Il trapianto allogenico da donatore alternativo dopo condizionamento











![Decessi Follow-up post trapianto (mediana 6. 0 mesi [0. 4 – 25. 7]) Decessi Follow-up post trapianto (mediana 6. 0 mesi [0. 4 – 25. 7])](https://slidetodoc.com/presentation_image_h/75d23ad4ddd75022c66bb91c4003c1ef/image-26.jpg)

- Slides: 27

Il trapianto allogenico da donatore alternativo dopo condizionamento a ridotta intensità Alessandro Rambaldi

Is the donor type (related or unrelated) still an issue in the setting of RIC allograft? The role of the conditioning regimen and GVHD prophylaxis

Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders Perez-Simon et al: Blood. 2002; 100: 3121 -3127 Transplantation-related mortality

Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders Perez-Simon et al: Blood. 2002; 100: 3121 -3127 The conditioning regimen fludarabine 150 mg/m 2 + melphalan 140 mg/m 2 Event Free Survival

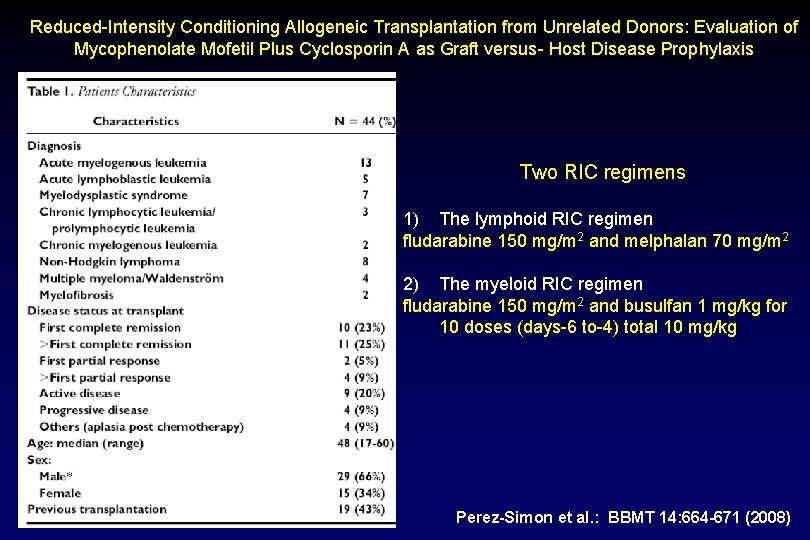
Reduced-Intensity Conditioning Allogeneic Transplantation from Unrelated Donors: Evaluation of Mycophenolate Mofetil Plus Cyclosporin A as Graft versus- Host Disease Prophylaxis Two RIC regimens 1) The lymphoid RIC regimen fludarabine 150 mg/m 2 and melphalan 70 mg/m 2 2) The myeloid RIC regimen fludarabine 150 mg/m 2 and busulfan 1 mg/kg for 10 doses (days-6 to-4) total 10 mg/kg Perez-Simon et al. : BBMT 14: 664 -671 (2008)

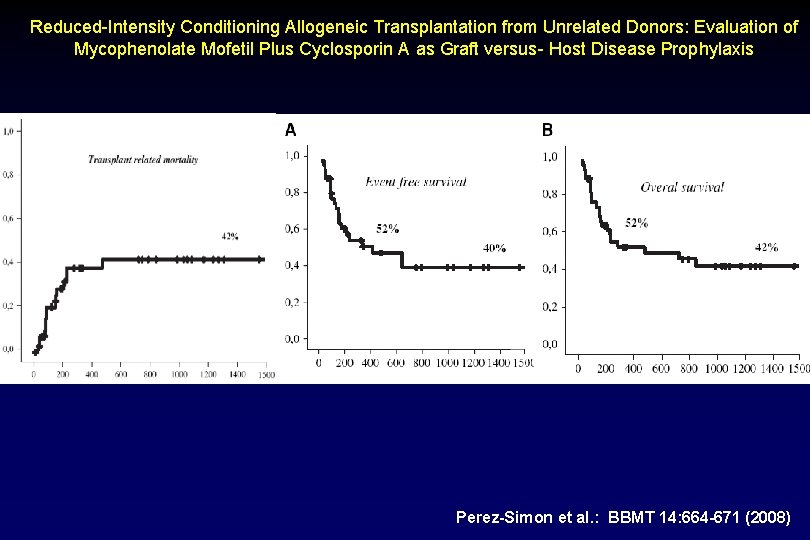
Reduced-Intensity Conditioning Allogeneic Transplantation from Unrelated Donors: Evaluation of Mycophenolate Mofetil Plus Cyclosporin A as Graft versus- Host Disease Prophylaxis Perez-Simon et al. : BBMT 14: 664 -671 (2008)

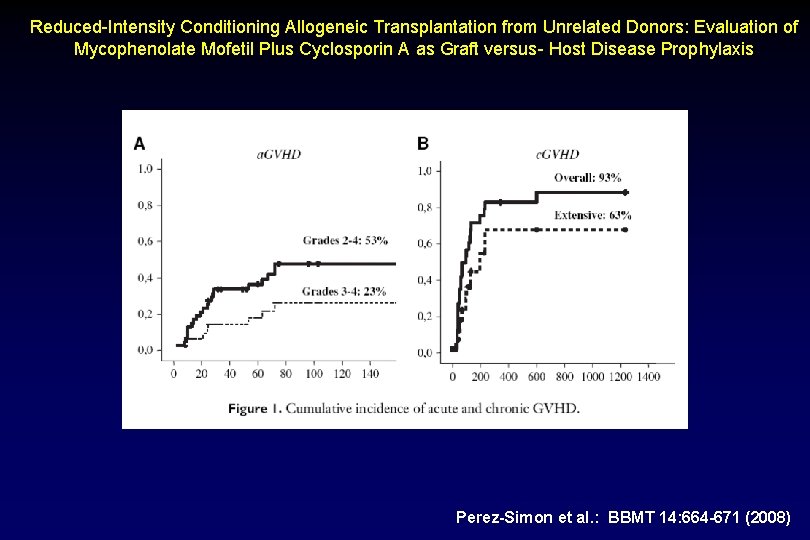
Reduced-Intensity Conditioning Allogeneic Transplantation from Unrelated Donors: Evaluation of Mycophenolate Mofetil Plus Cyclosporin A as Graft versus- Host Disease Prophylaxis Perez-Simon et al. : BBMT 14: 664 -671 (2008)

OS for patients over age 50 after nonmyeloablative or myeloablative transplantation Alyea, E. P. et al. Blood 2005; 105: 1810 -1814 Copyright © 2005 American Society of Hematology. Copyright restrictions may apply.

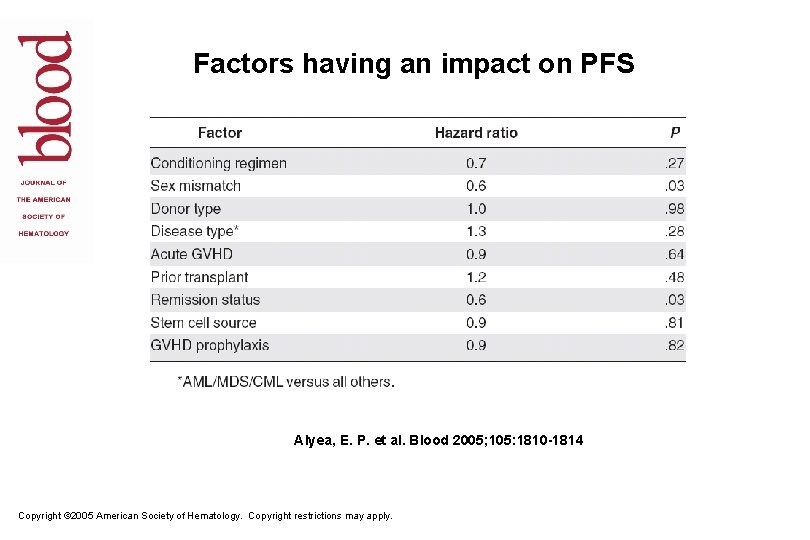
Factors having an impact on PFS Alyea, E. P. et al. Blood 2005; 105: 1810 -1814 Copyright © 2005 American Society of Hematology. Copyright restrictions may apply.

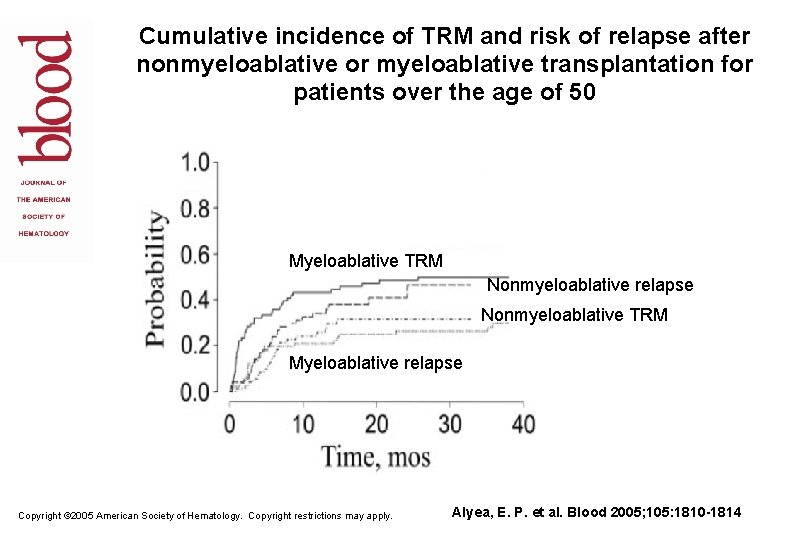
Cumulative incidence of TRM and risk of relapse after nonmyeloablative or myeloablative transplantation for patients over the age of 50 Myeloablative TRM Nonmyeloablative relapse Nonmyeloablative TRM Myeloablative relapse Copyright © 2005 American Society of Hematology. Copyright restrictions may apply. Alyea, E. P. et al. Blood 2005; 105: 1810 -1814

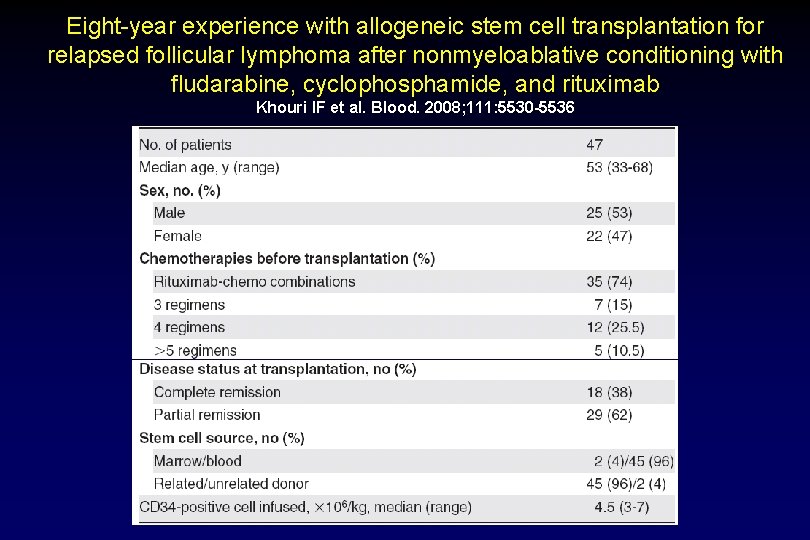
Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab Khouri IF et al. Blood. 2008; 111: 5530 -5536

Unrelated hematopoietic stem cell transplantation with reduced intensity regimens in high-risk patients for age or disease: results from two independent prospective GITMO studies for Gruppo Italiano Trapianti di Midollo Osseo (GITMO) and Italian Bone Marrow Donor Registry (IBMDR)

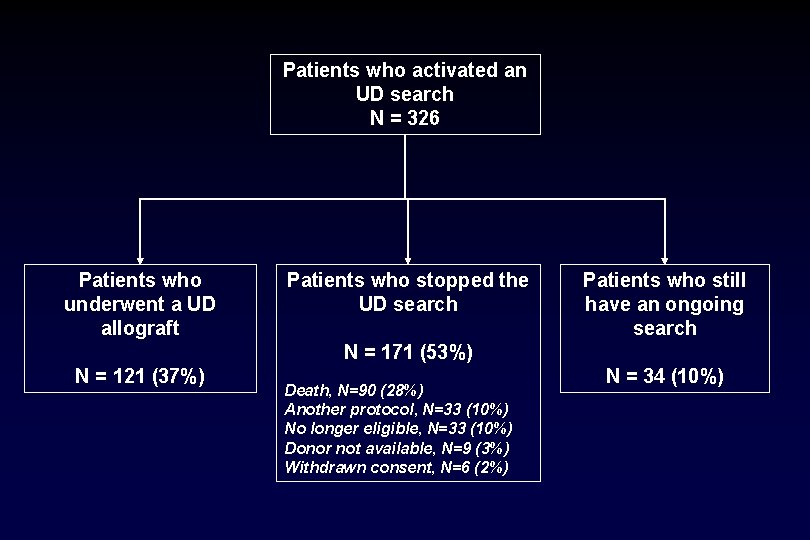
Patients who activated an UD search N = 326 Patients who underwent a UD allograft Patients who stopped the UD search Patients who still have an ongoing search N = 171 (53%) N = 121 (37%) Death, N=90 (28%) Another protocol, N=33 (10%) No longer eligible, N=33 (10%) Donor not available, N=9 (3%) Withdrawn consent, N=6 (2%) N = 34 (10%)

Patients characteristics by treatment strategy Univariate analyses. *Non parametric test for the medians; § Fisher exact test

Results of 121 patients actually allografted

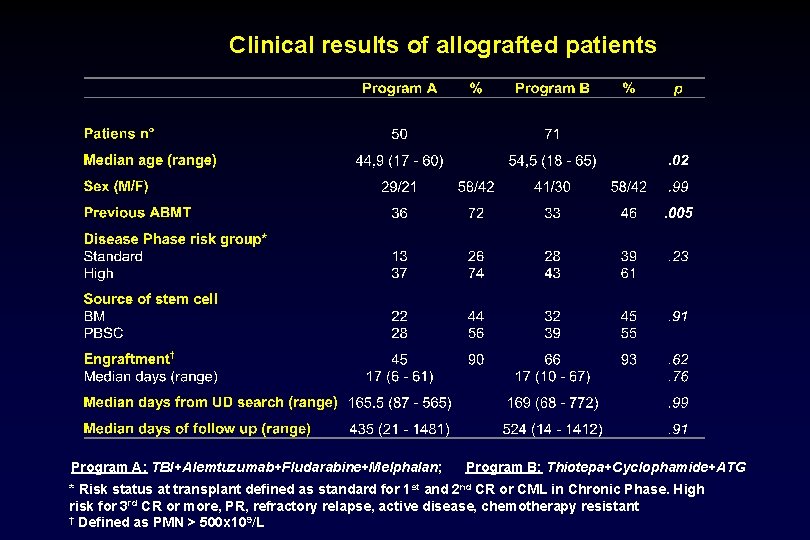
Clinical results of allografted patients Program A: TBI+Alemtuzumab+Fludarabine+Melphalan; Program B: Thiotepa+Cyclophamide+ATG * Risk status at transplant defined as standard for 1 st and 2 nd CR or CML in Chronic Phase. High risk for 3 rd CR or more, PR, refractory relapse, active disease, chemotherapy resistant † Defined as PMN > 500 x 109/L

Clinical results of allografted patients Program A: TBI+Alemtuzumab+Fludarabine+Melphalan; Program B: Thiotepa+Cyclophamide+ATG

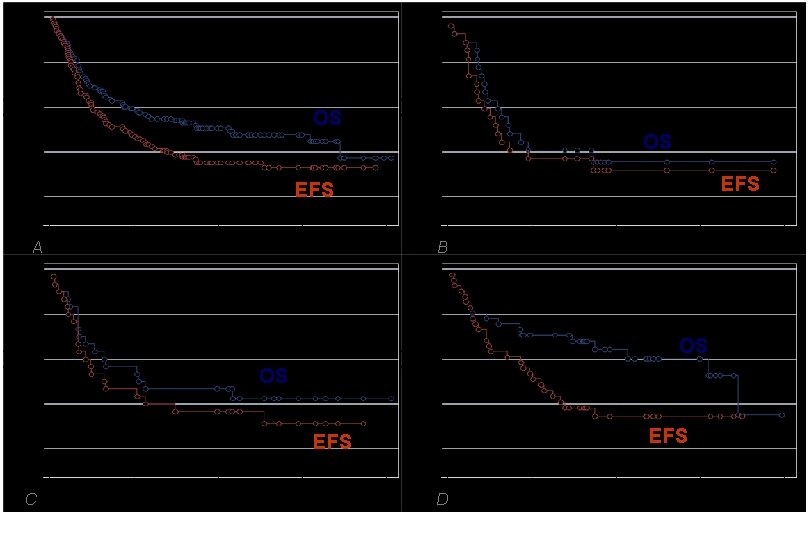
All patients (N=121) Acute Leukemias (N=27) OS OS EFS A B Non Hodgkin Lymphoma (N=30) Hodgkin Disease (N=41) OS OS EFS C D

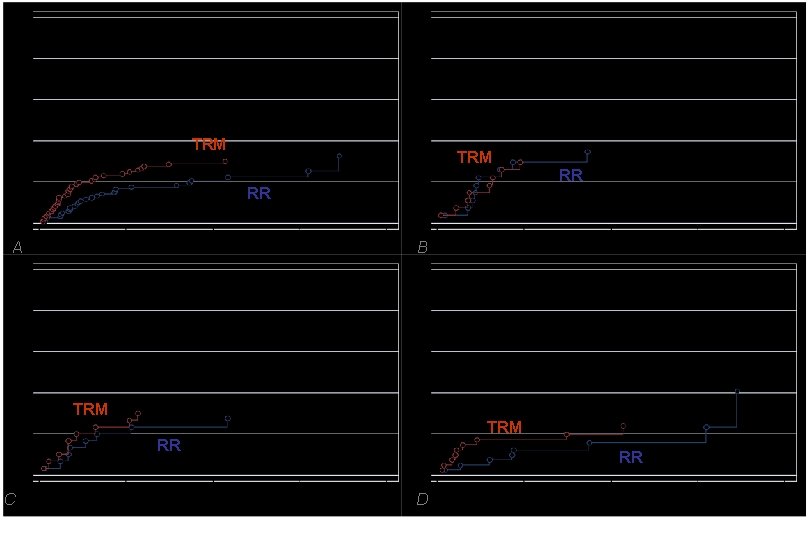
All patients (N=121) Acute Leukemias (N=27) TRM RR RR A B Non Hodgkin Lymphoma (N=30) Hodgkin Disease (N=41) TRM RR C RR D

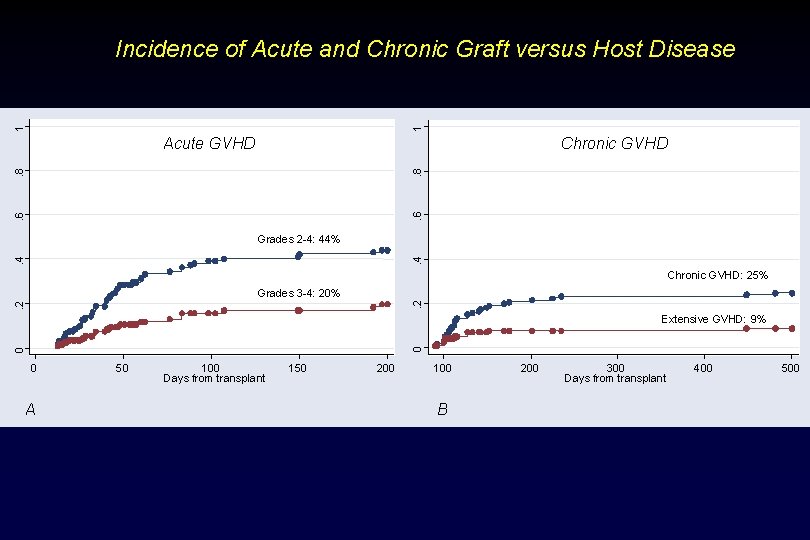
1 1 Incidence of Acute and Chronic Graft versus Host Disease Chronic GVHD . 6 . 8 Acute GVHD . 4 Grades 2 -4: 44% Chronic GVHD: 25%. 2 Grades 3 -4: 20% 0 0 Extensive GVHD: 9% 0 A 50 100 Days from transplant 150 200 100 B 200 300 Days from transplant 400 500

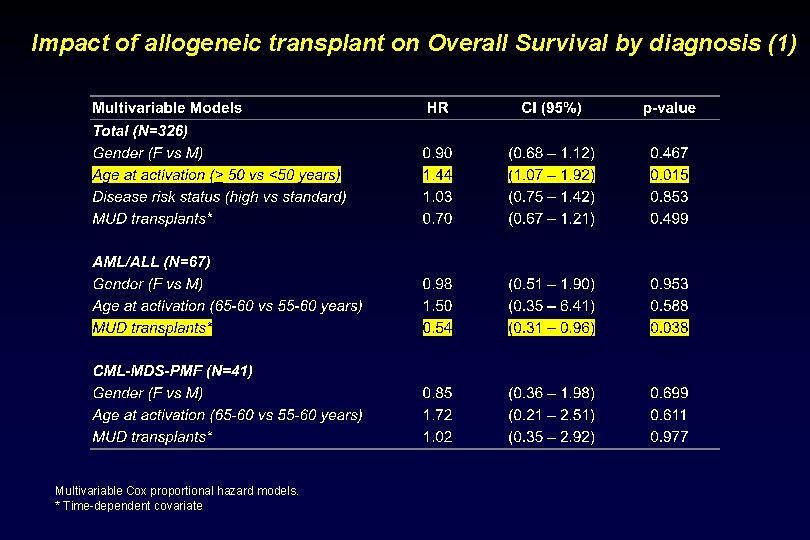
Impact of allogeneic transplant on Overall Survival by diagnosis (1) Multivariable Cox proportional hazard models. * Time-dependent covariate

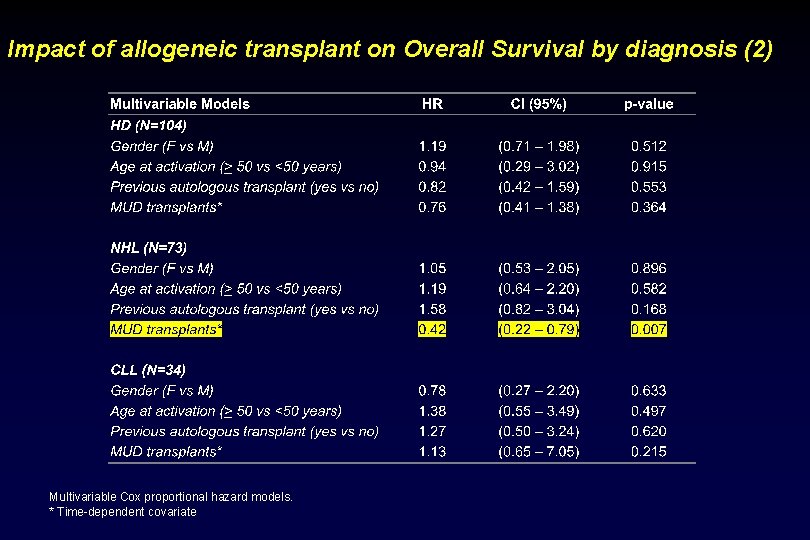
Impact of allogeneic transplant on Overall Survival by diagnosis (2) Multivariable Cox proportional hazard models. * Time-dependent covariate

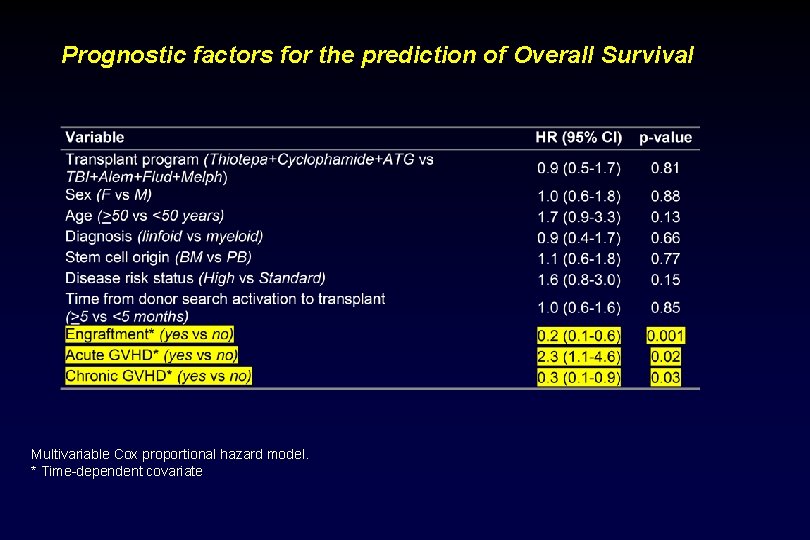
Prognostic factors for the prediction of Overall Survival Multivariable Cox proportional hazard model. * Time-dependent covariate

Protocollo Glob. Al Confronto randomizzato tra regimi di condizionamento a ridotta intensità contenenti rispettivamente Globulina Anti Linfocitaria verso Alemtuzumab nel trapianto allogenico da donatore non familiare Sponsorizzato dal GITMO Gruppo Italiano Trapianto Midollo Osseo

Curve di reclutamento 250 Randomisation (N=103) Registration (N=215) Il protocollo prevede l’arruolamento di 100 -150 pazienti. 150 I risultati della analisi ad interim saranno disponibili a metà Novembre e permetteranno di prendere una 100 decisione sulla opportunità di chiudere la fase di reclutamento. 50 ar m -05 ay -0 ju 5 lse 05 pno 05 v 0 ja 5 nm 06 ar m -06 ay -0 ju 6 lse 06 pno 06 v 0 ja 6 nm 07 ar m -07 ay -0 ju 7 lse 07 pno 07 v 0 ja 7 nm 08 ar m -08 ay -0 ju 8 lse 08 p 08 0 m Number of patients 200 Months
![Decessi Followup post trapianto mediana 6 0 mesi 0 4 25 7 Decessi Follow-up post trapianto (mediana 6. 0 mesi [0. 4 – 25. 7])](https://slidetodoc.com/presentation_image_h/75d23ad4ddd75022c66bb91c4003c1ef/image-26.jpg)
Decessi Follow-up post trapianto (mediana 6. 0 mesi [0. 4 – 25. 7])

Conclusions • The conditioning regimen • The GVHD prophylaxis • The patients you are selecting for the allograft
 Alessandro rambaldi
Alessandro rambaldi Empty
Empty Avanti e dopo cristo
Avanti e dopo cristo Botox vistabex
Botox vistabex Linea del tempo medioevo
Linea del tempo medioevo Diarrea dopo gelato artigianale
Diarrea dopo gelato artigianale La sera di quello stesso giorno il primo dopo il sabato
La sera di quello stesso giorno il primo dopo il sabato Dopo la quaresima
Dopo la quaresima Cortisone dopo estrazione dente
Cortisone dopo estrazione dente La quiete dopo la tempesta poesia
La quiete dopo la tempesta poesia Matting dopo sclerosanti
Matting dopo sclerosanti Valutazione obesità modena
Valutazione obesità modena Enunciativa imperativa
Enunciativa imperativa Dopo la tricodinia ricrescono i capelli
Dopo la tricodinia ricrescono i capelli George steiner dopo babele
George steiner dopo babele Chi è lo storico
Chi è lo storico Que es un modelo alternativo
Que es un modelo alternativo Dfd nivel 1 ejemplos
Dfd nivel 1 ejemplos Modelo didactico tecnologico caracteristicas
Modelo didactico tecnologico caracteristicas Trascrizione nei procarioti
Trascrizione nei procarioti Progetto alternativo alla religione cattolica
Progetto alternativo alla religione cattolica Deuda externa uruguay
Deuda externa uruguay Splicing genetico
Splicing genetico Rock alternativo
Rock alternativo Voto batismal alternativo
Voto batismal alternativo Paradigma alternativo características
Paradigma alternativo características 選択的スプライシング
選択的スプライシング Que es un modelo alternativo
Que es un modelo alternativo