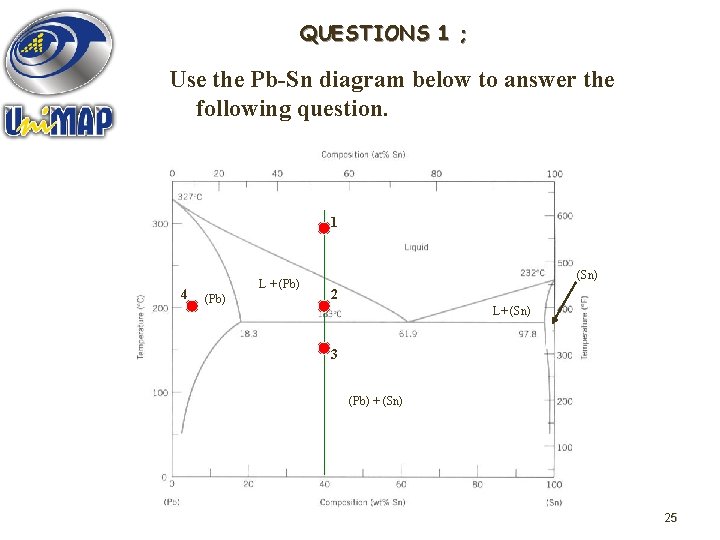
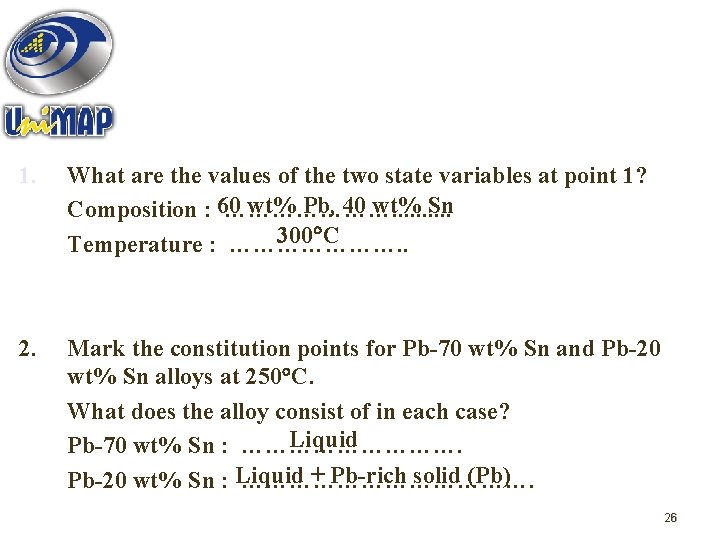
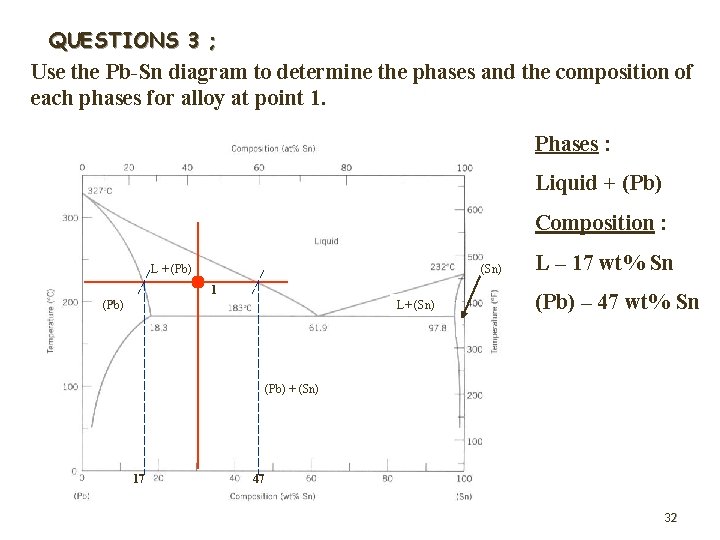
QUESTIONS 1 ; Use the Pb-Sn diagram below to answer the following question. 1 4 L + (Pb) (Sn) 2 L+ (Sn) 3 (Pb) + (Sn) 25

1. What are the values of the two state variables at point 1? wt% Pb, 40 wt% Sn Composition : 60 …………………. . 300 C Temperature : …………………. . 2. Mark the constitution points for Pb-70 wt% Sn and Pb-20 wt% Sn alloys at 250 C. What does the alloy consist of in each case? Liquid Pb-70 wt% Sn : ……………. + Pb-rich solid (Pb) Pb-20 wt% Sn : Liquid ………………. 26

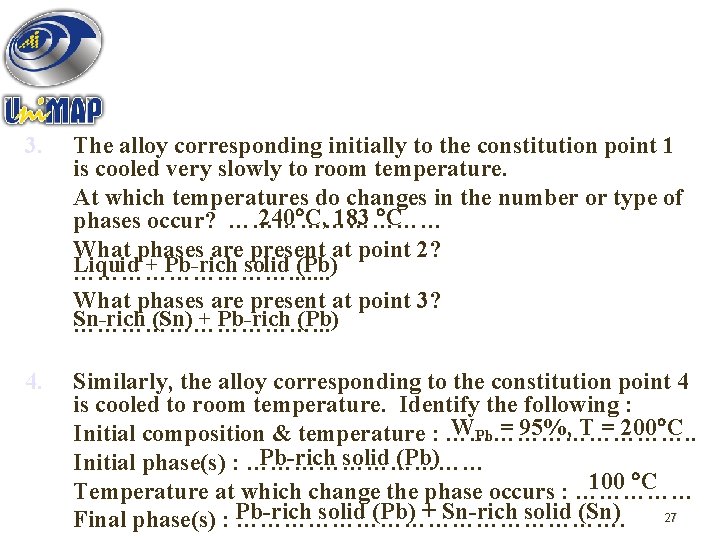
3. The alloy corresponding initially to the constitution point 1 is cooled very slowly to room temperature. At which temperatures do changes in the number or type of 240 C, 183 C phases occur? …………… What phases are present at point 2? Liquid + Pb-rich solid (Pb) ……………. . . . What phases are present at point 3? Sn-rich (Sn) + Pb-rich (Pb) ……………. . . 4. Similarly, the alloy corresponding to the constitution point 4 is cooled to room temperature. Identify the following : WPb = 95%, T = 200 C Initial composition & temperature : ……………. . Pb-rich solid (Pb) Initial phase(s) : …………… 100 C Temperature at which change the phase occurs : …………… solid (Pb) + Sn-rich solid (Sn) 27 Final phase(s) : Pb-rich …………………….

(B) Composition of the phases In a single-phase region, the phase composition and alloy composition coincide. In a two-phase region, the compositions of the phases are found at the ends of the isothermal line spanning the field through the constitution point. This line is called the tie-line. 28

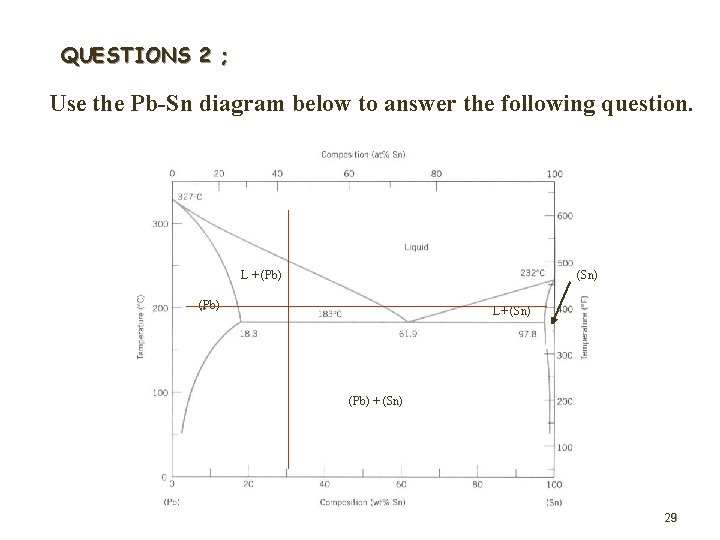
QUESTIONS 2 ; Use the Pb-Sn diagram below to answer the following question. L + (Pb) (Sn) (Pb) L+ (Sn) (Pb) + (Sn) 29

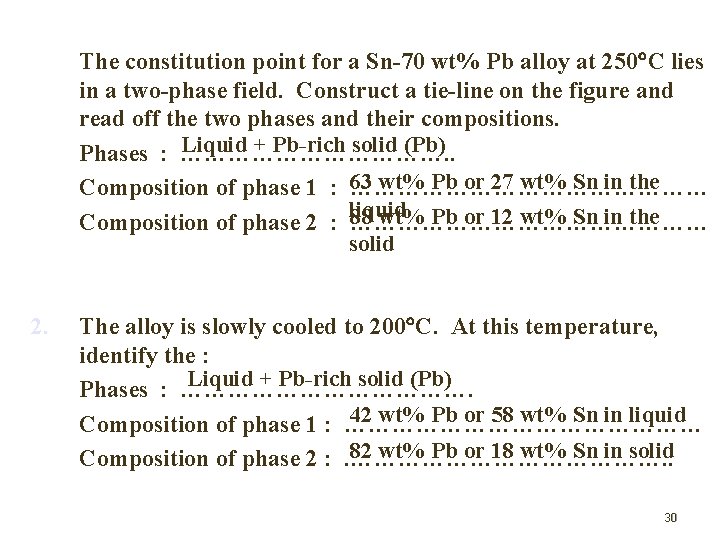
1. The constitution point for a Sn-70 wt% Pb alloy at 250 C lies in a two-phase field. Construct a tie-line on the figure and read off the two phases and their compositions. Liquid + Pb-rich solid (Pb) Phases : ………………. . wt% Pb or 27 wt% Sn in the Composition of phase 1 : 63 …………………… liquid wt% Pb or 12 wt% Sn in the Composition of phase 2 : 88 …………………… solid 2. The alloy is slowly cooled to 200 C. At this temperature, identify the : Liquid + Pb-rich solid (Pb) Phases : ………………. 42 wt% Pb or 58 wt% Sn in liquid Composition of phase 1 : …………………… 82 wt% Pb or 18 wt% Sn in solid Composition of phase 2 : . …………………. . 30

3. The alloy is cooled further to 150 C. At this temperature, identify the : Pb-rich solid (Pb) + Sn-rich solid (Sn) Phases : …………………… wt% Pb or 98 wt% Sn in (Sn) Composition of phase 1 : 2………………. . . 91 wt% Pb or 9 wt% Sn in (Pb) Composition of phase 2 : ……………… 31

QUESTIONS 3 ; Use the Pb-Sn diagram to determine the phases and the composition of each phases for alloy at point 1. Phases : Liquid + (Pb) Composition : L + (Pb) (Sn) 1 (Pb) L+ (Sn) L – 17 wt% Sn (Pb) – 47 wt% Sn (Pb) + (Sn) 17 47 32

(C) Proportion of the phases (Amount of phases) in two-phase alloys This will achieved by using LEVER RULE. (Lever rule – a useful tool for determining the weight fraction for each phases in a two-phase field) Steps in finding the weight percentages of the phases in a two-phases region. 1. construct a tie-line through the constitution point 2. read off the three compositions (for the alloy, and the two ends of the tie-line) 3. apply the lever rule. Note : Wphase 1 + Wphase 2 = 1 @ 100% 33

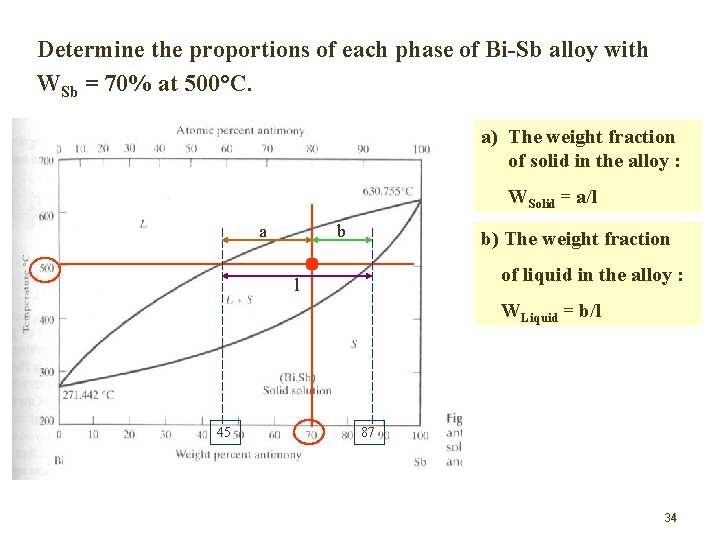
Determine the proportions of each phase of Bi-Sb alloy with WSb = 70% at 500 C. a) The weight fraction of solid in the alloy : WSolid = a/l a b b) The weight fraction of liquid in the alloy : l WLiquid = b/l 45 87 34

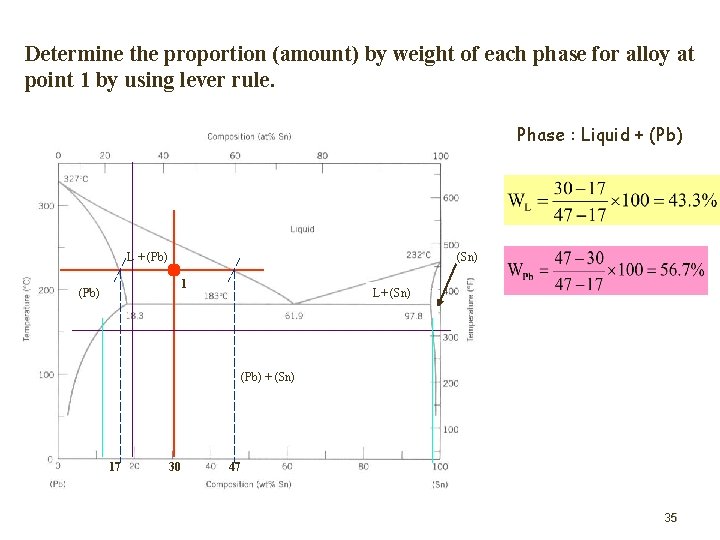
Determine the proportion (amount) by weight of each phase for alloy at point 1 by using lever rule. Phase : Liquid + (Pb) L + (Pb) (Sn) 1 (Pb) L+ (Sn) (Pb) + (Sn) 17 30 47 35

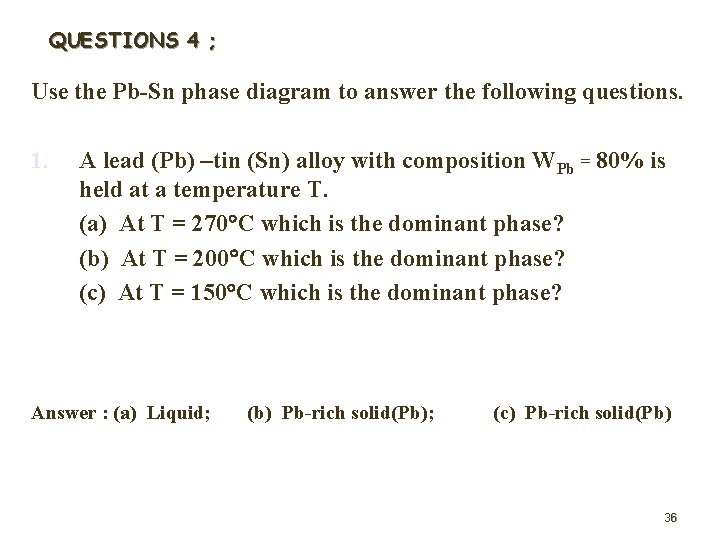
QUESTIONS 4 ; Use the Pb-Sn phase diagram to answer the following questions. 1. A lead (Pb) –tin (Sn) alloy with composition WPb = 80% is held at a temperature T. (a) At T = 270 C which is the dominant phase? (b) At T = 200 C which is the dominant phase? (c) At T = 150 C which is the dominant phase? Answer : (a) Liquid; (b) Pb-rich solid(Pb); (c) Pb-rich solid(Pb) 36

2. The alloy is cooled to 150 C. (a) How many phases are present? (b) Give the approximate composition of the phase(s). (c) Which is the dominant phase? (d) What (roughly) are the proportions by weight of each phase? Answer : (a) Two (b) WPb = 2% in (Sn) , WPb = 90% in (Pb) (c) Pb-rich solid (Pb) (d) Sn-rich solid (WSn) = 11 wt% ; Pb-rich solid (WPb) = 89 wt% 37

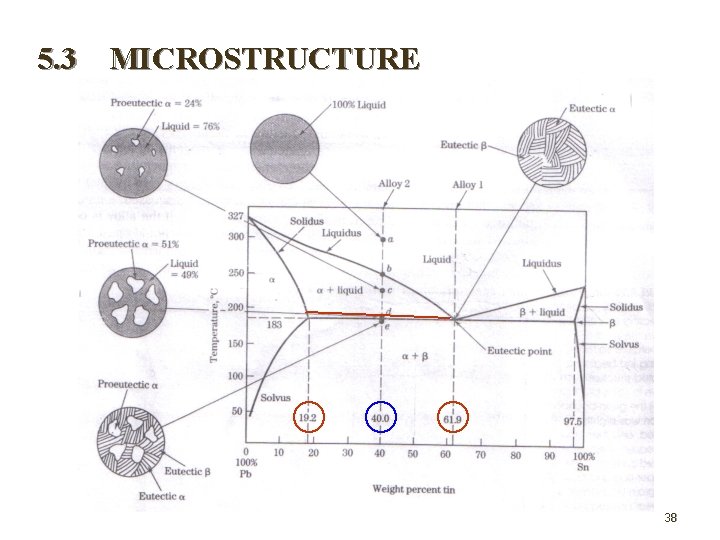
5. 3 MICROSTRUCTURE 38

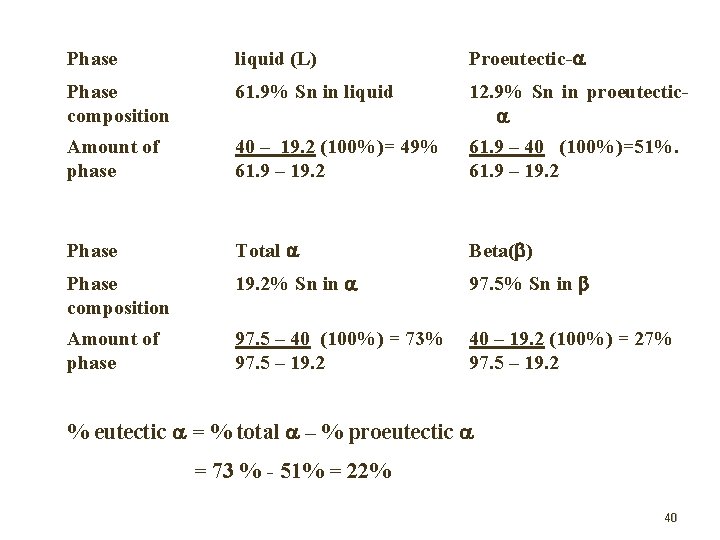
Problems Consider the Pb-Sn diagram, make a phase analysis for the plumbum alloy equilibrium solidification for each composition below ; (i) Point d with 40% Sn at 183 C + T. (ii) Point e with 40% Sn at 183 C - T. 39

(i) Phase liquid (L) Proeutectic- Phase composition 61. 9% Sn in liquid 12. 9% Sn in proeutectic Amount of phase 40 – 19. 2 (100%)= 49% 61. 9 – 19. 2 61. 9 – 40 (100%)=51%. 61. 9 – 19. 2 Total Beta( ) Phase composition 19. 2% Sn in 97. 5% Sn in Amount of phase 97. 5 – 40 (100%) = 73% 97. 5 – 19. 2 40 – 19. 2 (100%) = 27% 97. 5 – 19. 2 (ii) Phase % eutectic = % total – % proeutectic = 73 % - 51% = 22% 40

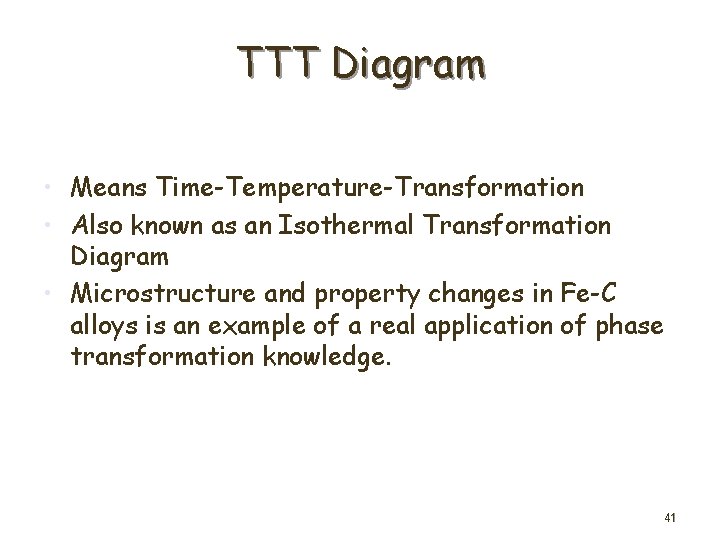
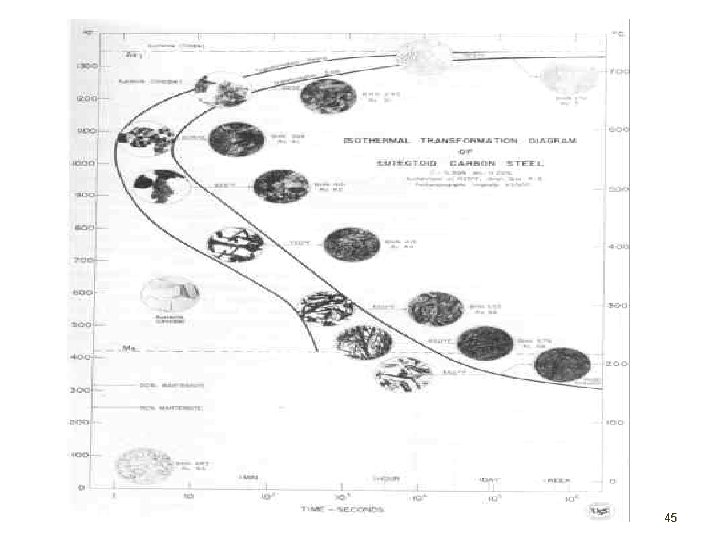
TTT Diagram • Means Time-Temperature-Transformation • Also known as an Isothermal Transformation Diagram • Microstructure and property changes in Fe-C alloys is an example of a real application of phase transformation knowledge. 41

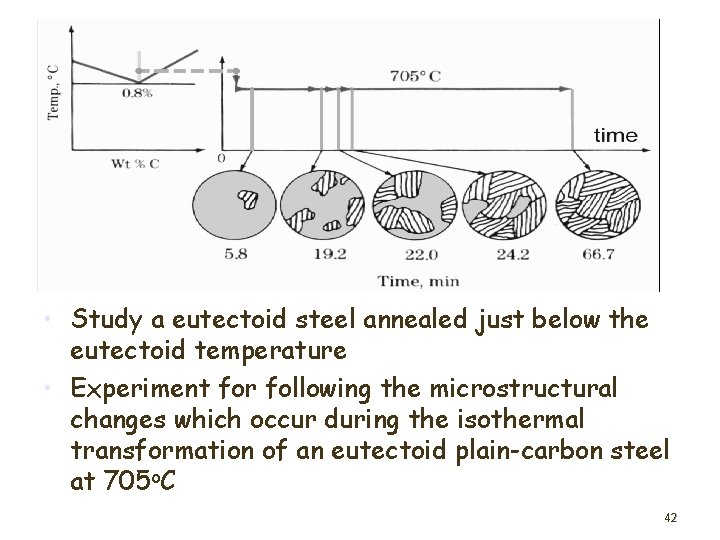
• Study a eutectoid steel annealed just below the eutectoid temperature • Experiment for following the microstructural changes which occur during the isothermal transformation of an eutectoid plain-carbon steel at 705 o. C 42

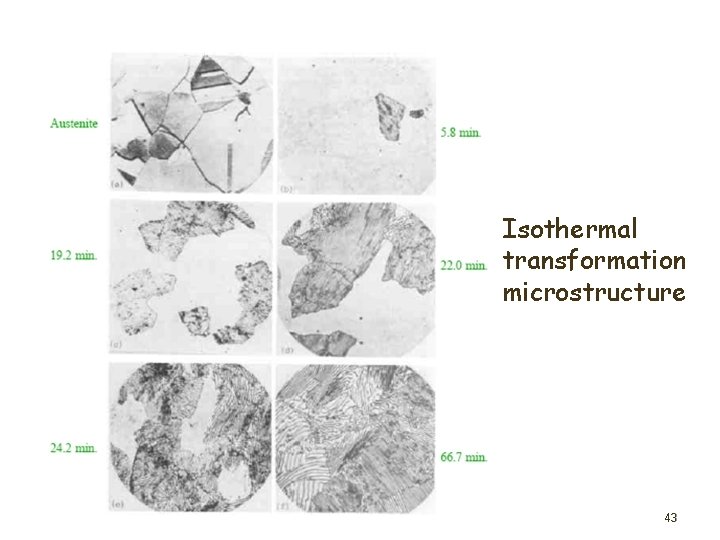
Isothermal transformation microstructure 43

• Valid only for eutectoid steels, and constant temperature transformations • Two solid line : start and end of transformation • Dashed line : 50% transformation • Eutectoid temperature a horizontal line (above which is austenite) • Transformation rate increases with decreasing temperature 44

45

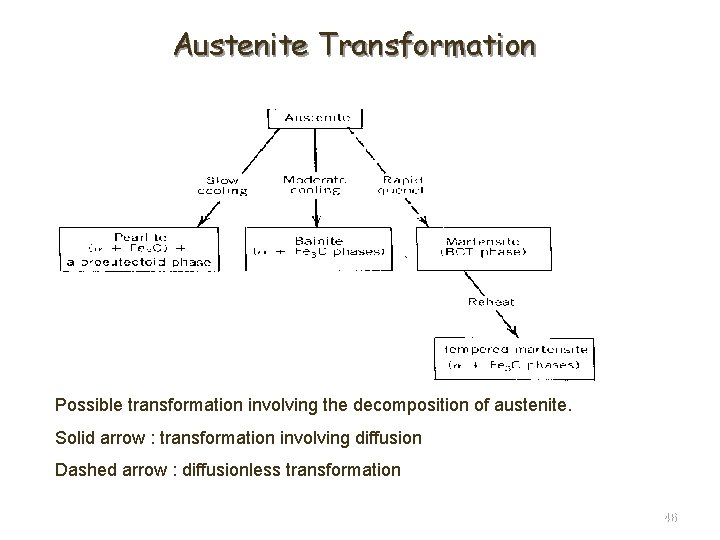
Austenite Transformation Possible transformation involving the decomposition of austenite. Solid arrow : transformation involving diffusion Dashed arrow : diffusionless transformation 46

• Slow cooling, coarse pearlite formed with thick ferrite and cementite layers • Increased cooling rate, lamellar thickness decreases • Increased cooling rate still allows formation of bainite • Faster cooling rate allow formation of martensite 47

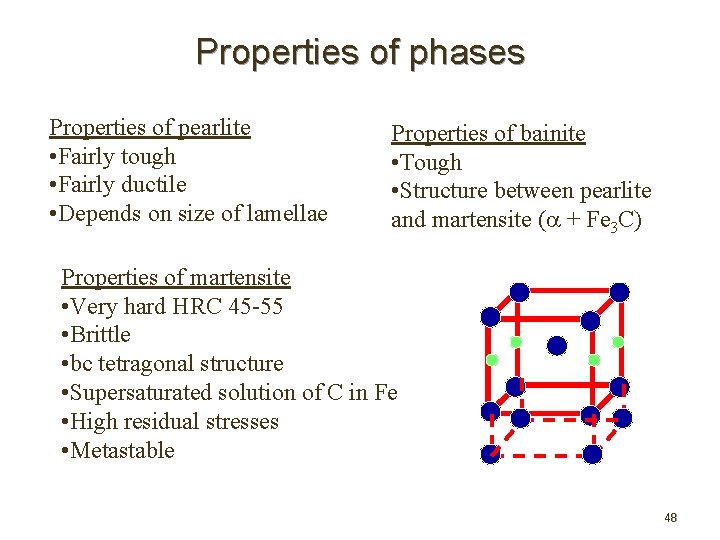
Properties of phases Properties of pearlite • Fairly tough • Fairly ductile • Depends on size of lamellae Properties of bainite • Tough • Structure between pearlite and martensite ( + Fe 3 C) Properties of martensite • Very hard HRC 45 -55 • Brittle • bc tetragonal structure • Supersaturated solution of C in Fe • High residual stresses • Metastable 48

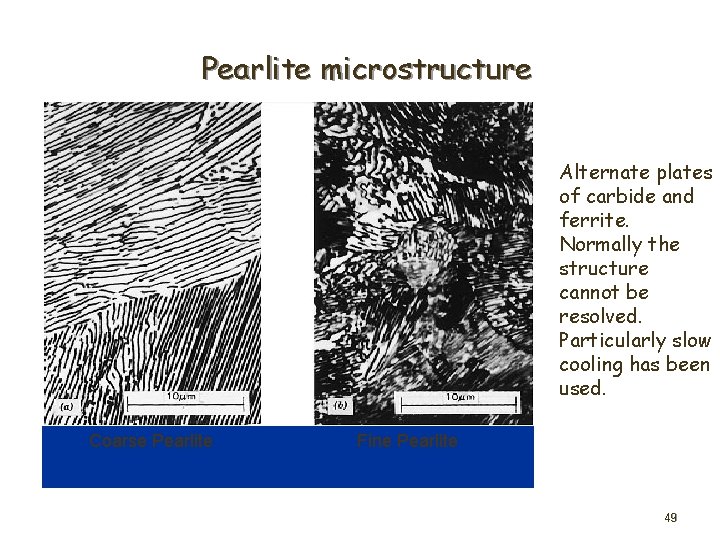
Pearlite microstructure Alternate plates of carbide and ferrite. Normally the structure cannot be resolved. Particularly slow cooling has been used. Coarse Pearlite Fine Pearlite 49

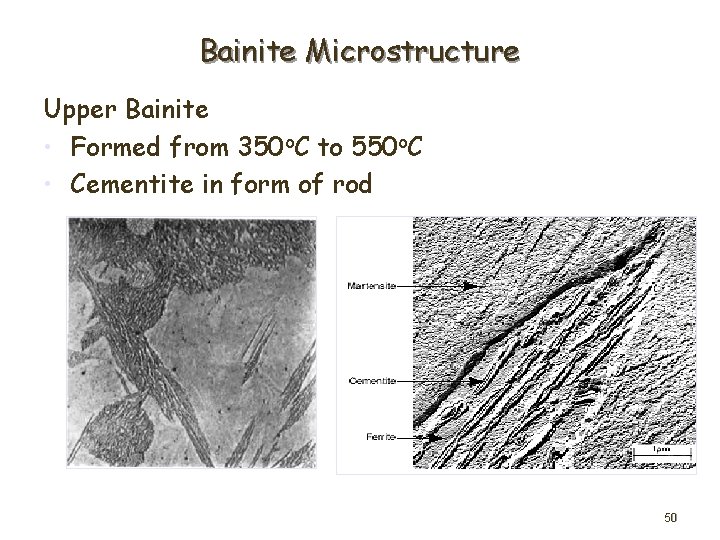
Bainite Microstructure Upper Bainite • Formed from 350 o. C to 550 o. C • Cementite in form of rod 50

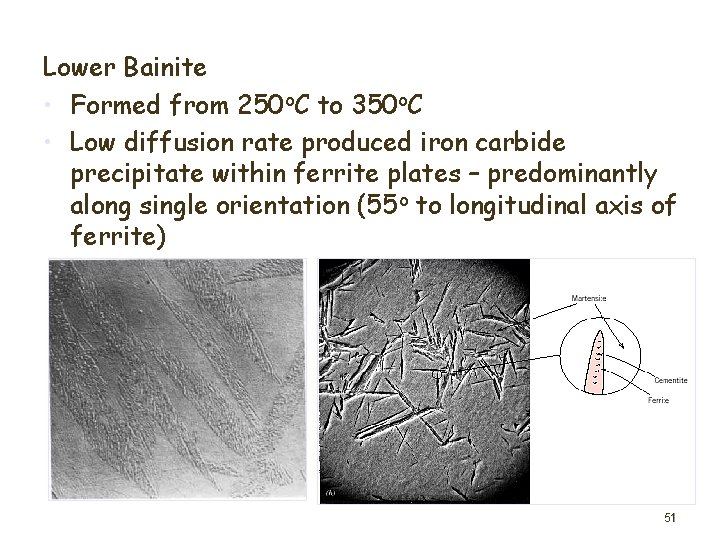
Lower Bainite • Formed from 250 o. C to 350 o. C • Low diffusion rate produced iron carbide precipitate within ferrite plates – predominantly along single orientation (55 o to longitudinal axis of ferrite) 51

Formation of martensite • High cooling rates - quenching – Rapid cooling with water or oil • Diffusion of carbon inhibited – Neither ferrite nor pearlite have sufficient time to form • Shear transformation – Body centred tetragonal structure – Carbon evenly distributed 52

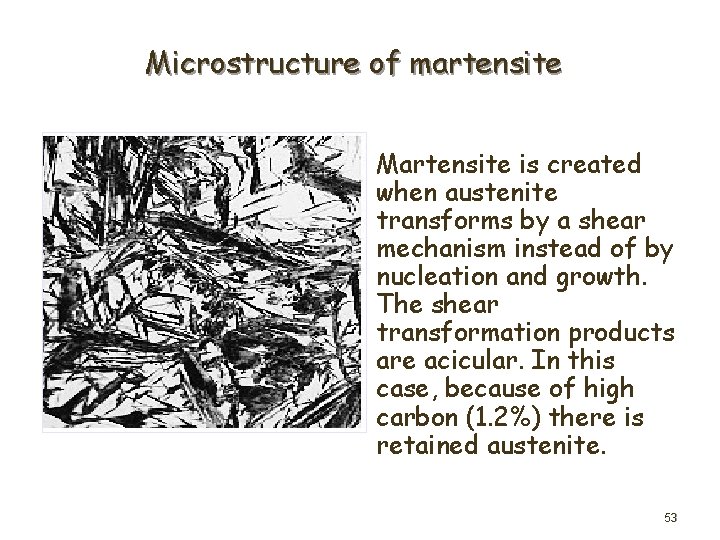
Microstructure of martensite Martensite is created when austenite transforms by a shear mechanism instead of by nucleation and growth. The shear transformation products are acicular. In this case, because of high carbon (1. 2%) there is retained austenite. 53

Heat treatment • Definition of heat treatment is a controlled heating and cooling cycles intended to adjust the microstructure and mechanical properties of a material for a specific purpose. • Examples: annealing, normalizing, quenching and tempering 54

Annealing • Material exposed to elevated temperature for extended period of time, and then slowly cooled. • Used to relieve stresses, increase softness, ductility and toughness, or to • produce specific microstructure • Full annealing : Transformation to austenite, then furnace cooled to coarse pearlite (relatively soft and ductile material) 55

Normalizing • Used to refine grains and produce more uniform distribution in steels which have been plastically deformed (e. g. rolling) resulting in tougher steel. • Complete transformation to austenite, then air cooled to a fine pearlite 56

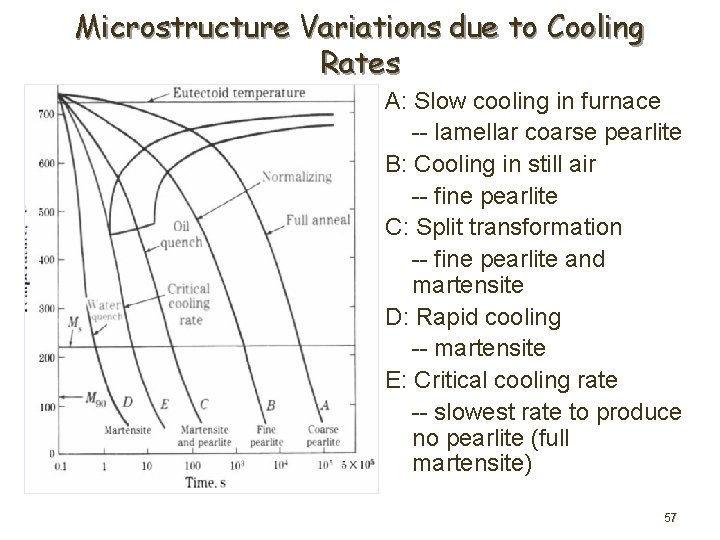
Microstructure Variations due to Cooling Rates A: Slow cooling in furnace -- lamellar coarse pearlite B: Cooling in still air -- fine pearlite C: Split transformation -- fine pearlite and martensite D: Rapid cooling -- martensite E: Critical cooling rate -- slowest rate to produce no pearlite (full martensite) 57

Tempering • Heating martensite to between 100 & 600˚C • Softens & toughens martensite • Effects dependent on temperature and include – Stress relief – Epsilon carbide precipitates from martensite – Cementite precipitates from martensite – Epsilon carbide converts to cementite – Retained austenite transforms to bainite 58

Effect of tempering • Increases ductility and toughness • Reduces hardness and strength • Solid solution elements have little effect on tempering – Ni, Si, Al, Mn • Strong carbide formers raise tempering temperature for equivalent hardness – Cr, Mo, V 59

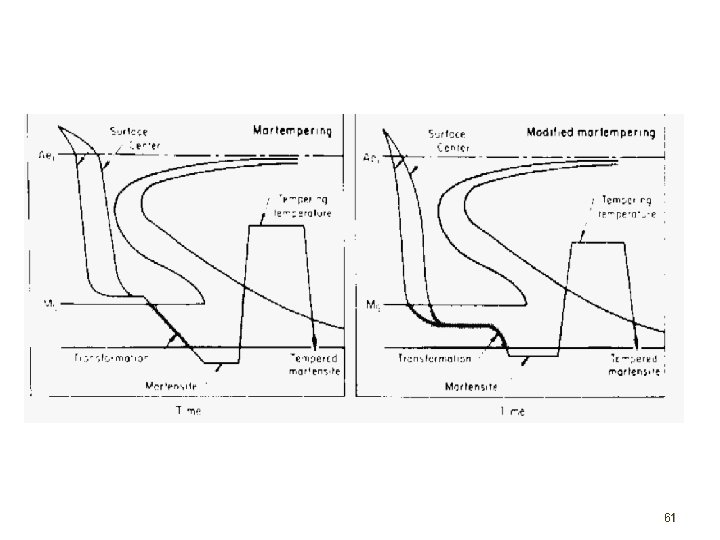
Martempering (Marquenching) Modified quench to minimize distortion of heat treated steel: 1) austenitize steel 2) quench in hot oil or molten salt just above martensite start temperature 3) hold in quenching medium until uniform temperature in steel (but before austenite to bainite transformation begins) 4) cool at moderate to prevent thermal gradients ====> usually, parts are later tempered to toughen steel 60

61

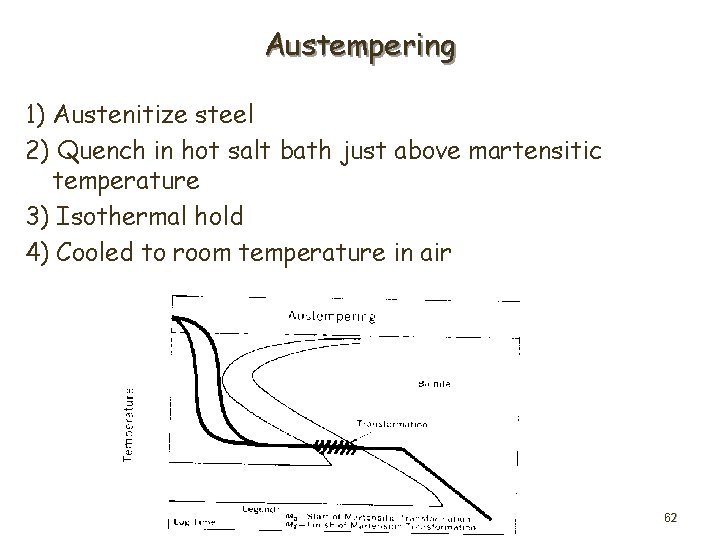
Austempering 1) Austenitize steel 2) Quench in hot salt bath just above martensitic temperature 3) Isothermal hold 4) Cooled to room temperature in air 62

• Isothermal heat treatment process to produce Bainite • Alternative to quenching and tempering • Improved ductility and impact strength for particular hardness • Decreased cracking and distortion quenching • Particularly advantageous for thin sections (<3/8") {thicker sections have non-uniform properties due to different cooling rates} 63