Gas laws The flatulant facts http www mhhe












- Slides: 21

Gas laws The flatulant facts

• http: //www. mhhe. com/physsci/chemistry/ess entialchemistry/flash/vaporv 3. swf • vapor pressure simulation • https: //www. youtube. com/watch? v=re 9 r 0 kz. Q p_M • vapor pressure explanation video

Kinetic theory • Kinetic theory states that atoms and molecules are in a constant random motion. • With this chaotic movement gas particles exhibit predictable, measurable actions that collectively we call the gas laws • **Constant chaotic motion

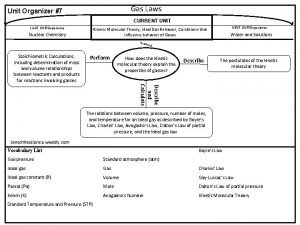
The relationships • The behavior and effect of a gas on its environment is controlled by 4 major things. • Pressure • Temperature • Volume • Number of gas molecules

Pressure Force exerted per unit area Symbol - P Units – mm. Hg, atm, torr, bar, psi, k. Pa 760 mm. Hg = 1 atm = 760 torr = 1. 0133 bar = 14. 7 psi = 101. 3 k. Pa • A barometer is a device used for measuring ambient pressure. • •

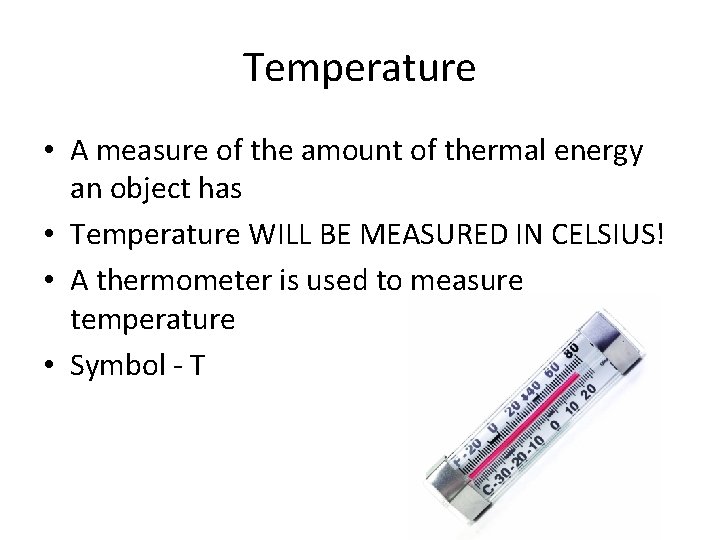
Temperature • A measure of the amount of thermal energy an object has • Temperature WILL BE MEASURED IN CELSIUS! • A thermometer is used to measure temperature • Symbol - T

Volume • • How much space an object takes up. Unite - Liters and cm 3 Symbol – V 1 Liter = 1000 m. L =. 001 m 3 = 1000 cm 3

Amount of gas molecules • The number of gaseous atoms or molecules that are contained in the system • Measured in moles • Symbol - n

STP • STP stands for standard temperature and pressure. • This value is used for a reference and starting point involving many examinations of matter. • STP is equal to 0*C and 1 atm pressure.

The gas laws • Direct relationships between these 4 variables allows for the calculation of missing variables and predicting effects that a gas will have on its environment. 4 exist • Boyles Law • Charles Law • Gay-Lussac’s Law • Combined gas Law • Ideal gas Law

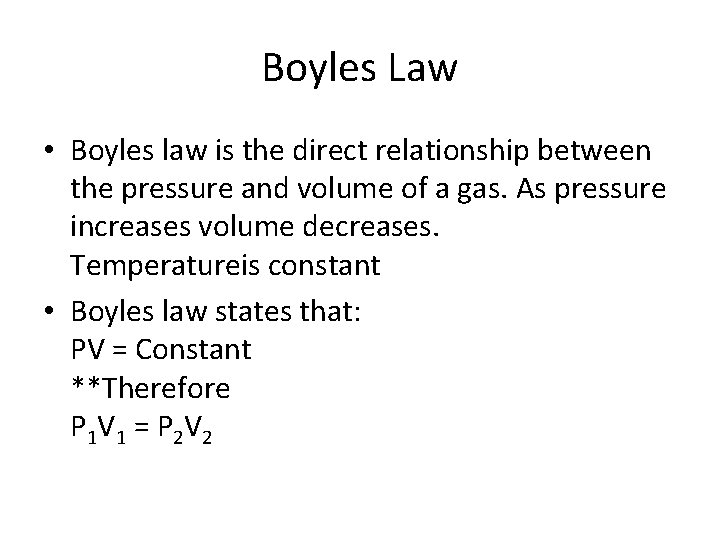
Boyles Law • Boyles law is the direct relationship between the pressure and volume of a gas. As pressure increases volume decreases. Temperatureis constant • Boyles law states that: PV = Constant **Therefore P 1 V 1 = P 2 V 2


• http: //www. physics-chemistry-interactiveflashanimation. com/matter_change_state_measur ement_mass_volume/pressure_volume_boyle _mariotte_law_ideal_gas_closed_system_MC Q. htm • p. HET simulation – gas behavior. Constant temperature

• https: //www. youtube. com/watch? v=R 037 j. VY Ow. OU • https: //www. youtube. com/watch? v=EB 9 Yn 2 z Jldw • https: //www. youtube. com/watch? v=Rjkrq. M m 52 JI • https: //www. youtube. com/watch? v=X 7 roaf 8 k ck. M

Charles law • Charles law is the direct relationship between temperature and volume As temperature increases volume increases. Pressure is constant • No handy memory phrase • Charles law states that V/T = constant **Therefore V 1/T 1 = V 2/T 2

• http: //group. chem. iastate. edu/Greenbowe/se ctions/projectfolder/flashfiles/gaslaw/charles _law. html • p. HET simulation – gas behavior. Constant pressure.

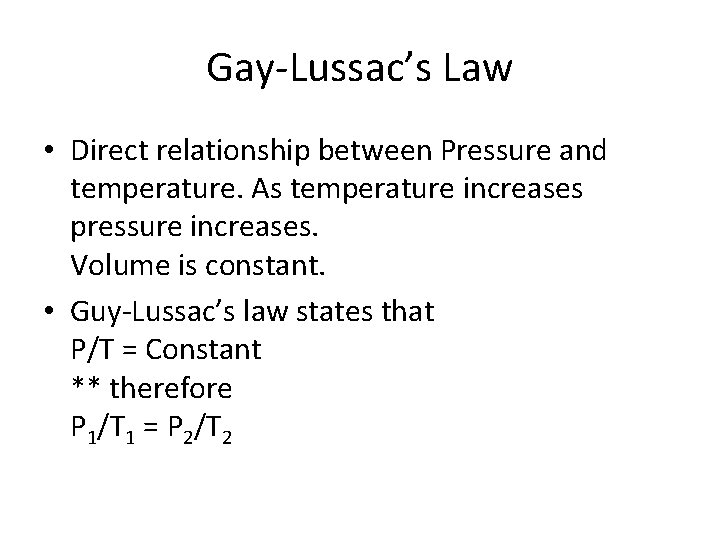
Gay-Lussac’s Law • Direct relationship between Pressure and temperature. As temperature increases pressure increases. Volume is constant. • Guy-Lussac’s law states that P/T = Constant ** therefore P 1/T 1 = P 2/T 2

Combined gas law • When Boyles law and Charles law are combined, the combined gas law relates all 3 variables • P 1 V 1/T 1 = P 2 V 2/T 2 • Given any 2 variables the 3 rd variable can be found.

• https: //phet. colorado. edu/en/simulation/gasproperties

Avogadro's law • Equal volumes of any 2 gases at the same temperature and pressure contain the same amount of molecules • Molar gas volume is the volume that one mole of gas occupiues • Avogadros law states that Vm = 22. 4 L/mol at STP

Ideal gas law • The ideal gas law is an equation used to examine a gas under non realistic (ideal) conditions. This equation loses integrity at very high temperatures and very low pressures • The ideal gas law states: PV = n. RT where R = a constant
 Www.mhhe.com/yacht 2019
Www.mhhe.com/yacht 2019 Facts about montesquieu
Facts about montesquieu Gas laws crash course
Gas laws crash course Direct indirect relationship
Direct indirect relationship Gas laws
Gas laws Ley general de los gases
Ley general de los gases Bourdon gauge gas law
Bourdon gauge gas law Different gas laws
Different gas laws Avogrados law
Avogrados law Gas law conceptual questions
Gas law conceptual questions Boyle's law finding v2
Boyle's law finding v2 Gas laws formula
Gas laws formula Boyle's gas law formula
Boyle's gas law formula Different gas laws
Different gas laws Combined gas laws
Combined gas laws 13-4 practice problems chemistry
13-4 practice problems chemistry State charle's law.
State charle's law. Empirical gas laws
Empirical gas laws Ap chemistry gas laws
Ap chemistry gas laws Gas laws graphic organizer
Gas laws graphic organizer Gas laws hot air balloon
Gas laws hot air balloon Volume and pressure formula
Volume and pressure formula