Nuclear Chemistry http www mhhe comphysscichemistryessentialchemistryflashradioa 7 swf


















- Slides: 28

Nuclear Chemistry

http: //www. mhhe. com/physsci/chemistry/essentialchemistry/flash/radioa 7. swf


Radioactive decay movie n http: //streaming. discoveryeducation. co m/index. cfm

Radioactivity: n n Decay of, and emission of electromagnetic radiation and particles from the nuclei of certain elements that spontaneously desintegrate. Atoms with Atomic Number greater than 83 (unstable)

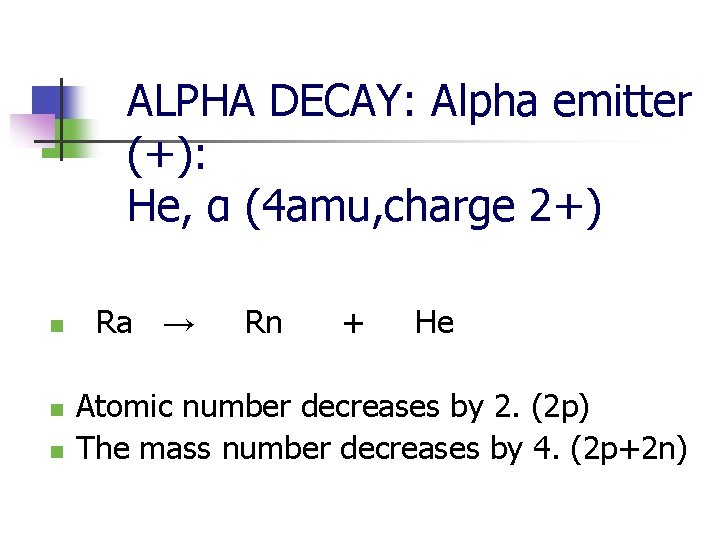
ALPHA DECAY: Alpha emitter (+): He, α (4 amu, charge 2+) n n n Ra → Rn + He Atomic number decreases by 2. (2 p) The mass number decreases by 4. (2 p+2 n)

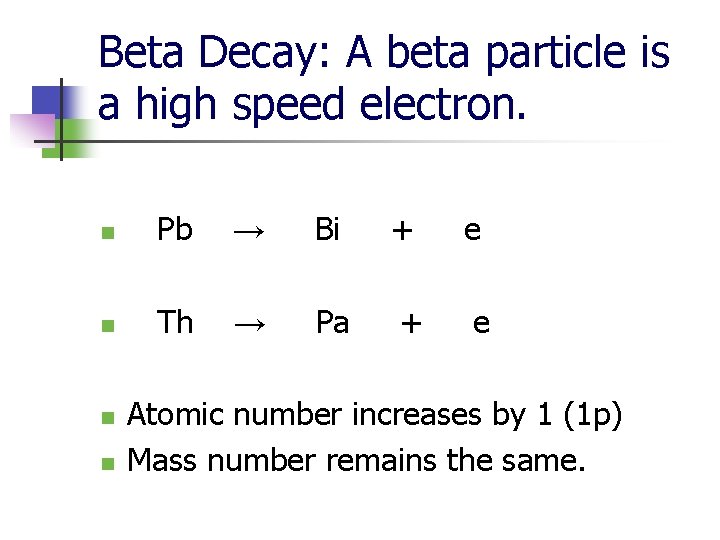
Beta Decay: A beta particle is a high speed electron. n Pb → Bi + e n Th → Pa + e n n Atomic number increases by 1 (1 p) Mass number remains the same.

GAMMA RADIATION (γ) Are not particles: no mass, no charge. Similar to X-rays, but greater energy.

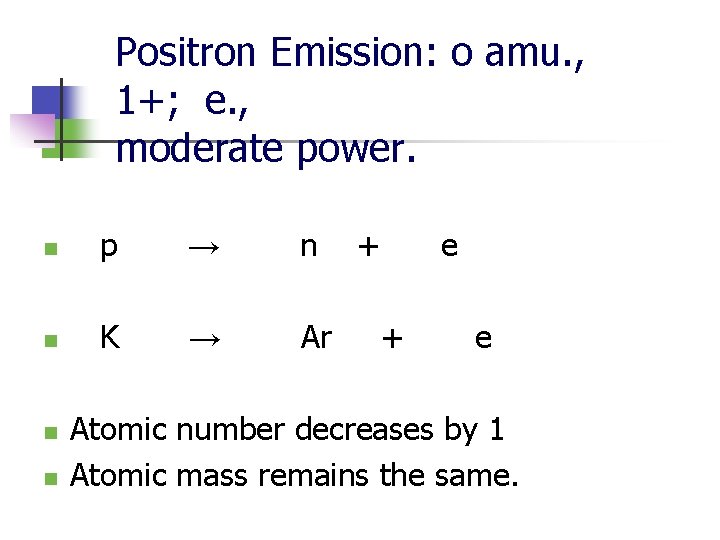
Positron Emission: o amu. , 1+; e. , moderate power. n p → n n K → Ar n n + e Atomic number decreases by 1 Atomic mass remains the same.

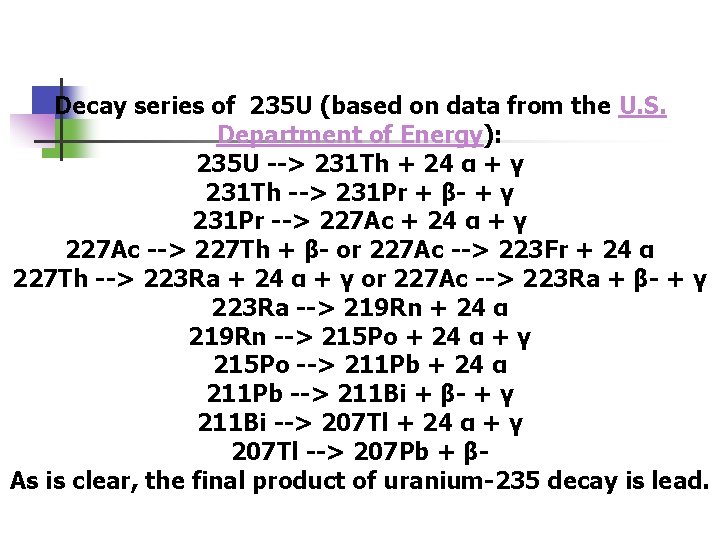
Decay series of 235 U (based on data from the U. S. Department of Energy): 235 U --> 231 Th + 24 α + γ 231 Th --> 231 Pr + β- + γ 231 Pr --> 227 Ac + 24 α + γ 227 Ac --> 227 Th + β- or 227 Ac --> 223 Fr + 24 α 227 Th --> 223 Ra + 24 α + γ or 227 Ac --> 223 Ra + β- + γ 223 Ra --> 219 Rn + 24 α 219 Rn --> 215 Po + 24 α + γ 215 Po --> 211 Pb + 24 α 211 Pb --> 211 Bi + β- + γ 211 Bi --> 207 Tl + 24 α + γ 207 Tl --> 207 Pb + βAs is clear, the final product of uranium-235 decay is lead.

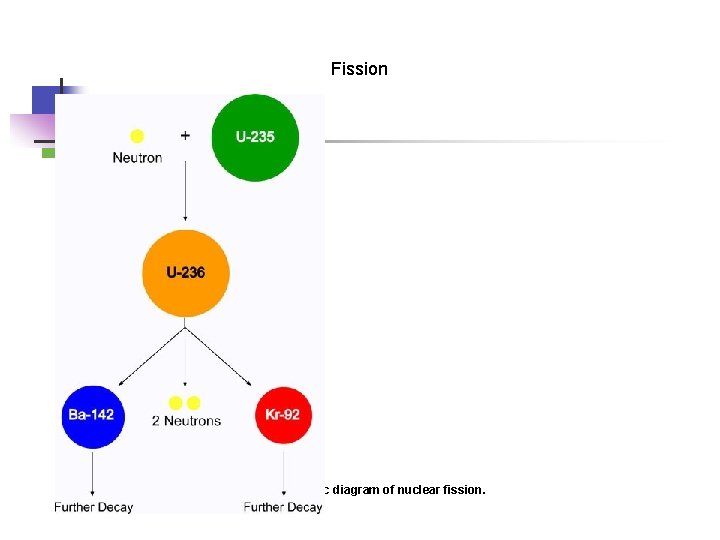
Fission Figure 6: A basic diagram of nuclear fission.





– Nuclear Decay. Practice Write equations for the following nuclear decay reactions. Make sure that both mass numbers and atomic numbers are balanced on each side. 1. Decay of polonium-218 by alpha ( ) emission. 2. Decay of carbon-14 by beta ( -) emission. 3. Decay of chlorine-32 by positron ( +) emission. 4. Decay of promethium-142 by electron capture.

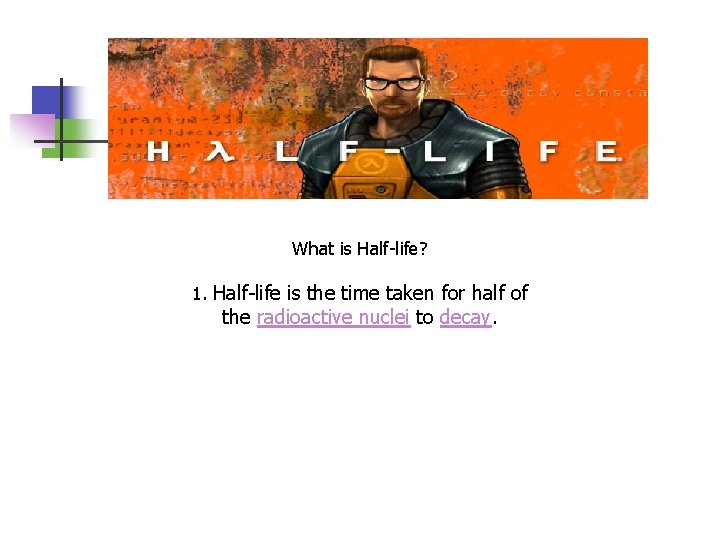
What is Half-life? 1. Half-life is the time taken for half of the radioactive nuclei to decay.

Half-life The half-life of phosphorous-30 is 2. 5 min. If you start with 35 g of phosphorus-30, how many grams would remain after 20. 0 min? The half-life of polonium-210 is 138. 4 days. How many milligrams of polonium-210 remain after 415. 2 days if you start with 2. 0 mg of the isotope? 20. 0 g of a radioactive isotope are present at 1: 00 p. m. , and 5. 0 g remain at 2: 00 p. m. How many half-lives have gone by? _____________ How long is the half-life of the isotope? _____________ Predict how many grams will be left at 2: 30 p. m. _____________

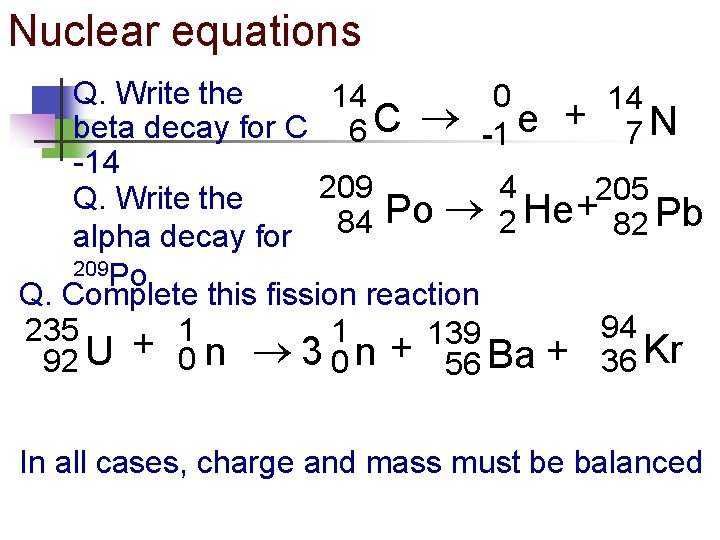
Nuclear equations Q. Write the 14 0 14 beta decay for C 6 C -1 e + 7 N -14 209 4 205 Q. Write the + Po He Pb 84 2 82 alpha decay for 209 Po Q. Complete this fission reaction 94 1 235 1 139 92 U + 0 n 3 0 n + 56 Ba + 36 Kr In all cases, charge and mass must be balanced

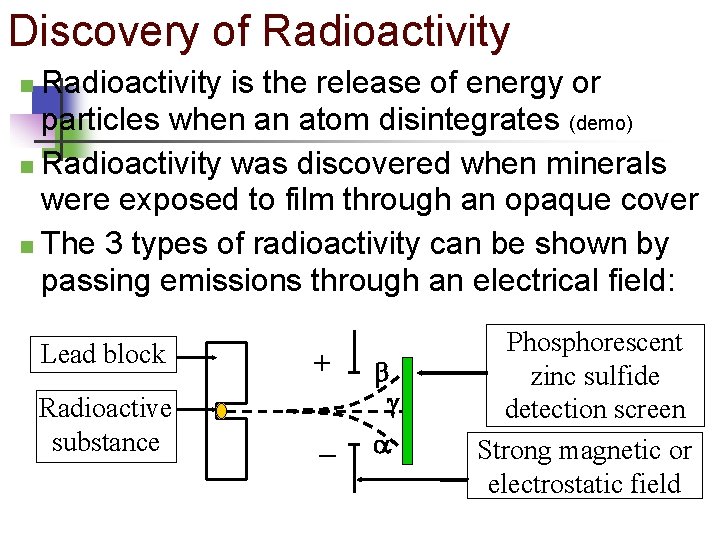
Discovery of Radioactivity is the release of energy or particles when an atom disintegrates (demo) n Radioactivity was discovered when minerals were exposed to film through an opaque cover n The 3 types of radioactivity can be shown by passing emissions through an electrical field: n Lead block + Radioactive substance – Phosphorescent zinc sulfide detection screen Strong magnetic or electrostatic field

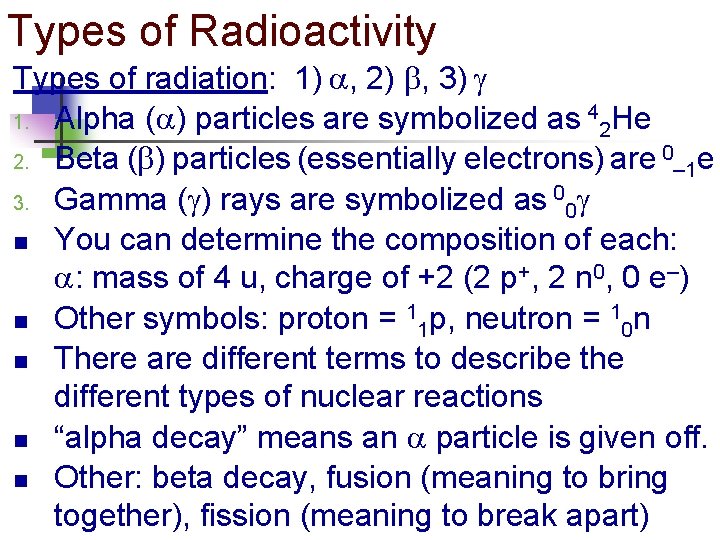
Types of Radioactivity Types of radiation: 1) , 2) , 3) 1. Alpha ( ) particles are symbolized as 42 He 2. Beta ( ) particles (essentially electrons) are 0– 1 e 3. Gamma ( ) rays are symbolized as 00 n You can determine the composition of each: : mass of 4 u, charge of +2 (2 p+, 2 n 0, 0 e–) n Other symbols: proton = 11 p, neutron = 10 n n There are different terms to describe the different types of nuclear reactions n “alpha decay” means an particle is given off. n Other: beta decay, fusion (meaning to bring together), fission (meaning to break apart)

Risk of Radioactivity n n n Biological exposure; (mutations, cancer) Radioactive waste ( Isotopes w/long half live) Danger of accidents (Chernobyl, Three Mile Island, Fukushima Daiichi plant. )

Chernobyl https: //www. youtube. com/watch? v=i. LJu. Q QDPY 8 Y#t=161. 939396

Benefits n n n Nuclear power- electricity. Tracers: I-131, Carbon-14(short half lives) Food: used in food preservation Geology and Archeology: Dating Rocks: U-238/Pb-206 Dating Fossils; Carbon dating C-14/C-12




 Http://dendro.cnre.vt.edu/forestbiology/photosynthesis.swf
Http://dendro.cnre.vt.edu/forestbiology/photosynthesis.swf Www.mhhe.com/yacht 2019
Www.mhhe.com/yacht 2019 Advantages of swf
Advantages of swf Elves swf
Elves swf Swf
Swf Connectathon
Connectathon Signal molecule
Signal molecule Rob savoye
Rob savoye Materi pelatihan packaging ppt
Materi pelatihan packaging ppt Swf investigator
Swf investigator Plik swf
Plik swf Lesson 15 nuclear quest nuclear reactions
Lesson 15 nuclear quest nuclear reactions Fisión nuclear vs fision nuclear
Fisión nuclear vs fision nuclear Chernobyl webquest answer key
Chernobyl webquest answer key Nuclear chemistry
Nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Application of nuclear chemistry
Application of nuclear chemistry Effective nuclear charge trend
Effective nuclear charge trend 12x12x12x12x12x12x12
12x12x12x12x12x12x12 Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Nuclear chemistry review worksheet answer key
Nuclear chemistry review worksheet answer key 25 m/s
25 m/s Nuclear chemistry
Nuclear chemistry Nuclear chemistry
Nuclear chemistry Chapter 21 review nuclear chemistry
Chapter 21 review nuclear chemistry Nuclear chemistry
Nuclear chemistry Nuclear chemistry
Nuclear chemistry Irradiated food
Irradiated food Chapter 25 nuclear chemistry
Chapter 25 nuclear chemistry