Il trapianto di sangue cordonale dopo regime di




- Slides: 55

Il trapianto di sangue cordonale dopo regime di condizionamento ad intensità ridotta Franco Locatelli, MD Oncoematologia Pediatrica Fondazione IRCCS Policlinico San Matteo, Università di Pavia f. locatelli@smatteo. pv. it

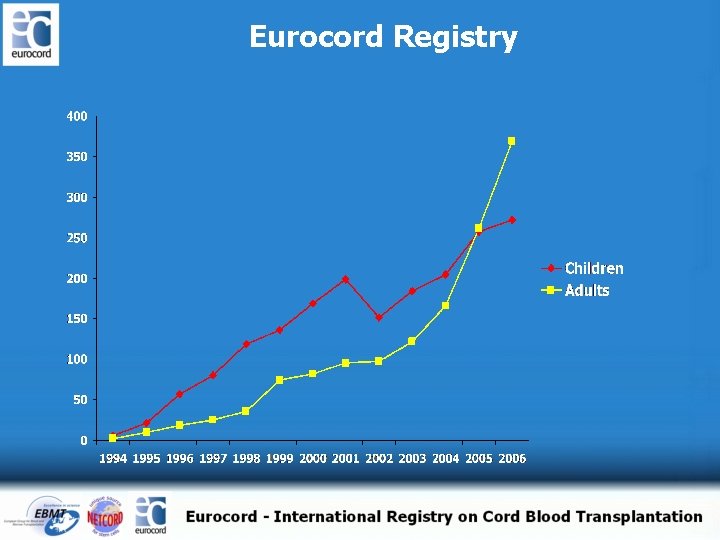
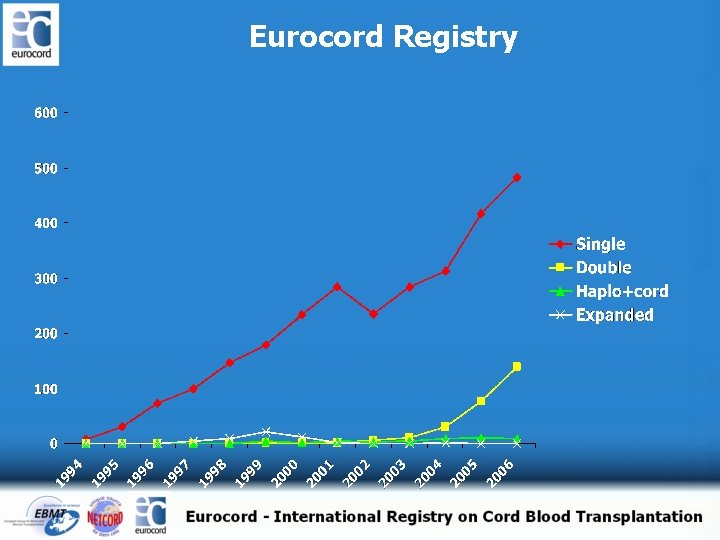
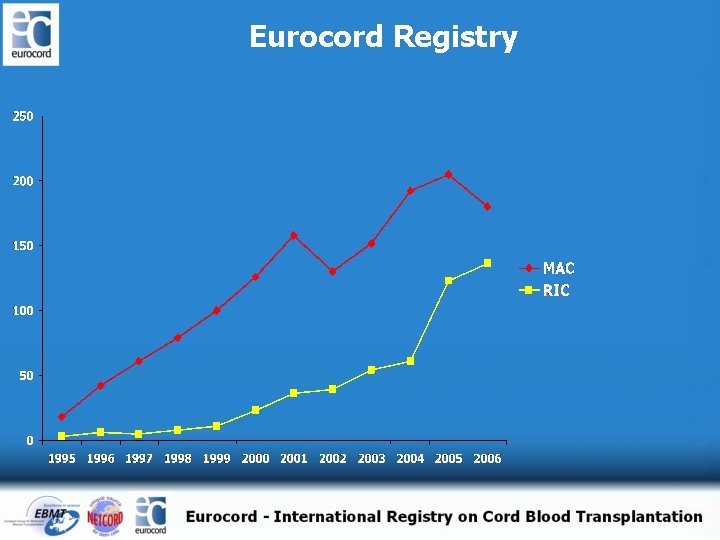
Eurocord Registry

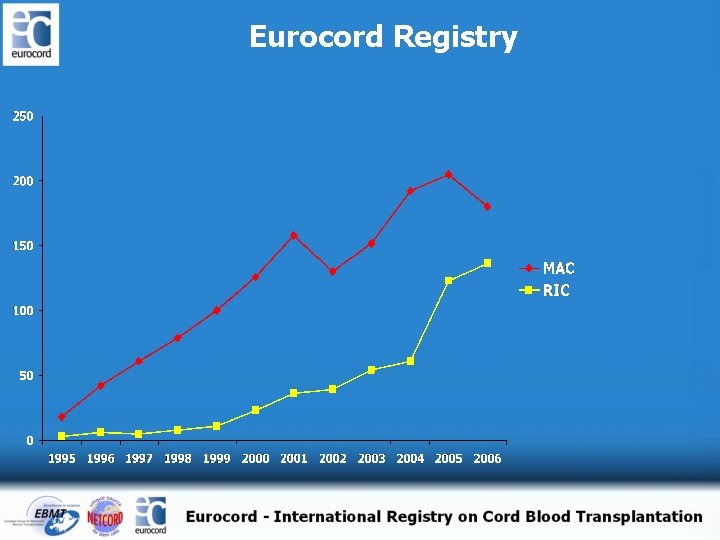
Eurocord Registry

Eurocord Registry

Learning curve in UCBT for adults

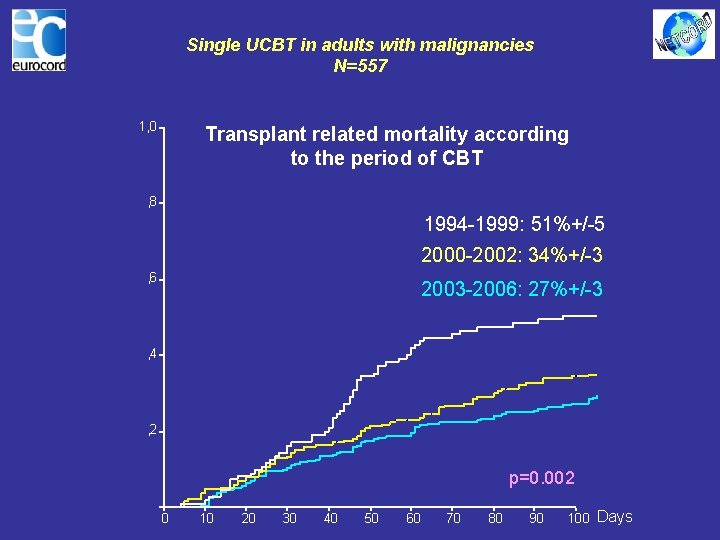
Single UCBT in adults with malignancies N=557 1, 0 Transplant related mortality according to the period of CBT , 8 1994 -1999: 51%+/-5 2000 -2002: 34%+/-3 , 6 2003 -2006: 27%+/-3 , 4 , 2 p=0. 002 0 10 20 30 40 50 60 70 80 90 100 Days

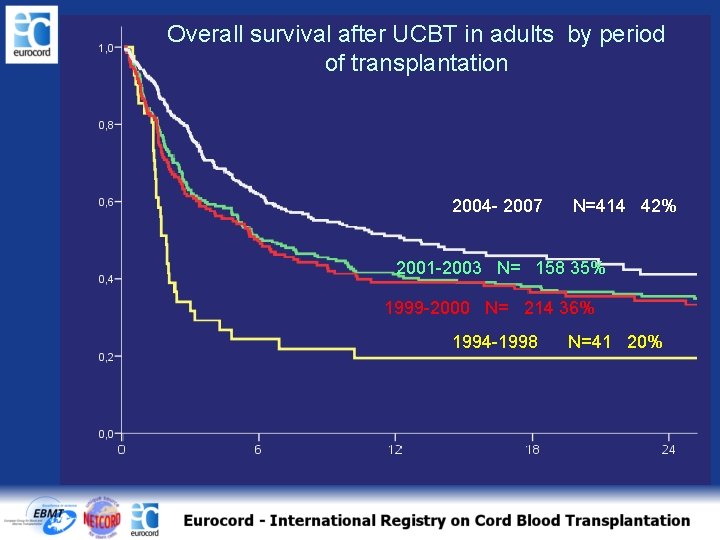
Overall survival after UCBT in adults by period of transplantation 2004 - 2007 N=414 42% 2001 -2003 N= 158 35% 1999 -2000 N= 214 36% 1994 -1998 N=41 20%

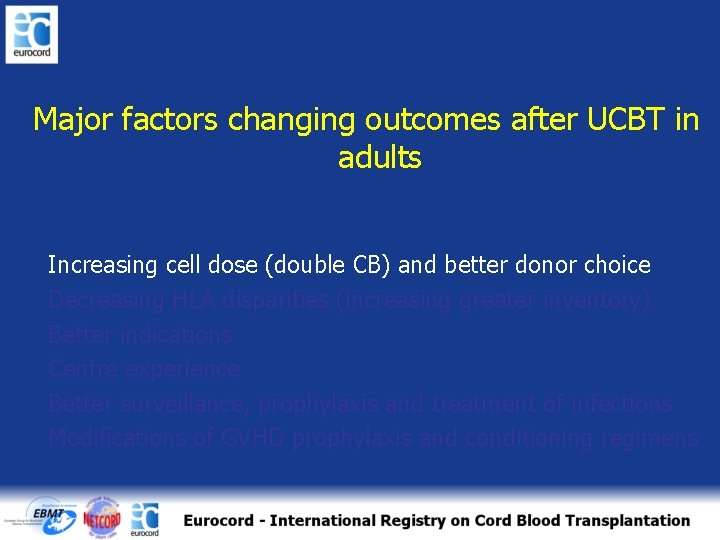
Major factors changing outcomes after UCBT in adults Increasing cell dose (double CB) and better donor choice Decreasing HLA disparities (increasing greater inventory) Better indications Centre experience Better surveillance, prophylaxis and treatment of infections Modifications of GVHD prophylaxis and conditioning regimens

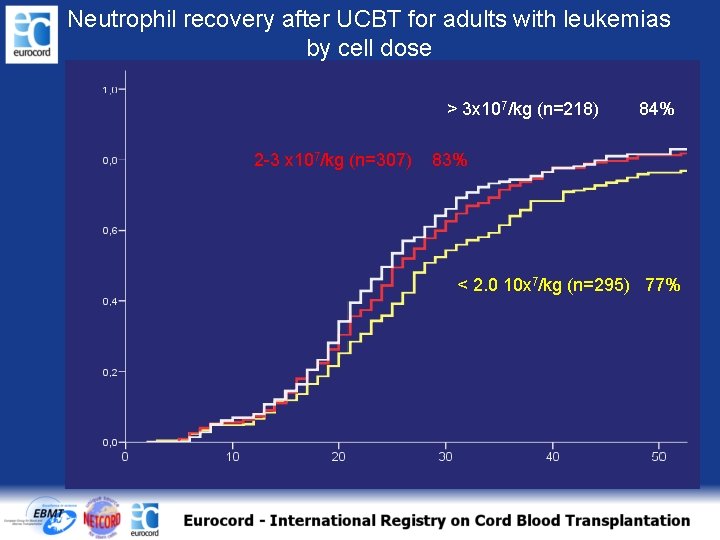
Neutrophil recovery after UCBT for adults with leukemias by cell dose > 3 x 107/kg (n=218) 2 -3 x 107/kg (n=307) 84% 83% < 2. 0 10 x 7/kg (n=295) 77%

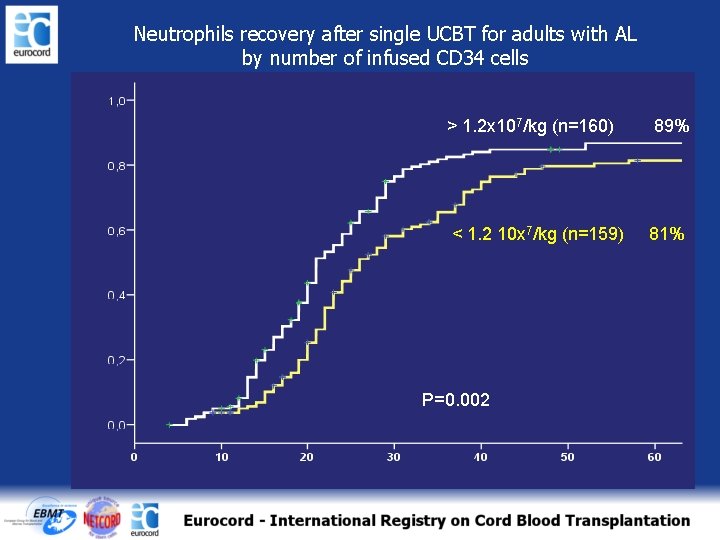
Neutrophils recovery after single UCBT for adults with AL by number of infused CD 34 cells > 1. 2 x 107/kg (n=160) 89% < 1. 2 10 x 7/kg (n=159) 81% P=0. 002

Major factors changing outcomes after UCBT in adults Increasing cell dose (double CB) and better donor choice Decreasing HLA disparities (increasing inventory of CBB) Better indications Centre experience Better surveillance, prophylaxis and treatment of infections Modifications of GVHD prophylaxis and conditioning regimens

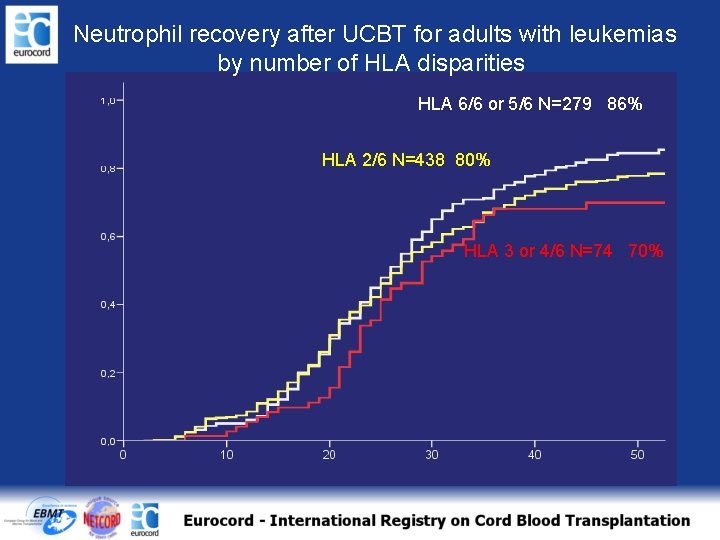
Neutrophil recovery after UCBT for adults with leukemias by number of HLA disparities HLA 6/6 or 5/6 N=279 86% HLA 2/6 N=438 80% HLA 3 or 4/6 N=74 70%

Major factors changing outcomes after UCBT in adults Increasing cell dose (double CB) Decreasing HLA disparities (increasing greater inventory) Better indications Better surveillance, prophylaxis and treatment of infections Modifications of GVHD prophylaxis and conditioning regimens

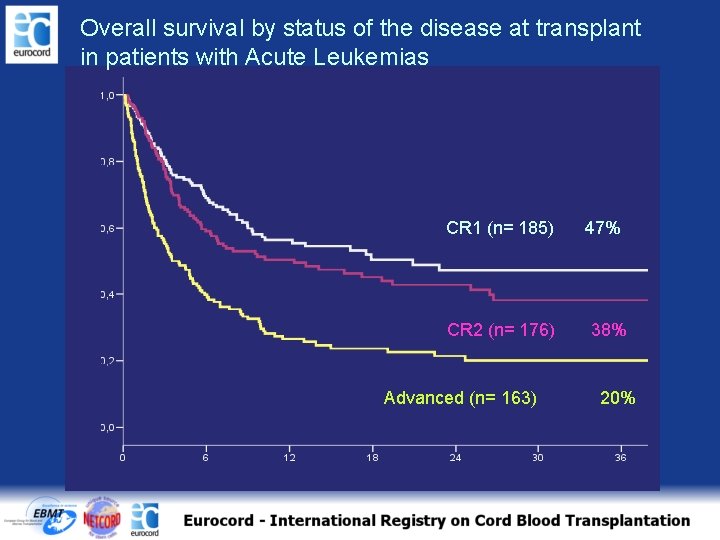
Overall survival by status of the disease at transplant in patients with Acute Leukemias CR 1 (n= 185) CR 2 (n= 176) Advanced (n= 163) 47% 38% 20%

Major factors changing outcomes after UCBT in adults Increasing cell dose (double CB) Decreasing HLA disparities (increasing greater inventory) Better indications Better surveillance, prophylaxis and treatment of infections Modifications of GVHD prophylaxis and conditioning regimens

Rationale for using RIC in patients given CBT • The mortality and morbidity associated with conventional myeloablative transplantation curtails the number of patients who can be treated with an allogeneiv HSCT;

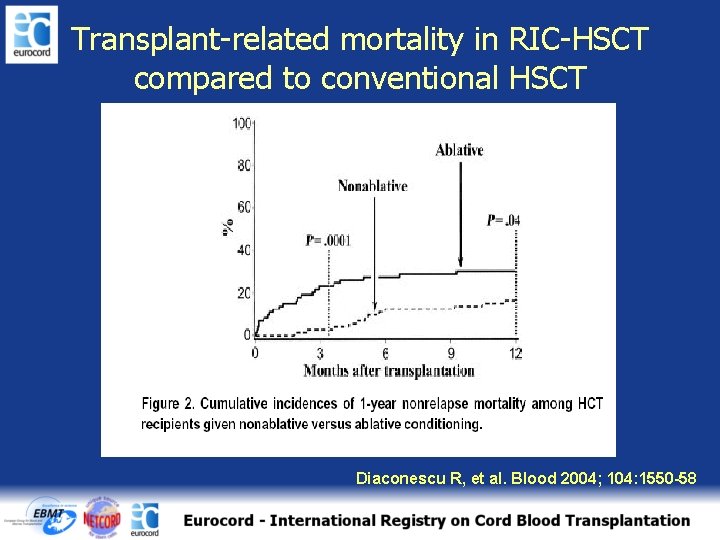
Transplant-related mortality in RIC-HSCT compared to conventional HSCT Diaconescu R, et al. Blood 2004; 104: 1550 -58

Rationale for using RIC in patients given CBT The mortality and morbidity associated with conventional myeloablative transplantation curtails the number of patients who can be treated with an allogeneic HSCT; The presence of residual host cells may also contribute to the reduction in incidence and severity of Gv. HD

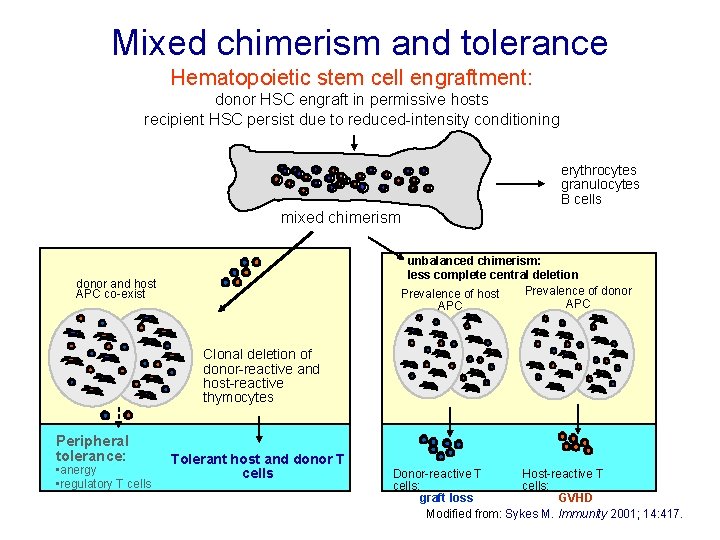
Mixed chimerism and tolerance Hematopoietic stem cell engraftment: donor HSC engraft in permissive hosts recipient HSC persist due to reduced-intensity conditioning erythrocytes granulocytes B cells mixed chimerism unbalanced chimerism: less complete central deletion Prevalence of donor Prevalence of host APC donor and host APC co-exist Clonal deletion of donor-reactive and host-reactive thymocytes Peripheral tolerance: • anergy • regulatory T cells Tolerant host and donor T cells Donor-reactive T Host-reactive T cells: graft loss GVHD Modified from: Sykes M. Immunity 2001; 14: 417.

Eurocord Registry

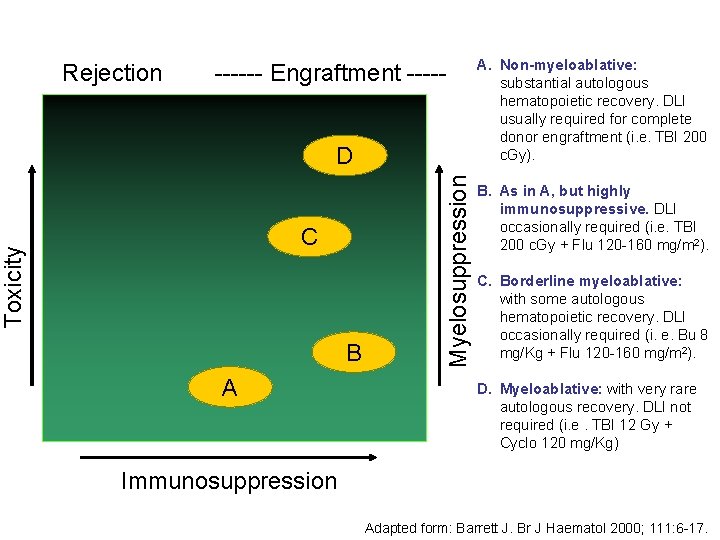
Rejection ------ Engraftment ----- Toxicity C B A Myelosuppression D A. Non-myeloablative: substantial autologous hematopoietic recovery. DLI usually required for complete donor engraftment (i. e. TBI 200 c. Gy). B. As in A, but highly immunosuppressive. DLI occasionally required (i. e. TBI 200 c. Gy + Flu 120 -160 mg/m 2). C. Borderline myeloablative: with some autologous hematopoietic recovery. DLI occasionally required (i. e. Bu 8 mg/Kg + Flu 120 -160 mg/m 2). D. Myeloablative: with very rare autologous recovery. DLI not required (i. e. TBI 12 Gy + Cyclo 120 mg/Kg) Immunosuppression Adapted form: Barrett J. Br J Haematol 2000; 111: 6 -17.

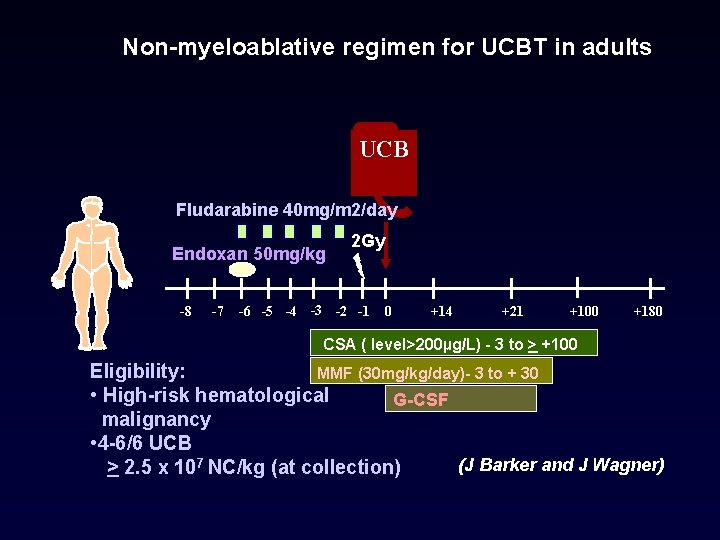
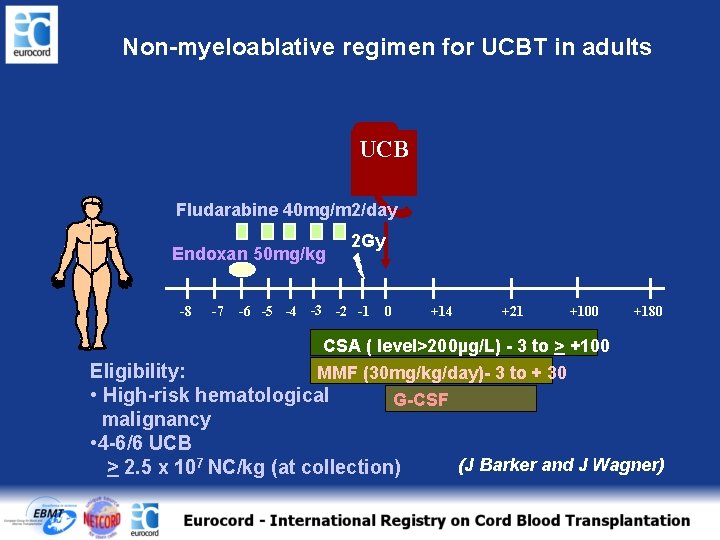
Non-myeloablative regimen for UCBT in adults UCB Fludarabine 40 mg/m 2/day Endoxan 50 mg/kg -8 -7 2 Gy -6 -5 -4 -3 -2 -1 0 +14 +21 +100 +180 CSA ( level>200µg/L) - 3 to > +100 Eligibility: MMF (30 mg/kg/day)- 3 to + 30 • High-risk hematological G-CSF malignancy • 4 -6/6 UCB (J Barker and J Wagner) > 2. 5 x 107 NC/kg (at collection)

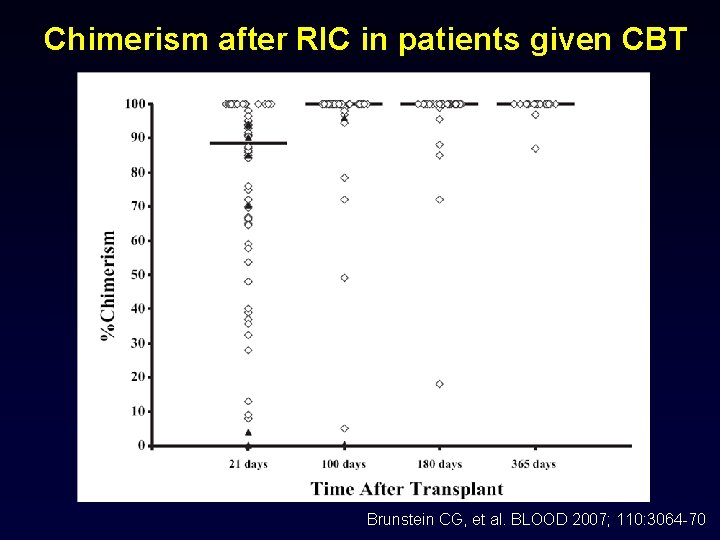
Chimerism after RIC in patients given CBT Brunstein CG, et al. BLOOD 2007; 110: 3064 -70

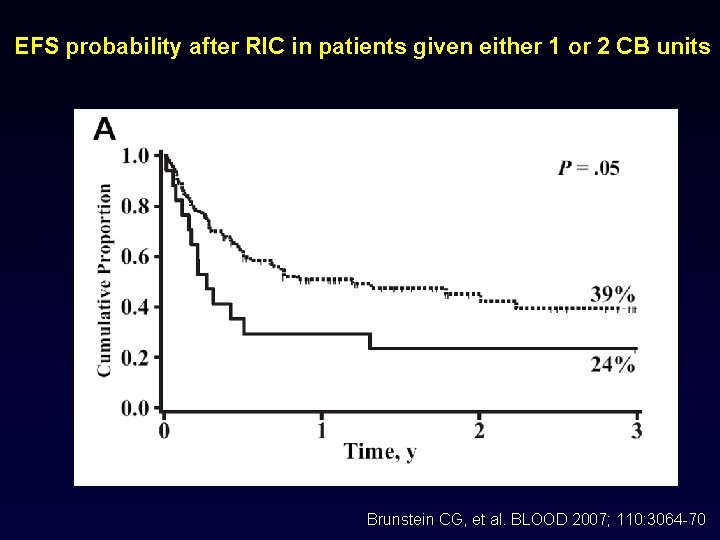
EFS probability after RIC in patients given either 1 or 2 CB units Brunstein CG, et al. BLOOD 2007; 110: 3064 -70

RIC before single unrelated CBT for adults with hematological maligancies An Eurocord-Netcord, SFGM-TC and Minnesota group analysis

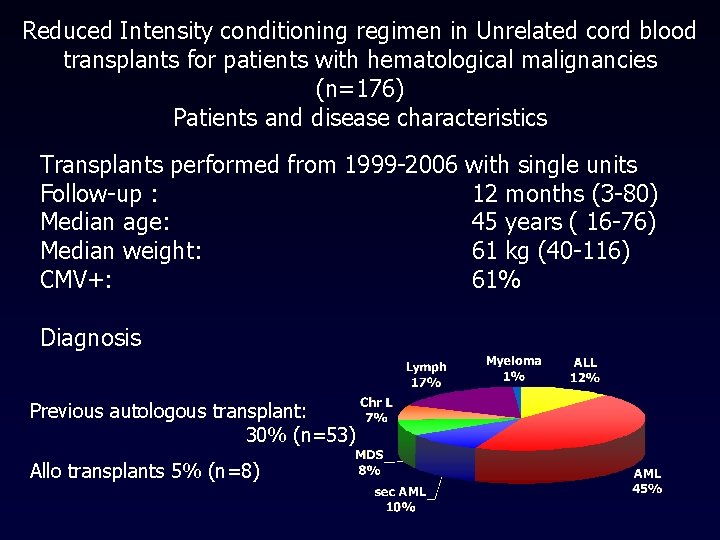
Reduced Intensity conditioning regimen in Unrelated cord blood transplants for patients with hematological malignancies (n=176) Patients and disease characteristics Transplants performed from 1999 -2006 with single units Follow-up : 12 months (3 -80) Median age: 45 years ( 16 -76) Median weight: 61 kg (40 -116) CMV+: 61% Diagnosis Previous autologous transplant: 30% (n=53) Allo transplants 5% (n=8)

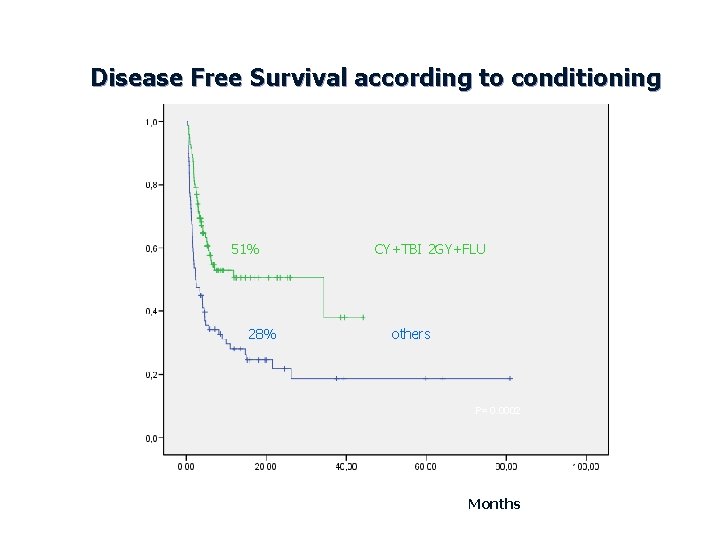
Disease Free Survival according to conditioning 51% 28% CY+TBI 2 GY+FLU others P= 0. 0002 Months

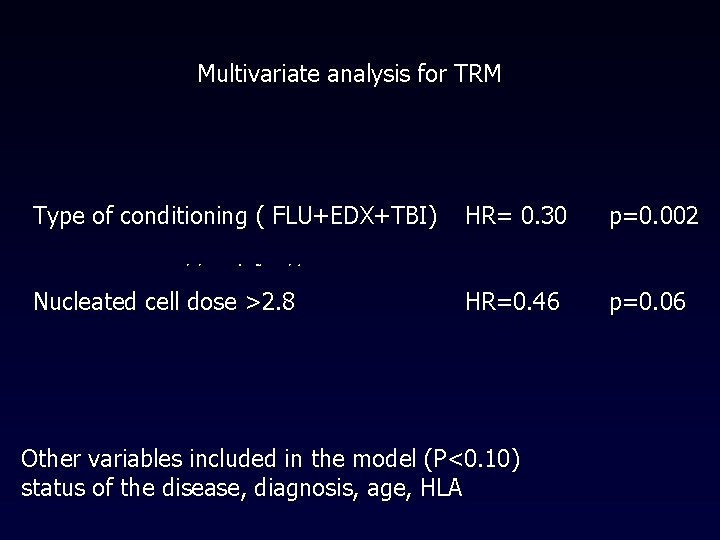
Multivariate analysis for TRM Type of conditioning ( FLU+EDX+TBI) HR= 0. 30 p=0. 002 Nucleated cell dose >2. 8 HR=0. 46 p=0. 06 Other variables included in the model (P<0. 10) status of the disease, diagnosis, age, HLA

Unrelated Cord Blood transplants in adults with hematological malignancies after Cyclophosphamide-Fludarabine-TBI reduced intensity conditioning regimen B Rio on behalf of Eurocord-Netcord and SFGM-TC group analysis

Patients and disease characteristics 155 patients (96 single CBT and 59 double CBT) have data on outcomes and were analysed Median Follow-up : Median age: Median weight: CMV+: Previous Tx: auto: 35%(n=54) allo: 1% (n=1) 18 months (1. 6 -56) 46, 7 years ( 19 -69) 65 kg (42 -125) 56%

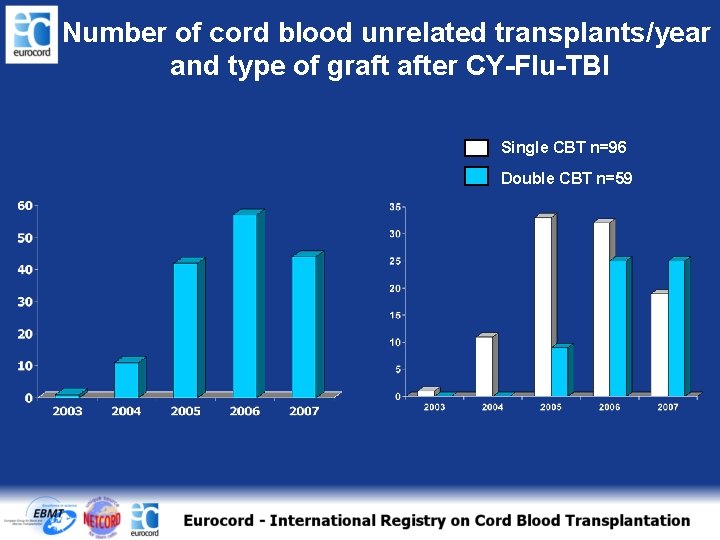
Number of cord blood unrelated transplants/year and type of graft after CY-Flu-TBI Single CBT n=96 Double CBT n=59

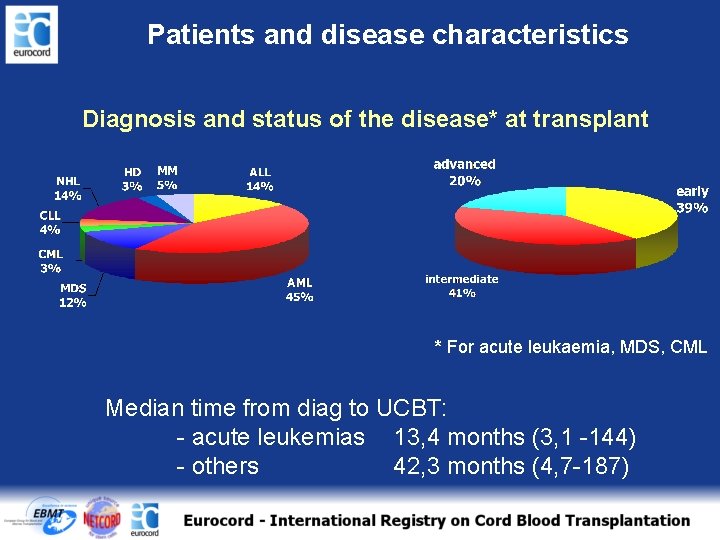
Patients and disease characteristics Diagnosis and status of the disease* at transplant * For acute leukaemia, MDS, CML Median time from diag to UCBT: - acute leukemias 13, 4 months (3, 1 -144) - others 42, 3 months (4, 7 -187)

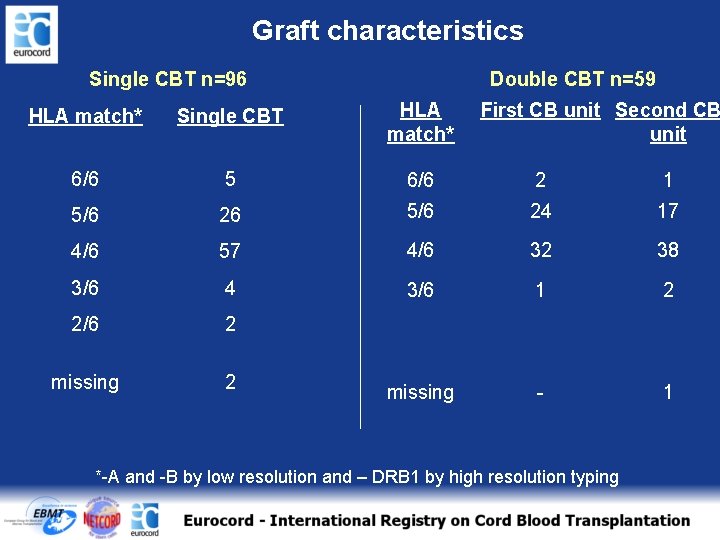
Graft characteristics Single CBT n=96 Double CBT n=59 HLA match* Single CBT HLA match* First CB unit Second CB unit 6/6 5 6/6 2 1 5/6 26 5/6 24 17 4/6 57 4/6 32 38 3/6 4 3/6 1 2 2/6 2 missing - 1 *-A and -B by low resolution and – DRB 1 by high resolution typing

Non-myeloablative regimen for UCBT in adults UCB Fludarabine 40 mg/m 2/day Endoxan 50 mg/kg -8 -7 2 Gy -6 -5 -4 -3 -2 -1 0 +14 +21 +100 +180 CSA ( level>200µg/L) - 3 to > +100 Eligibility: MMF (30 mg/kg/day)- 3 to + 30 • High-risk hematological G-CSF malignancy • 4 -6/6 UCB > 2. 5 x 107 NC/kg (at collection) (J Barker and J Wagner)

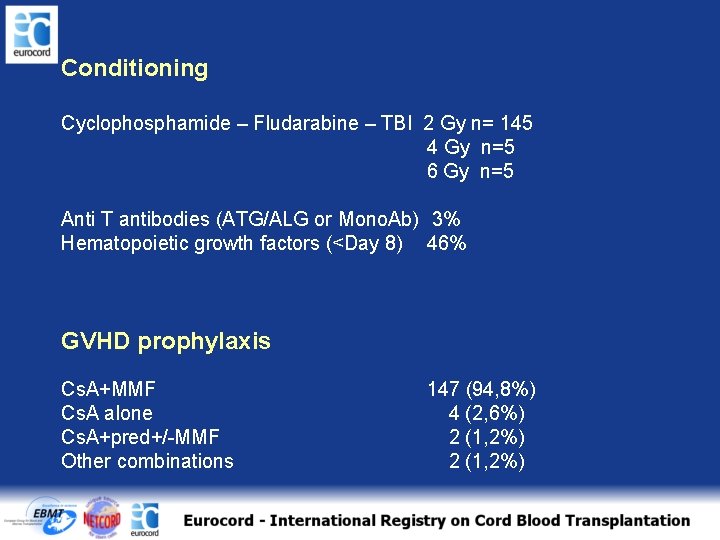
Conditioning Cyclophosphamide – Fludarabine – TBI 2 Gy n= 145 4 Gy n=5 6 Gy n=5 Anti T antibodies (ATG/ALG or Mono. Ab) 3% Hematopoietic growth factors (<Day 8) 46% GVHD prophylaxis Cs. A+MMF Cs. A alone Cs. A+pred+/-MMF Other combinations 147 (94, 8%) 4 (2, 6%) 2 (1, 2%)

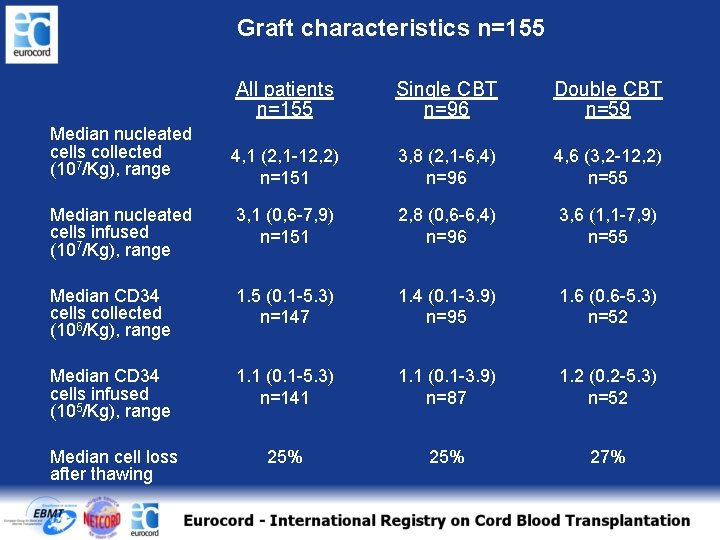
Graft characteristics n=155 All patients n=155 Single CBT n=96 Double CBT n=59 4, 1 (2, 1 -12, 2) n=151 3, 8 (2, 1 -6, 4) n=96 4, 6 (3, 2 -12, 2) n=55 Median nucleated cells infused (107/Kg), range 3, 1 (0, 6 -7, 9) n=151 2, 8 (0, 6 -6, 4) n=96 3, 6 (1, 1 -7, 9) n=55 Median CD 34 cells collected (106/Kg), range 1. 5 (0. 1 -5. 3) n=147 1. 4 (0. 1 -3. 9) n=95 1. 6 (0. 6 -5. 3) n=52 Median CD 34 cells infused (105/Kg), range 1. 1 (0. 1 -5. 3) n=141 1. 1 (0. 1 -3. 9) n=87 1. 2 (0. 2 -5. 3) n=52 Median cell loss after thawing 25% 27% Median nucleated cells collected (107/Kg), range

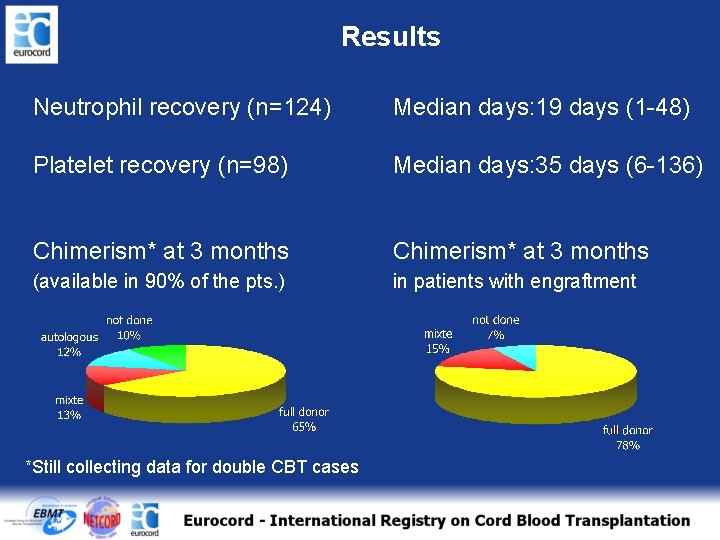
Results Neutrophil recovery (n=124) Median days: 19 days (1 -48) Platelet recovery (n=98) Median days: 35 days (6 -136) Chimerism* at 3 months (available in 90% of the pts. ) in patients with engraftment *Still collecting data for double CBT cases

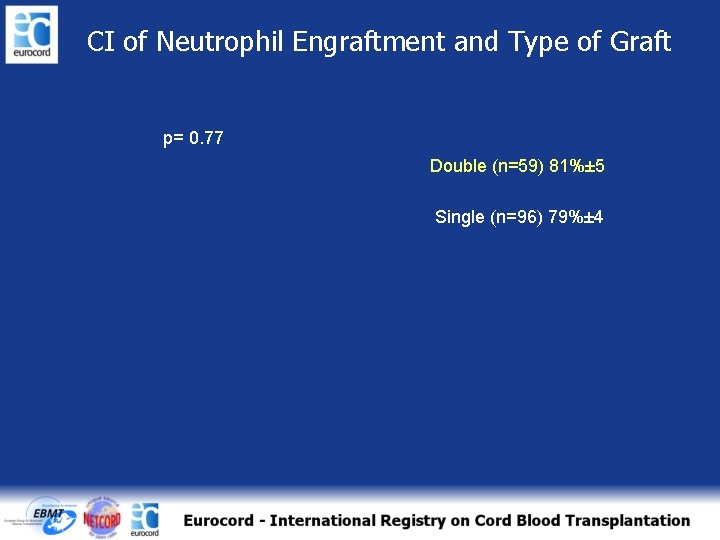
CI of Neutrophil Engraftment and Type of Graft p= 0. 77 Double (n=59) 81%± 5 Single (n=96) 79%± 4

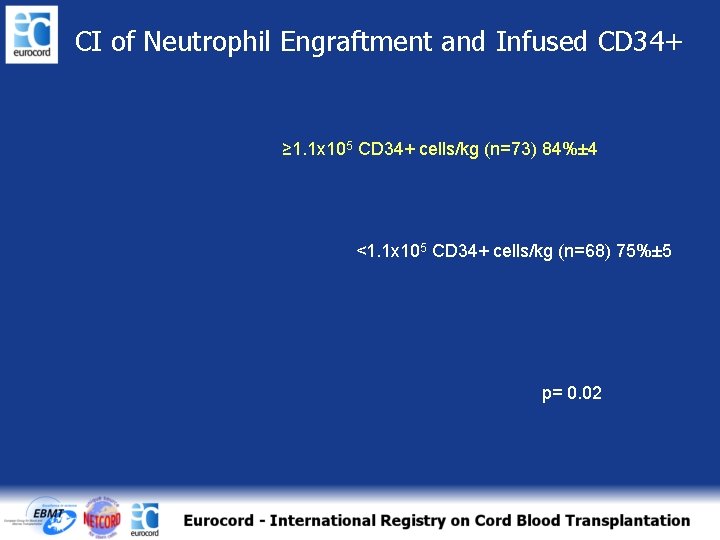
CI of Neutrophil Engraftment and Infused CD 34+ ≥ 1. 1 x 105 CD 34+ cells/kg (n=73) 84%± 4 <1. 1 x 105 CD 34+ cells/kg (n=68) 75%± 5 p= 0. 02

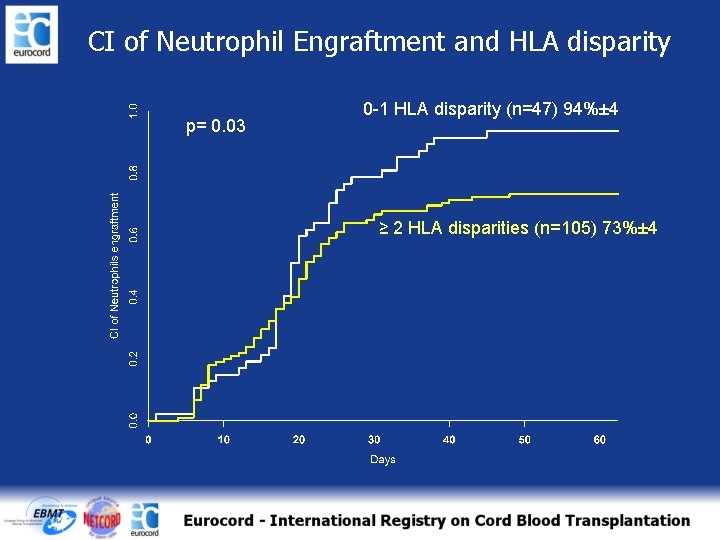
CI of Neutrophil Engraftment and HLA disparity p= 0. 03 0 -1 HLA disparity (n=47) 94%± 4 ≥ 2 HLA disparities (n=105) 73%± 4

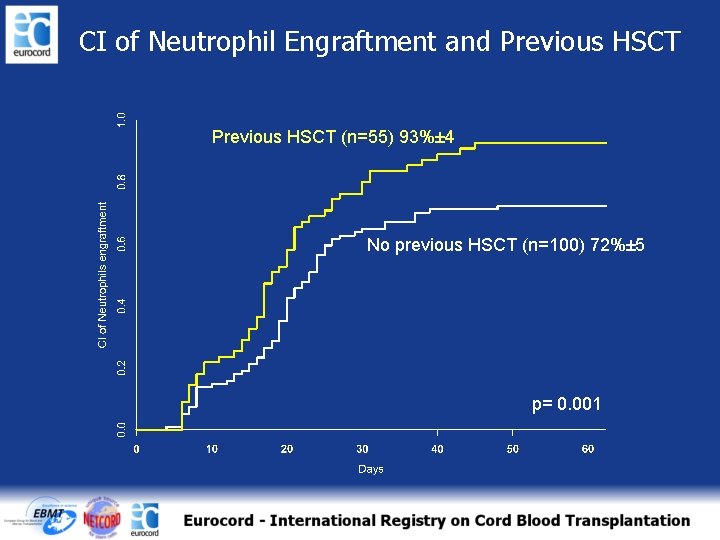
CI of Neutrophil Engraftment and Previous HSCT (n=55) 93%± 4 No previous HSCT (n=100) 72%± 5 p= 0. 001

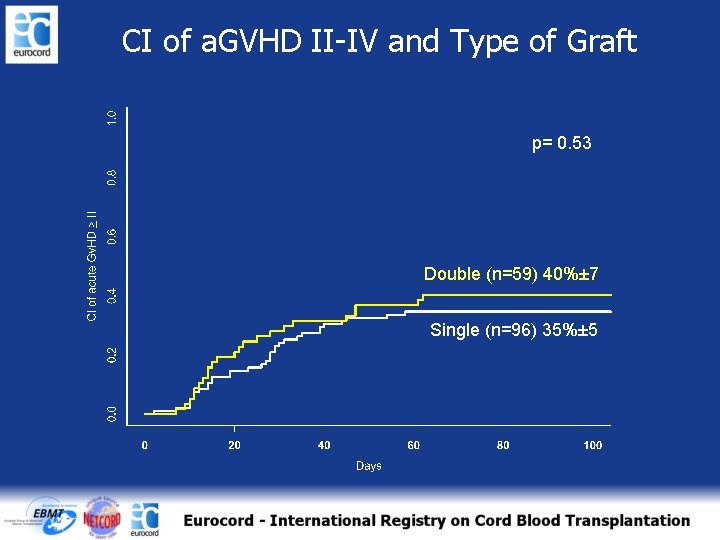
CI of a. GVHD II-IV and Type of Graft p= 0. 53 Double (n=59) 40%± 7 Single (n=96) 35%± 5

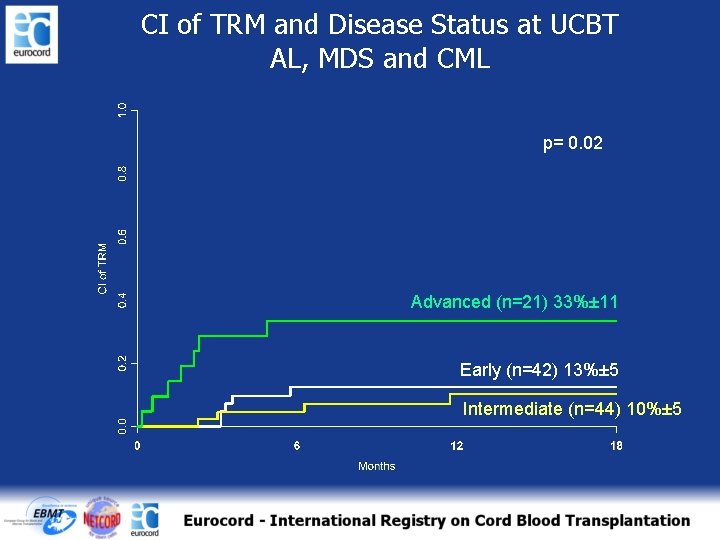
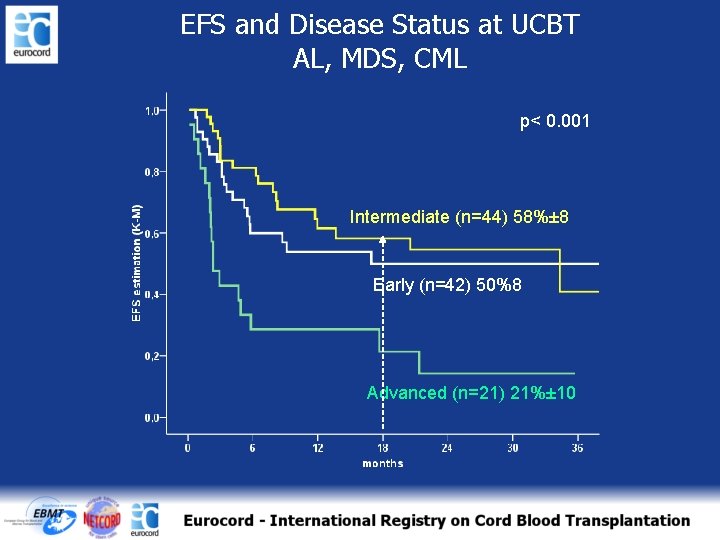
CI of TRM and Disease Status at UCBT AL, MDS and CML p= 0. 02 Advanced (n=21) 33%± 11 Early (n=42) 13%± 5 Intermediate (n=44) 10%± 5

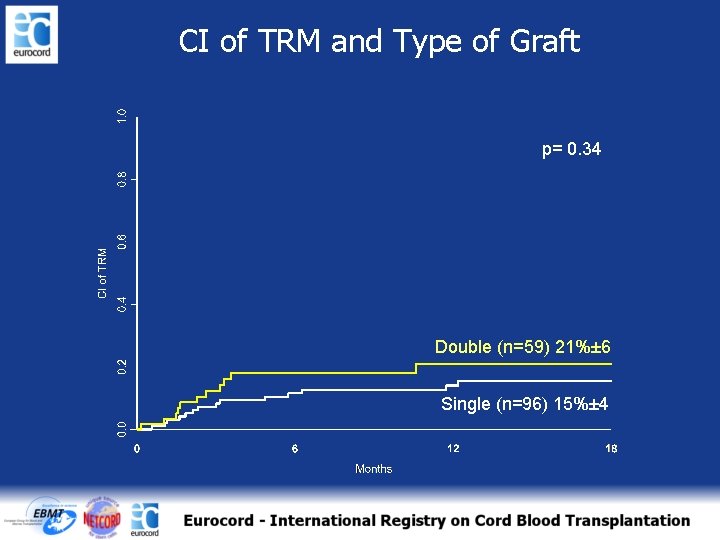
CI of TRM and Type of Graft p= 0. 34 Double (n=59) 21%± 6 Single (n=96) 15%± 4

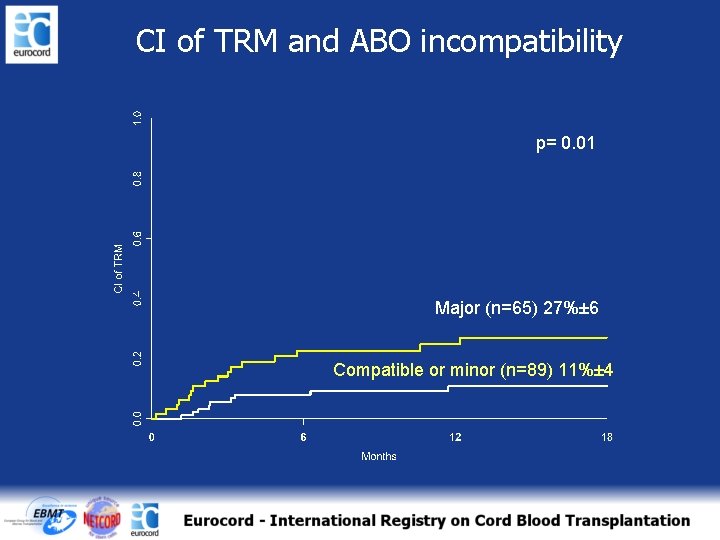
CI of TRM and ABO incompatibility p= 0. 01 Major (n=65) 27%± 6 Compatible or minor (n=89) 11%± 4

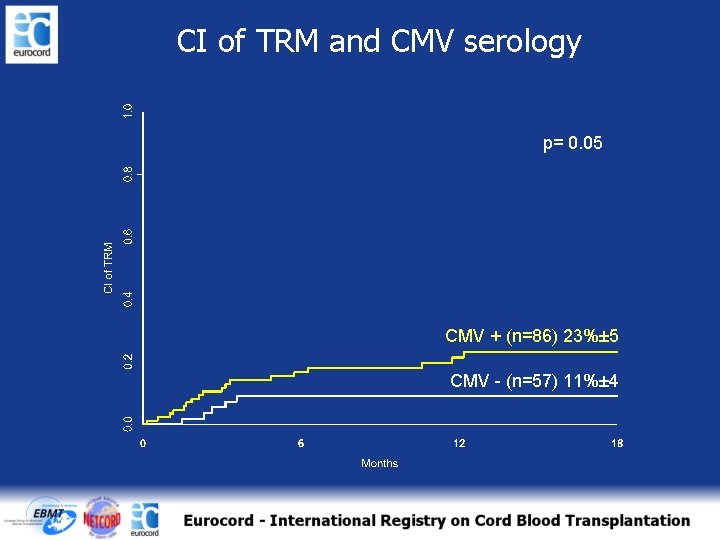
CI of TRM and CMV serology p= 0. 05 CMV + (n=86) 23%± 5 CMV - (n=57) 11%± 4

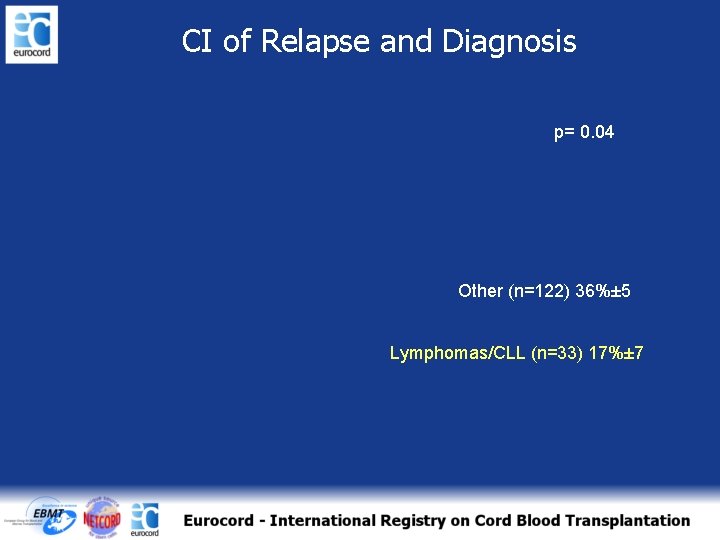
CI of Relapse and Diagnosis p= 0. 04 Other (n=122) 36%± 5 Lymphomas/CLL (n=33) 17%± 7

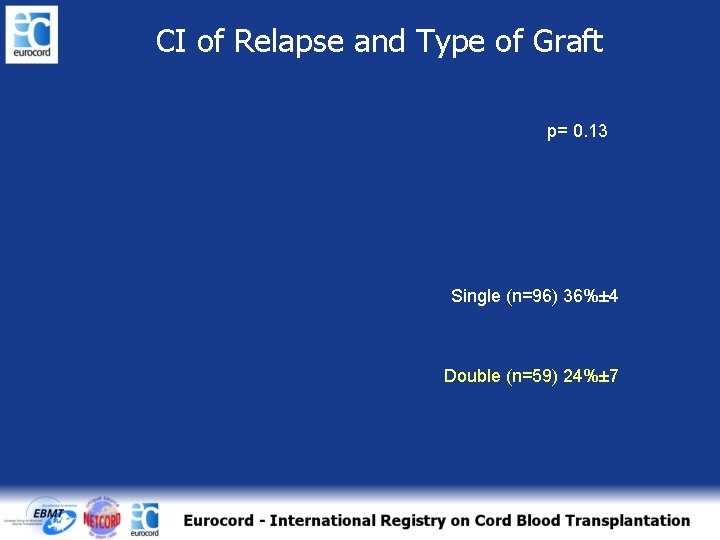
CI of Relapse and Type of Graft p= 0. 13 Single (n=96) 36%± 4 Double (n=59) 24%± 7

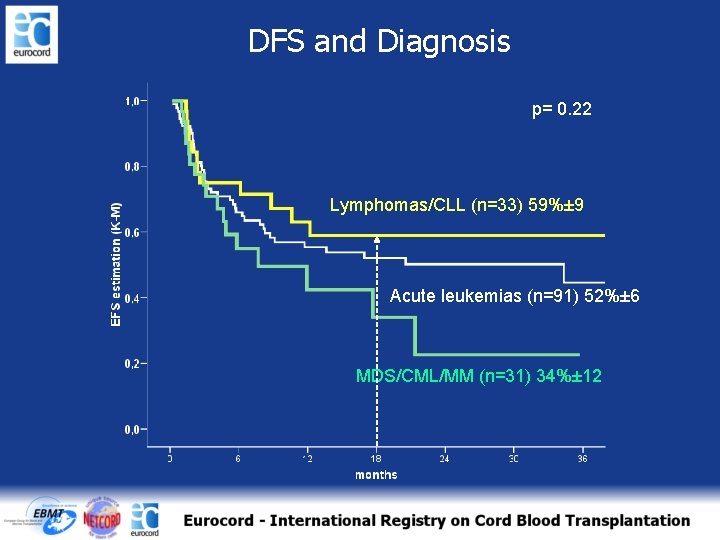
DFS and Diagnosis p= 0. 22 Lymphomas/CLL (n=33) 59%± 9 Acute leukemias (n=91) 52%± 6 MDS/CML/MM (n=31) 34%± 12

EFS and Disease Status at UCBT AL, MDS, CML p< 0. 001 Intermediate (n=44) 58%± 8 Early (n=42) 50%8 Advanced (n=21) 21%± 10

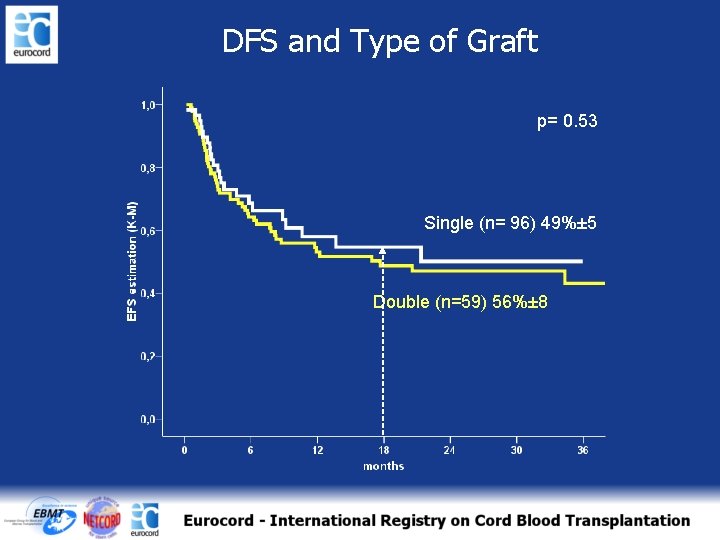
DFS and Type of Graft p= 0. 53 Single (n= 96) 49%± 5 Double (n=59) 56%± 8

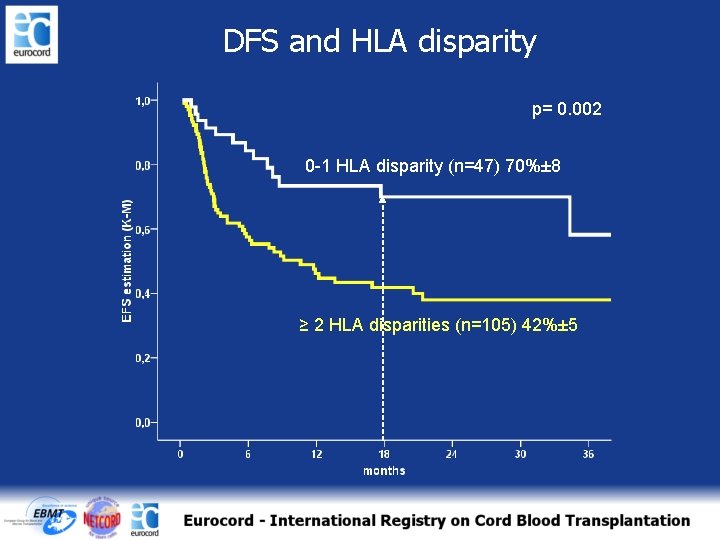
DFS and HLA disparity p= 0. 002 0 -1 HLA disparity (n=47) 70%± 8 ≥ 2 HLA disparities (n=105) 42%± 5

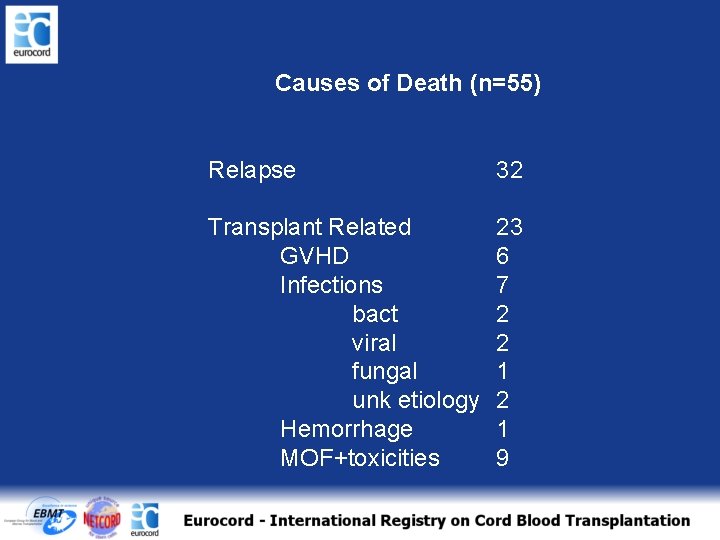
Causes of Death (n=55) Relapse 32 Transplant Related GVHD Infections bact viral fungal unk etiology Hemorrhage MOF+toxicities 23 6 7 2 2 1 9

Conclusions • Available evidence supports the use of CBT after RIC in adult patients either older than 45 years of age or not eligible to receive an allograft after a conventional preparative regimen. • The use of 2 units seems to decrease the risk of leukemia recurrence, although it remains to be definitively proved whether this translates into a better outcome. • Disease phase at time of transplantation is the most important factor predicting outcome. Better HLA matching of the CB unit(s) is associated with a better probability of DFS. • Particularly encouraging are the results obtained in patients with indolent lymphoproliferative disorders.

Eurocord team 2007 -2008 Eliane Gluckman Vanderson Rocha Irina Ionescu Federico Garnier David Pacheco Wagnara Chaves Karim Boujedir Andree Laure Herr Adriene Madureira Annalisa Ruggeri Nabil Kabbara Roel Willemze Samir Nabhan Juliana Rodrigues Celso Arrais Nabila Bechar Anne Leger Evelyne Thai
 Lercarnidipina
Lercarnidipina Avanti cristo
Avanti cristo Vistabex prima e dopo
Vistabex prima e dopo Linea del tempo medioevo
Linea del tempo medioevo Cortisone dopo estrazione dente
Cortisone dopo estrazione dente Diarrea gelato
Diarrea gelato La sera di quello stesso giorno il primo dopo il sabato
La sera di quello stesso giorno il primo dopo il sabato Dopo la quaresima
Dopo la quaresima O natura cortese son questi i doni tuoi
O natura cortese son questi i doni tuoi Flebectomia
Flebectomia Dieta semisolida sleeve
Dieta semisolida sleeve Imperativa frase
Imperativa frase Tinea capitis ricrescono i capelli
Tinea capitis ricrescono i capelli George steiner dopo babele
George steiner dopo babele Chi è lo storico
Chi è lo storico Albertis regime
Albertis regime Régime it cpi
Régime it cpi The ancien regime
The ancien regime Ancient regime
Ancient regime Ochsner sherren regime
Ochsner sherren regime Gik regime
Gik regime Simulazione energetica in regime dinamico
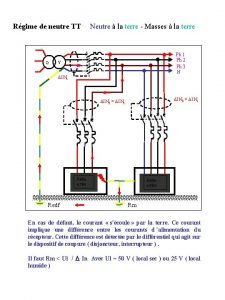
Simulazione energetica in regime dinamico Régime de neutre tns et tnc
Régime de neutre tns et tnc Régime hypoprotidique exemple
Régime hypoprotidique exemple Regime alimentar da águia
Regime alimentar da águia Regime permanente senoidal
Regime permanente senoidal What is the old regime in france
What is the old regime in france Forma de governo
Forma de governo Prince of philippines church music
Prince of philippines church music Antigo regime e iluminismo
Antigo regime e iluminismo Absolutismo
Absolutismo Régime de change au maroc
Régime de change au maroc Matematica finanziaria formule inverse
Matematica finanziaria formule inverse Describe the causes of french revolution
Describe the causes of french revolution The ancien regime
The ancien regime Regime diferenciado disciplinar
Regime diferenciado disciplinar O que é regime de colaboração
O que é regime de colaboração Propagande vichy
Propagande vichy Regime demografico moderno
Regime demografico moderno Dissection artere mesenterique
Dissection artere mesenterique Mass flow fermenter
Mass flow fermenter Neutre impédant
Neutre impédant Tableau régime alimentaire sous avk
Tableau régime alimentaire sous avk Regime jenny gray
Regime jenny gray Carte mentale régime totalitaire stalinien
Carte mentale régime totalitaire stalinien Regime diferenciado disciplinar
Regime diferenciado disciplinar Legal regime
Legal regime The flexible exchange rate regime 1973-present
The flexible exchange rate regime 1973-present Divc regime
Divc regime Regime de competencia
Regime de competencia Regime
Regime Rzeczp
Rzeczp Guinier regime
Guinier regime Regime example
Regime example Regime sinusoidale
Regime sinusoidale Tubo convergente
Tubo convergente