Gases Liquids and Solids Ice water and mist





















- Slides: 29

Gases, Liquids, and Solids Ice, water, and mist are simultaneously present in this winter scene in Yellowstone National Park. © Steven Fuller/Peter Arnold, Inc.

Kinetic Molecular Theory of Matter • Gases are composed of particles (atoms/molecules w/ definite & characteristic sizes) within empty space. • Particles move in rapid, random, straight-line motion; therefore, have kinetic energy. • Particles interact through attractions & repulsions; therefore, have potential energy. • Particle transfer energy to each other through ELASTIC collisions.

PE vs. KE The water in the lake behind the dam has potential energy as a result of its position.

Energy Transfer Upon release, the steel ball on the left transmits kinetic energy through a series of elastic collisions to the ball on the right.

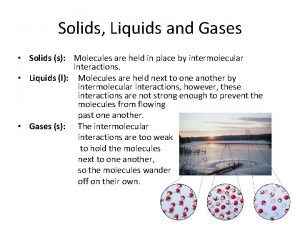
SOLIDS • High PE; low KE • Highly structured - many are crystalline • High degree of interparticle forces – Definite volume & shape – High density – Small compressibility – Very small thermal expansion

LIQUIDS • Relatively equal PE & KE • Not structured - particles flow readily • Significant degree of interparticle forces – Definite volume & indefinite shape – High density – Small compressibility – Small thermal expansion

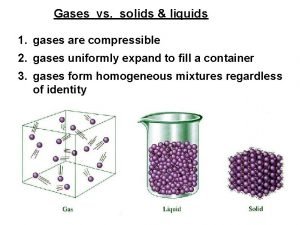
GASES • Low PE; High KE • Not structured - particles flow • Very low degree of interparticle forces – Indefinite volume & shape – Low density – Large compressibility – Moderate thermal expansion

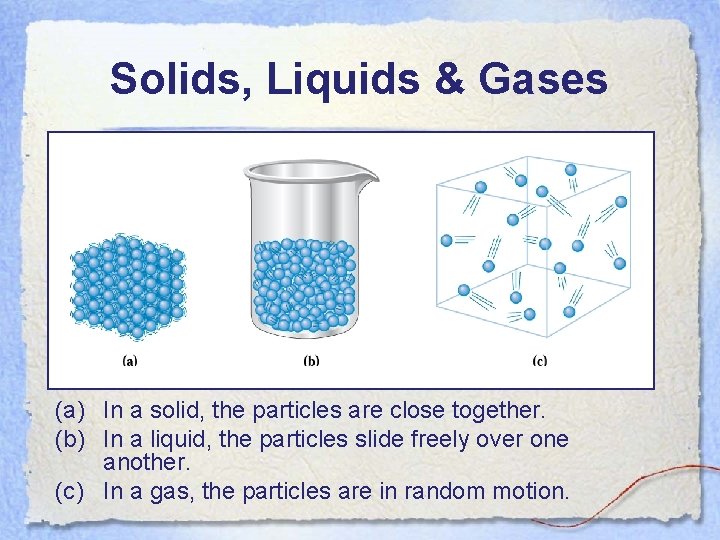
Solids, Liquids & Gases (a) In a solid, the particles are close together. (b) In a liquid, the particles slide freely over one another. (c) In a gas, the particles are in random motion.

Gas motion is affected by • Temperature – (high temp. = greater motion) • Pressure – (higher press. = more collisions) • Amount of particles and space

Temperature • Upper limit –NONE • Lower limit –When motion stops – 273˚C = 0 K

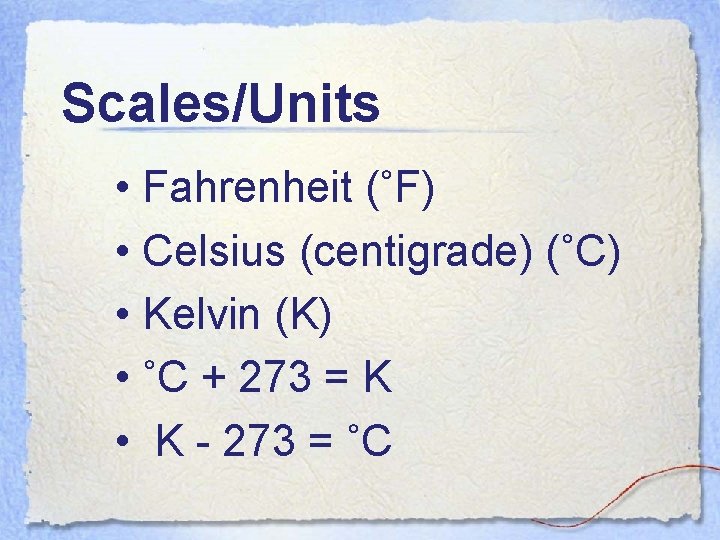
Scales/Units • Fahrenheit (˚F) • Celsius (centigrade) (˚C) • Kelvin (K) • ˚C + 273 = K • K - 273 = ˚C

Pressure • Measures the amount of collisions with the wall of a container. • Faster motion = more collisions • More particles = more collisions • Less space = more collisions

Measurement/Units • Barometers measure the number of collisions • Column of mercury (mm Hg) – 760 mm Hg = one standard atmospheric unit of pressure (atm) • 1 atm = 760 mm. Hg = 101. 4 k. Pa The essential components of a mercury barometer are a graduated glass tube, a glass dish, and liquid mercury.

Gas behavior • The pressure, temperature and volume of gases depend on each other. • Changes to one will affect the other(s) • Use GAS LAWS to predict the changes that occur.

Boyle’s Law • If the pressure on a gas is increased its volume will decrease. • P 1 V 1 = P 2 V 2

Application of Boyle’s Law

Charles’ Law • If the temperature on a gas is increased its volume will increase. • V 1/T 1 = V 2/T 2

Gay-Lussac’s Law • If the temperature on a gas is increased its pressure will increase. • P 1/T 1 = P 2/T 2

Combined Gas Law • P 1 V 1/T 1 =P 2 V 2/T 2 • This law is used when TWO sets of conditions are given with one missing variable.

Ideal Gases • In order to determine/use the moles/grams of gases we utilize the Ideal Gas Law. • This law is used when ONE set of conditions is given with one missing variable.

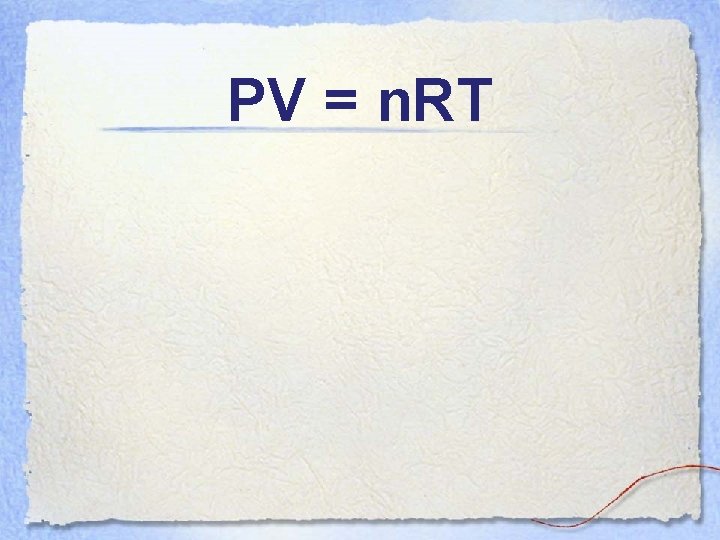
PV = n. RT

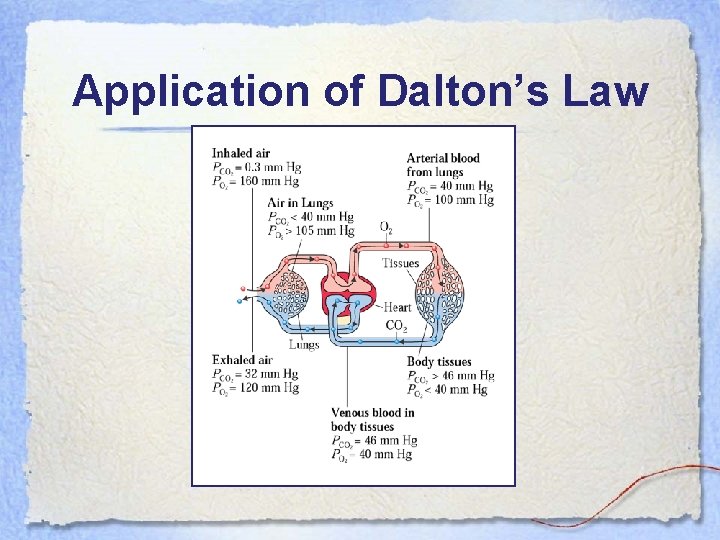
Dalton’s Law of Partial Pressure • The total pressure of a mixture of gases is equal to the sum of all of the individual gases’ partial pressures. • P 1 + P 2 + … + Pn = P T

Application of Dalton’s Law

Gas Stoichiometry • When 0. 091 g zinc react with sulfuric acid (H 2 SO 4), 33. 7 m. L of hydrogen gas are produced. What is the percent yield of the gas? • Steps for solving: – Write a balanced equation – Calculate theoretical yield • Amount of product you should get! – Determine the actual yield – Calculate the percent yield

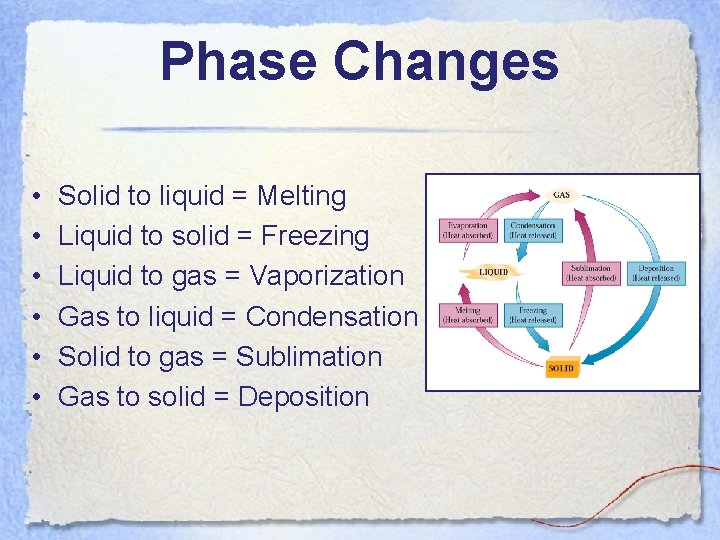
Phase Changes • • • Solid to liquid = Melting Liquid to solid = Freezing Liquid to gas = Vaporization Gas to liquid = Condensation Solid to gas = Sublimation Gas to solid = Deposition

Associated temperatures • Boiling point Bubbles of vapor form within a liquid when the temperature of the liquid reaches the liquid’s boiling point. • Freezing point

Pressure effects The converse of the pressure cooker “phenomenon” is that food cooks more slowly at reduced pressure.

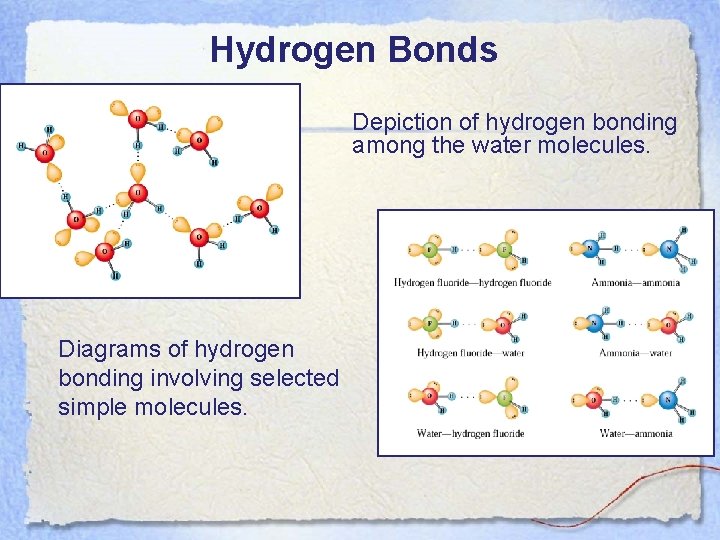
Hydrogen Bonds Depiction of hydrogen bonding among the water molecules. Diagrams of hydrogen bonding involving selected simple molecules.

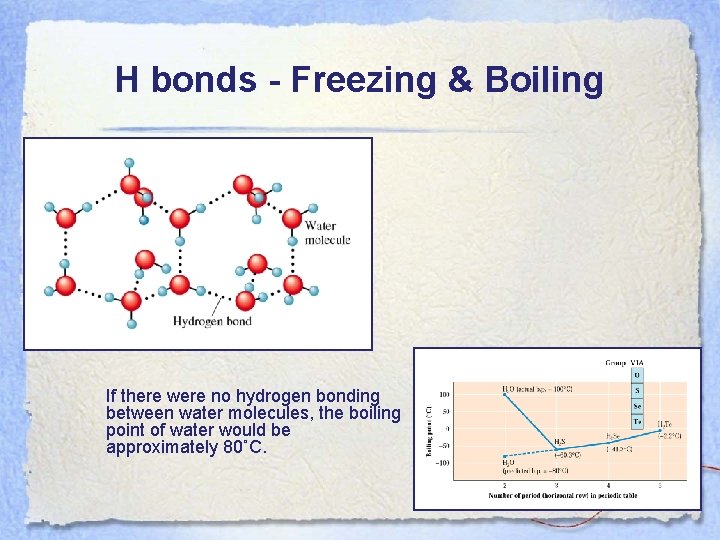
H bonds - Freezing & Boiling If there were no hydrogen bonding between water molecules, the boiling point of water would be approximately 80˚C.
 Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Venn diagram of liquid and gas
Venn diagram of liquid and gas 20 examples of liquids
20 examples of liquids Examples of solids liquids and gases pictures
Examples of solids liquids and gases pictures The science duo physical and chemical changes
The science duo physical and chemical changes Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of solids
Kinetic molecular theory of solids Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Liquid information
Liquid information Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Explain why gases are easier to compress than liquids.
Explain why gases are easier to compress than liquids. Water and water and water water
Water and water and water water Solid liquid gas information
Solid liquid gas information Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Liquids and solids menu
Liquids and solids menu Kesler science.com
Kesler science.com Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Molecular theory of gases and liquids
Molecular theory of gases and liquids Filter medium resistance formula
Filter medium resistance formula Fire sprinkler calculations
Fire sprinkler calculations Fike water mist
Fike water mist Water the elixir of life essay wikipedia
Water the elixir of life essay wikipedia Clear ice vs rime ice
Clear ice vs rime ice Clear ice vs rime ice
Clear ice vs rime ice Solubility of gases in water
Solubility of gases in water Messtechnik
Messtechnik Viscosity of syrup usp
Viscosity of syrup usp Bagian bagian hand sprayer
Bagian bagian hand sprayer