Lecture 18 Chapter 18 Kinetic Theory of Gases

















- Slides: 35

Lecture 18 Chapter 18 Kinetic Theory of Gases Now we look at temperature, pressure, and internal energy in terms of the motion of molecules and atoms? Relate to the 1 st Law of Thermodynamics Gas Solid

Todays Material Prelude Comments Ideal Gas Law First Law of Thermo and the Gas Law Microscopic kinetic theory of gases Specific heats for gasses Equipartition theorem Entropy – two equivalent definitions

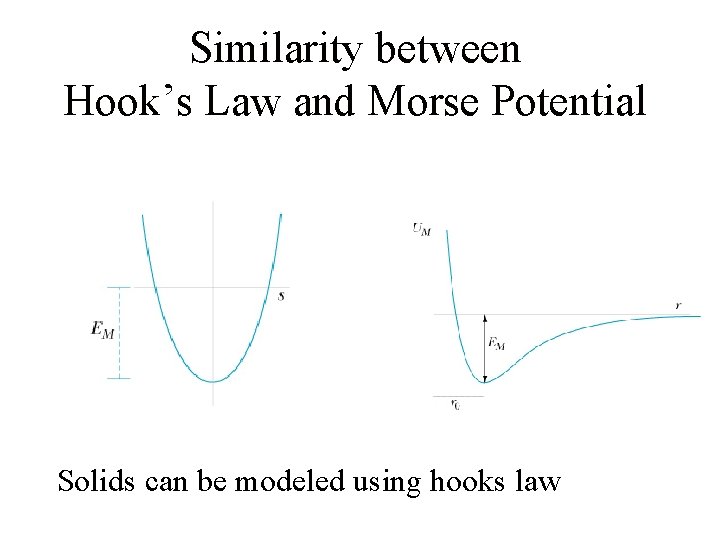
Similarity between Hook’s Law and Morse Potential Solids can be modeled using hooks law

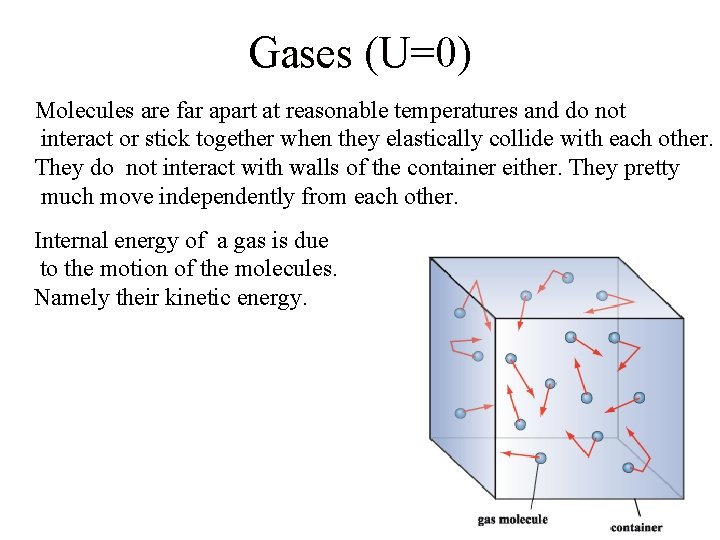
Gases (U=0) Molecules are far apart at reasonable temperatures and do not interact or stick together when they elastically collide with each other. They do not interact with walls of the container either. They pretty much move independently from each other. Internal energy of a gas is due to the motion of the molecules. Namely their kinetic energy.

Liquids Are inbetween solids and gases and are not at either extreme. They are more difficult to analyze from basic principles so we do not normally study them in detail because their motion is too complicated for an introductory course.

Avogadro’s Number How many molecules are there in a cubic meter of air at STP? First, find out how much mass there is. The mass is the density times the volume. m = 1. 2 kg/m 3 x 1 m 3 = 1. 2 kg. (or 2. 5 lbs) Now find the number of moles in the cubic meter and multiply by Avogadro’s number 1 m

Avogadro’s Number NA=6. 02 x 1023 atoms or molecules in one mole of any gas. One mole is the molecular weight in grams. It is also called the molar mass. 1 mole of air contains 29 gms Then the number of moles in 1. 2 kg is Then the number of molecules or atoms is Advogado’s number is also related to the Ideal Gas law.

Ideal Gases Experiment shows that 1 mole of any gas, such as helium, air, hydrogen, etc at the same volume and temperature has almost the same pressure. At low densities the pressures become even closer and obey the Ideal Gas Law: Called the gas constant

Ideal Gas Law in terms of Boltzman Constant At high gas densities you must add the Van der Waal corrections where a and b are constants. (Nobel prize 1 910) Lets use apply the 1 st law of Thermodynamics for reversible processes using the an ideal gas

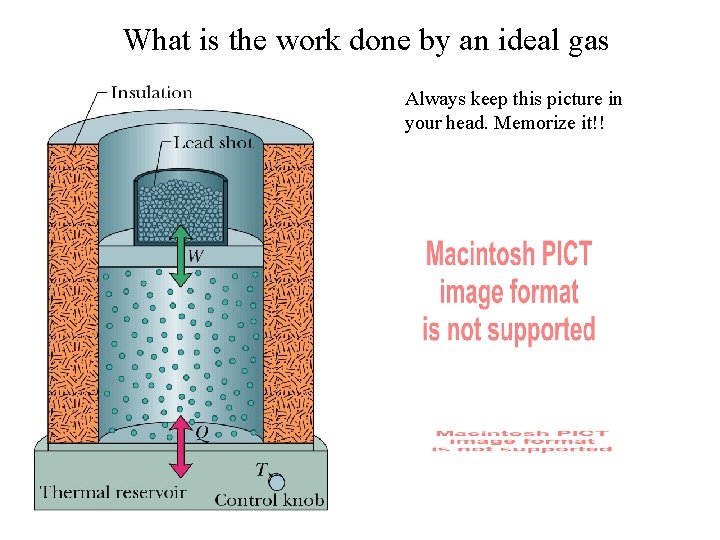
What is the work done by an ideal gas Always keep this picture in your head. Memorize it!!

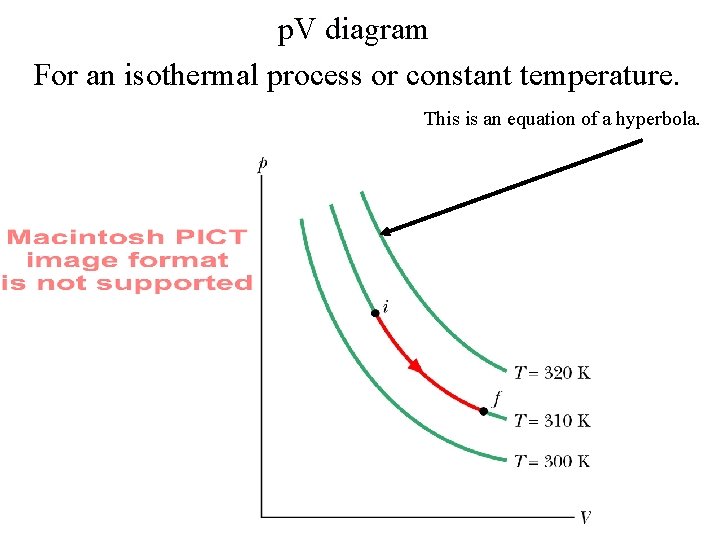
p. V diagram For an isothermal process or constant temperature. This is an equation of a hyperbola.

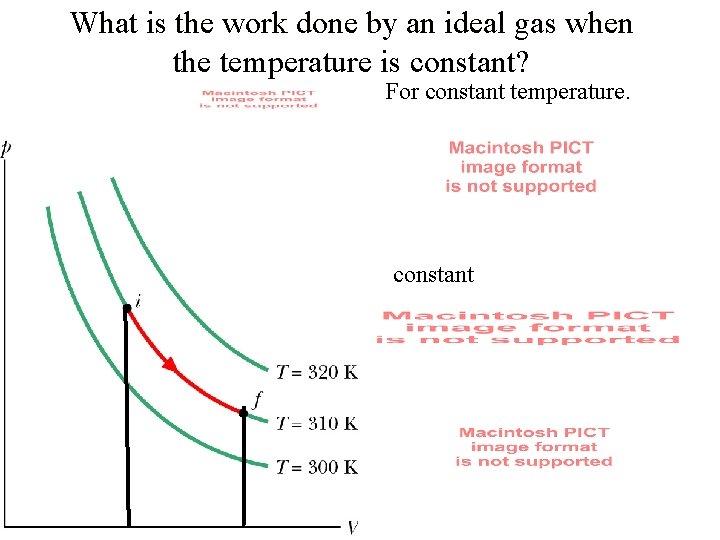
What is the work done by an ideal gas when the temperature is constant? For constant temperature. constant

What is the work done by an ideal gas when the temperature is constant? Isothermal expansion Isothermal compression Constant volume V f > Vi V f < Vi V f = Vi W>0 W<0 W=0 ln is positive ln is negative ln 1=0

Four situations where the work done by an ideal gas is very clear. t n ta r o p For a constant volume process: For a constant pressure process Im For a constant temperature process For an ideal gas undergoing any reversible thermodynamic process.

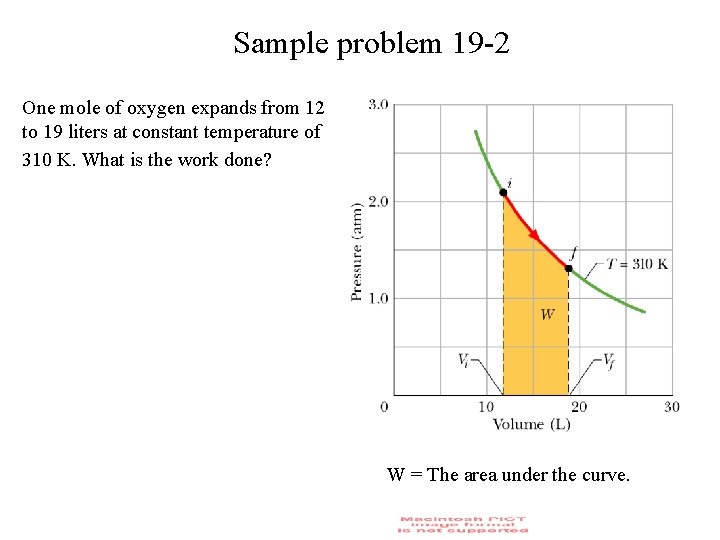
Sample problem 19 -2 One mole of oxygen expands from 12 to 19 liters at constant temperature of 310 K. What is the work done? W = The area under the curve.

Sample problem 19 -2 One mole of oxygen expands from 12 to 19 liters at constant temperature of 310 K. What is the work done? (Note volume units cancel) W = The area under the curve.

This is want we know so far. What’s Next We now know how to find W. If we can find the change in internal energy, then we know Q. Resort to a microscopic or kinetic theory of a gas.

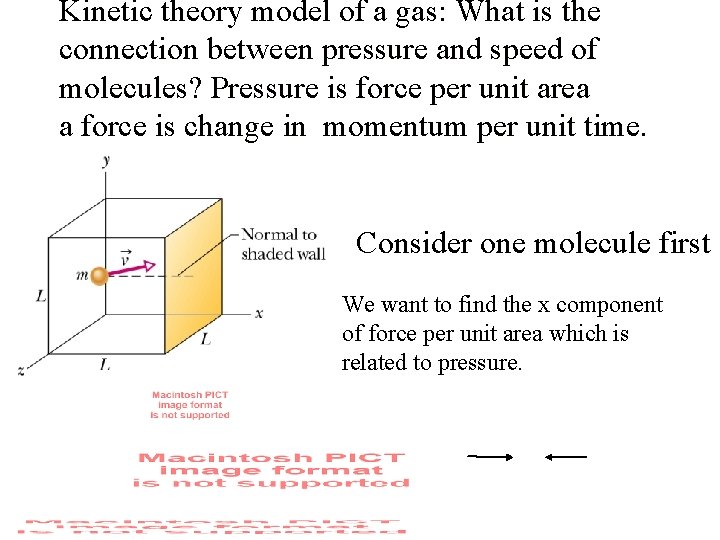
Kinetic theory model of a gas: What is the connection between pressure and speed of molecules? Pressure is force per unit area a force is change in momentum per unit time. Consider one molecule first We want to find the x component of force per unit area which is related to pressure.

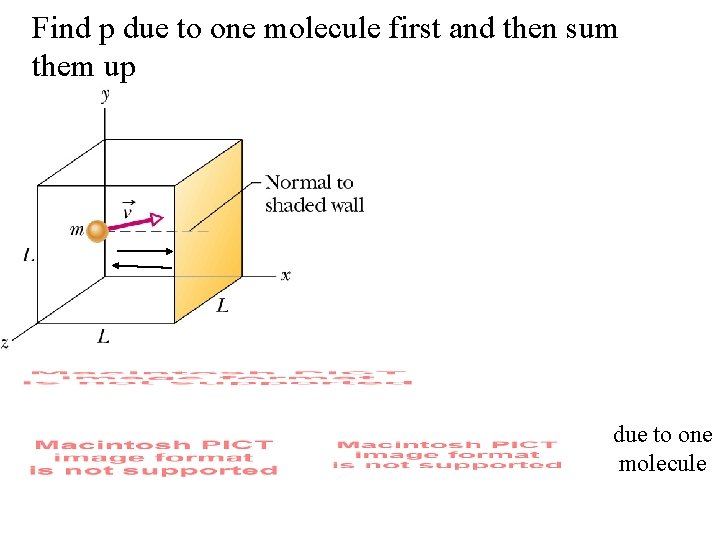
Find p due to one molecule first and then sum them up due to one molecule

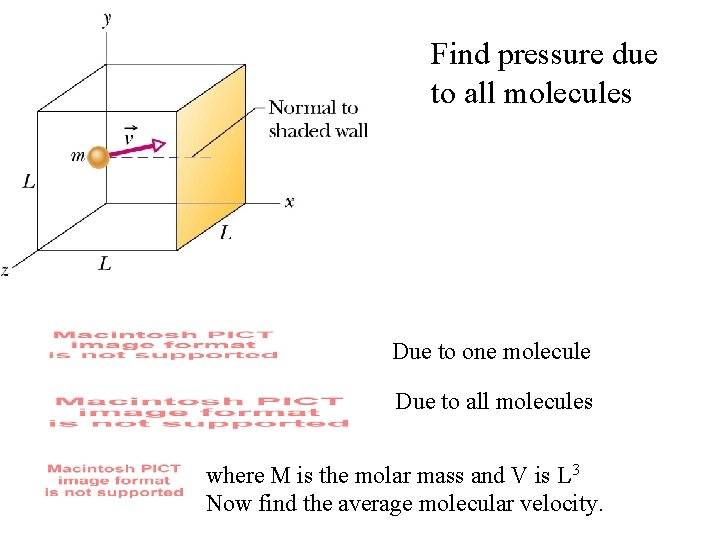
Find pressure due to all molecules Due to one molecule Due to all molecules where M is the molar mass and V is L 3 Now find the average molecular velocity.

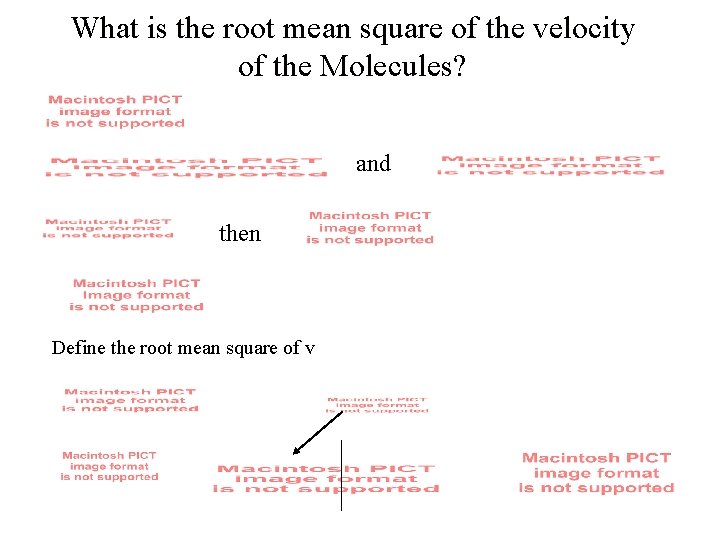
What is the root mean square of the velocity of the Molecules? and then Define the root mean square of v

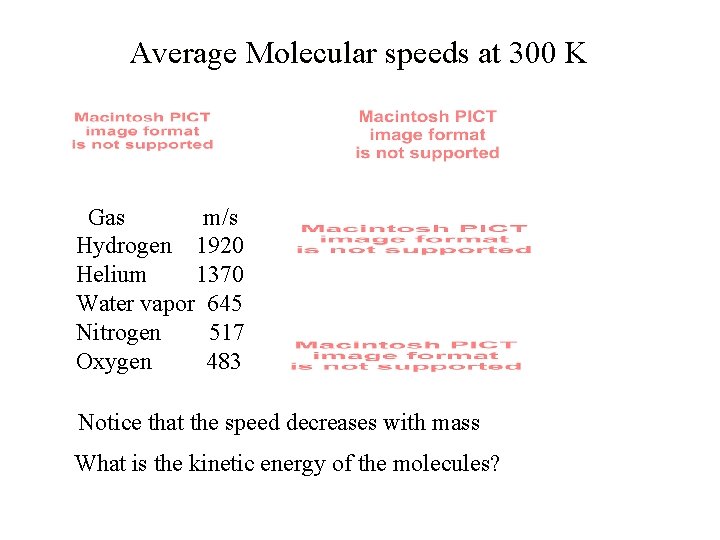
Average Molecular speeds at 300 K Gas m/s Hydrogen 1920 Helium 1370 Water vapor 645 Nitrogen 517 Oxygen 483 Notice that the speed decreases with mass What is the kinetic energy of the molecules?

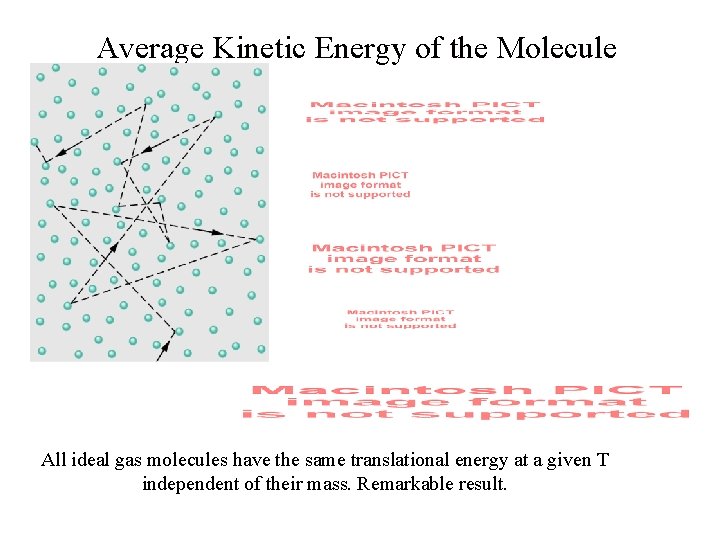
Average Kinetic Energy of the Molecule All ideal gas molecules have the same translational energy at a given T independent of their mass. Remarkable result.

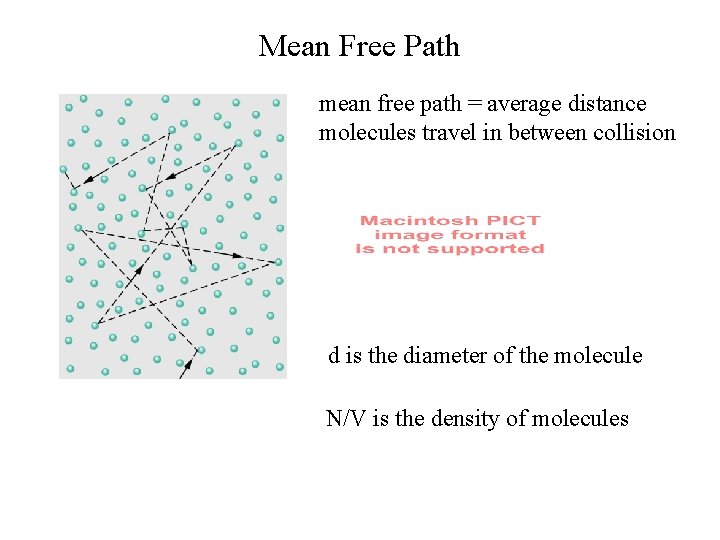
Mean Free Path mean free path = average distance molecules travel in between collision d is the diameter of the molecule N/V is the density of molecules

Problem. Suppose we have a oxygen molecule at 300 K at p =1 atm with a molecular diameter of d= 290 pm. What is λ, v, and f where f is defined as the frequency of collisions in an ideal gas? Find λ

Problem. Suppose we have a oxygen molecule at 300 K at p =1 atm with a molecular diameter of d= 290 pm. What is λ, v, and f where f is defined as the frequency of collisions in an ideal gas? Find λ

What is v, the speed of a molecule in an ideal gas? Use the rms speed. What is f, the frequency of collision? What is the time between collisions? f = vrms /λ

Demo comments 1. Jug O' Air 2. Boiling by Cooling (Ice on beaker) 3. Boiling by Reducing Pressure(Vacuum in Bell jar) 4. Dipping Duck Toy 5. Leslie cube and the laser themometer.

1. Jug of Air (Inflate with bike pump and watch temp. rise) p/T = n. R/V = constant Increase pressure using bicycle pump. To keep P/T constant temperature rises. Volume in jug remains constant.

2. Boiling by Reducing Pressure (Vacuum in Bell jar) See velocity distribution of molecules next subject

3. Boiling by Cooling • Heat up until water boils • Remove flame then cork flask quickly • Put cubes of ice on flask • Wait until temperature and pressure is lowered at constant volume until boiling starts again. P/T = constant

4. Dipping Duck Toy • Wet head cools as water evaporates off of it • Pressure drops inside head • Contained pressure pushes water up tube • When center of gravity is exceeded head tips • Exposes bottom of tube then pressure equalized • Starts all over again

5. Leslie cube and the laser thermometer. Mostly infrared - colors are misleading in optical regime

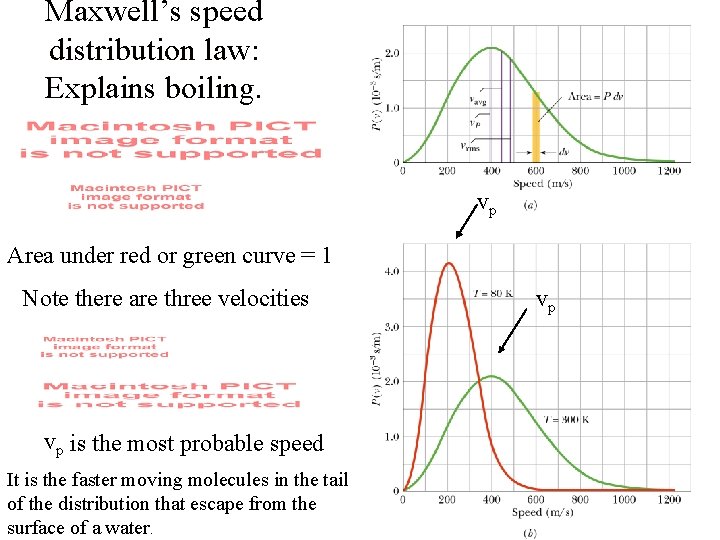
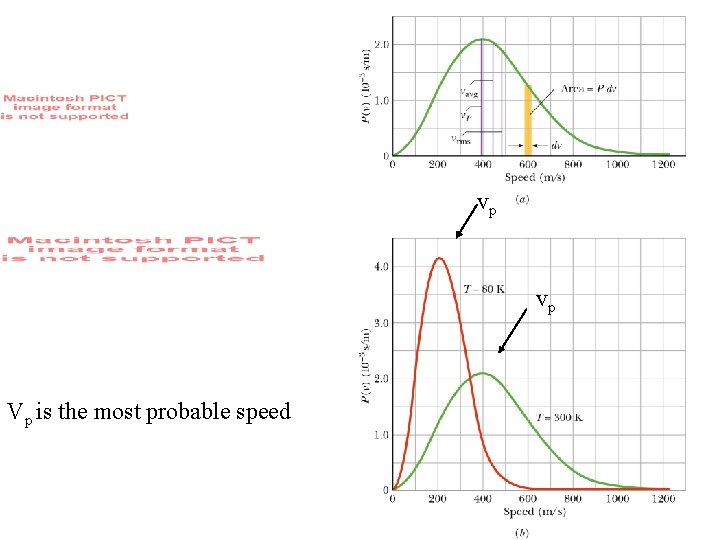
Maxwell’s speed distribution law: Explains boiling. vp Area under red or green curve = 1 Note there are three velocities vp is the most probable speed It is the faster moving molecules in the tail of the distribution that escape from the surface of a water. vp

vp vp Vp is the most probable speed
 Kinetic molecular theory
Kinetic molecular theory Kinetic particle model
Kinetic particle model Kinetic theory of gases
Kinetic theory of gases Postulate of kinetic theory
Postulate of kinetic theory Kinetic theory
Kinetic theory Kinetic molecular theory
Kinetic molecular theory Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Molecular theory of gases and liquids
Molecular theory of gases and liquids Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Define kinetic theory of matter
Define kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave The kinetic molecular theory
The kinetic molecular theory Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Postulates of kinetic molecular theory
Postulates of kinetic molecular theory Kinetic theory def
Kinetic theory def Timeline of kinetic molecular theory
Timeline of kinetic molecular theory Kinetic theory of solids
Kinetic theory of solids Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Ideal gas law examples
Ideal gas law examples What is a kinetic theory of matter
What is a kinetic theory of matter Pv=1/3nmc^2
Pv=1/3nmc^2 Kinetic molecular theory
Kinetic molecular theory Tenets of kinetic molecular theory
Tenets of kinetic molecular theory Natural language processing
Natural language processing Decision theory lecture notes
Decision theory lecture notes Sargur srihari
Sargur srihari Natural language processing lecture notes
Natural language processing lecture notes Avogadro's law relationship
Avogadro's law relationship Chapter 11 review gases section 1
Chapter 11 review gases section 1 Ideal gas law problems
Ideal gas law problems Chapter 14 the behavior of gases
Chapter 14 the behavior of gases


























































