Final Exam Review Semester 2 Chapters 8 9
















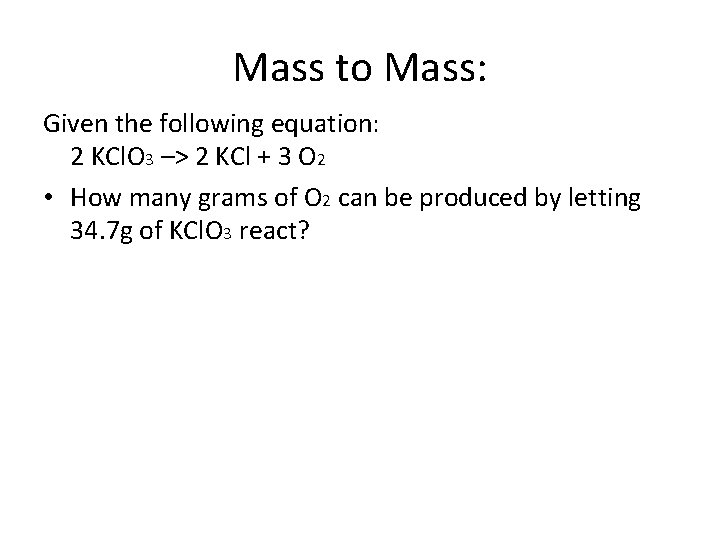






- Slides: 65

Final Exam Review Semester 2 Chapters: 8, 9, 10, 11, 13, 14

Chapter 8 Test Review

• Covalent bond • Endothermic reaction • Lewis Exothermic reaction • structure • Molecule • Pi bond • Sigma bond • Resonance • Structural formula • VESPR model • Polar covalent bond • Non-polar bond • Chemical equation • Chemical reaction Vocabulary • Coefficient • Product • Reactant • Combustion reaction • Decomposition reaction • Single replacement reaction • Double replacement reaction • Synthesis reaction • Precipitate • Aqueous • Complete ionic equation • Net ionic equation • Solute • Solvent • Spectator Ion

Covalent Bonds • How many covalent bonds can elements in the following groups form: – Group 1 (alkali metals) – Group 2 (alkali earth metals) – Group 3 – Group 4 – Group 5 – Group 6 – Group 7 (halogens) – Group 8 ( noble gases)

The Periodic Table of the Elements IA VIIIA 1 2 H 1. 008 He 4. 003 IIA 3 4 Li Be 6. 941 9. 012 IIIA 5 9 10 C N O F Ne 12. 01 14. 01 16. 00 19. 00 20. 18 11 12 17 18 Mg Al Si P S Cl Ar 22. 99 24. 31 26. 98 28. 09 30. 97 32. 07 35. 45 39. 95 IVB 22 VB VIIB VIIIB IB 15 8 VIIA B 14 7 VIA 10. 81 13 6 VA Na IIIB IVA 16 IIB 19 20 21 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39. 10 40. 08 44. 96 47. 87 50. 94 52. 00 54. 94 55. 85 58. 93 58. 69 63. 55 65. 39 69. 72 72. 61 74. 92 78. 96 79. 90 83. 80 37 38 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 85. 47 87. 62 88. 91 39 91. 22 92. 91 95. 94 (98) 101. 1 102. 9 106. 4 107. 9 112. 4 114. 8 118. 7 121. 8 127. 6 126. 9 131. 3 55 56 57 72 73 74 75 76 78 79 80 81 82 83 84 85 86 Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 132. 9 137. 3 138. 9 178. 5 180. 9 183. 8 186. 2 190. 2 192. 2 77 195. 1 197. 0 200. 6 204. 4 207. 2 209. 0 (209) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 Fr Ra Ac Rf Db Sg Bh Hs Mt Uun Uuu Uub (223) (226) (227) (261) (262) (266) (264) (269) (268) (271) (272) (277) 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 140. 1 140. 9 144. 2 (145) 150. 4 152. 0 157. 3 158. 9 162. 5 164. 9 167. 3 168. 9 173. 0 175. 0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr 232. 0 (231) 238. 0 (237) (244) (243) (247) (251) (252) (257) (258) (259) (262)

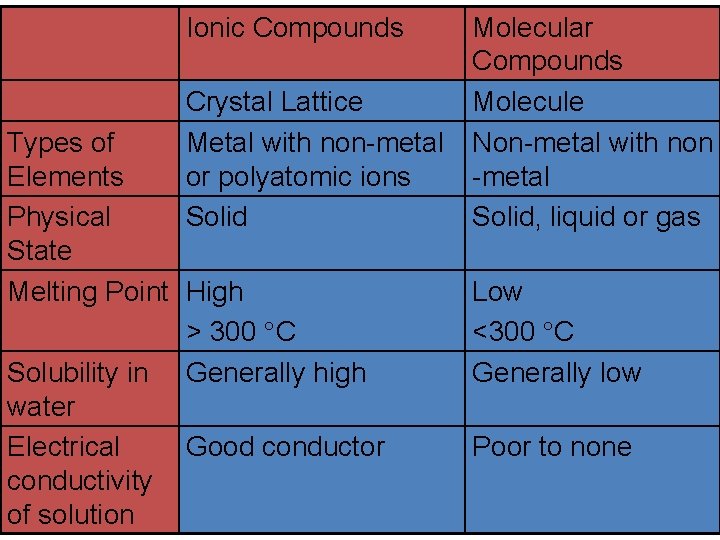
Ionic Compounds Crystal Lattice Metal with non-metal or polyatomic ions Solid Types of Elements Physical State Melting Point High > 300 C Solubility in Generally high water Electrical Good conductor conductivity of solution Molecular Compounds Molecule Non-metal with non -metal Solid, liquid or gas Low <300 C Generally low Poor to none

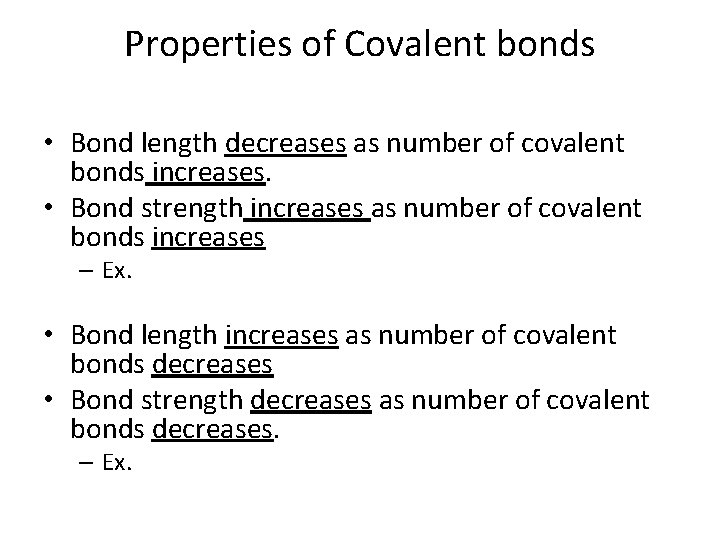
Properties of Covalent bonds • Bond length decreases as number of covalent bonds increases. • Bond strength increases as number of covalent bonds increases – Ex. • Bond length increases as number of covalent bonds decreases • Bond strength decreases as number of covalent bonds decreases. – Ex.

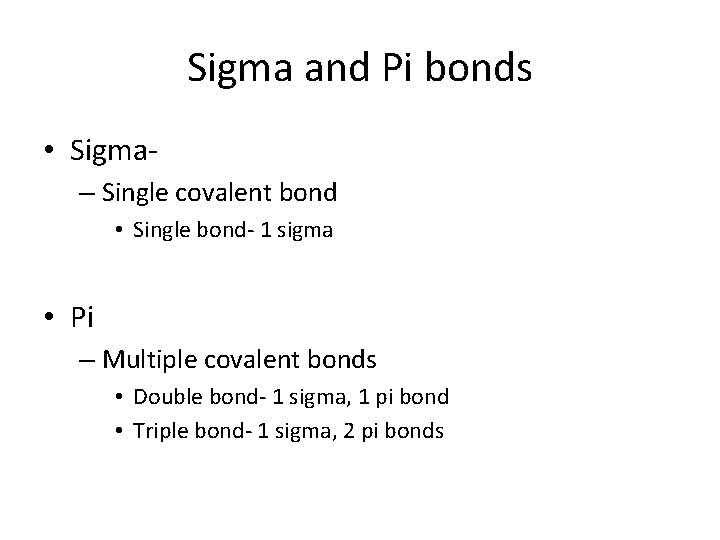
Sigma and Pi bonds • Sigma– Single covalent bond • Single bond- 1 sigma • Pi – Multiple covalent bonds • Double bond- 1 sigma, 1 pi bond • Triple bond- 1 sigma, 2 pi bonds

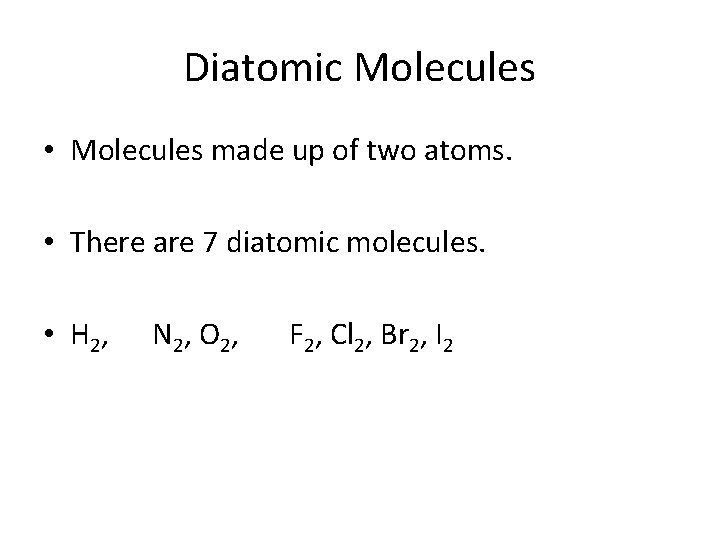
Diatomic Molecules • List the 7 diatomic molecules:

Diatomic Molecules • Molecules made up of two atoms. • There are 7 diatomic molecules. • H 2, N 2, O 2, F 2, Cl 2, Br 2, I 2

How many atoms in each formula? 1. 2. 3. 4. 5. 6. 7. 8. 9. CH 3 OH CH 4 PF 3 OF 2 NO 2 BH 3 SO 4 2 CNN 2 H 2

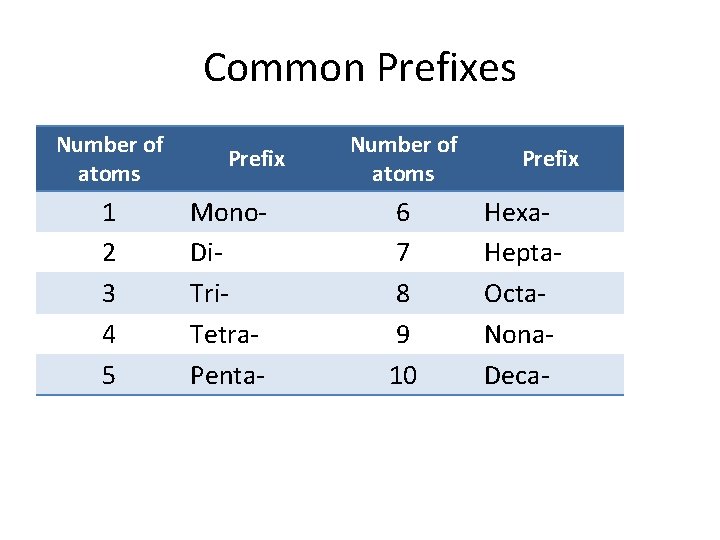
Common Prefixes Number of atoms 1 2 3 4 5 Prefix Mono- Di- Tri. Tetra. Penta- Number of atoms 6 7 8 9 10 Prefix Hexa. Hepta. Octa. Nona. Deca-

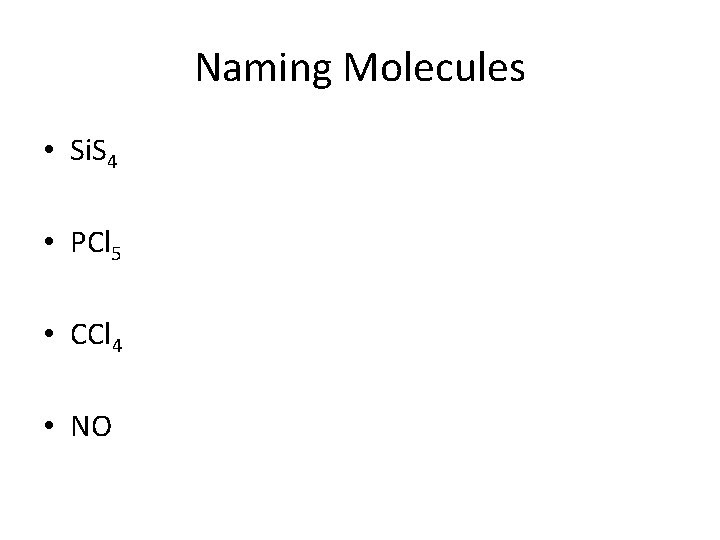
Naming Molecules • Si. S 4 • PCl 5 • CCl 4 • NO

Writing Formulas • Sulfur difluoride • Silicon tetrachloride • Chlorine trifluoride • Tetrasulfur heptanitride

Steps to doing lewis structures with covalent bonds 1. Count the valence electrons for all atoms 2. Put the least electronegative atom in the center. Hydrogen is always on outside 3. Assign 2 electrons to each atom 4. Complete octets on outside atoms 5. Put remaining electrons in pairs on central atom 6. If central atom doesn’t have an octet, move electrons from outer atoms to form double or triple bonds

Lewis Structures and Octet • • • Practice by drawing H 2 O 2 + N 2 H 2 O CO 2 - +

Lewis structures • CH 3 OH • BH 3 • N 2 H 2

Lewis Structures with polyatomic ions • SO 4 2 - • CN-

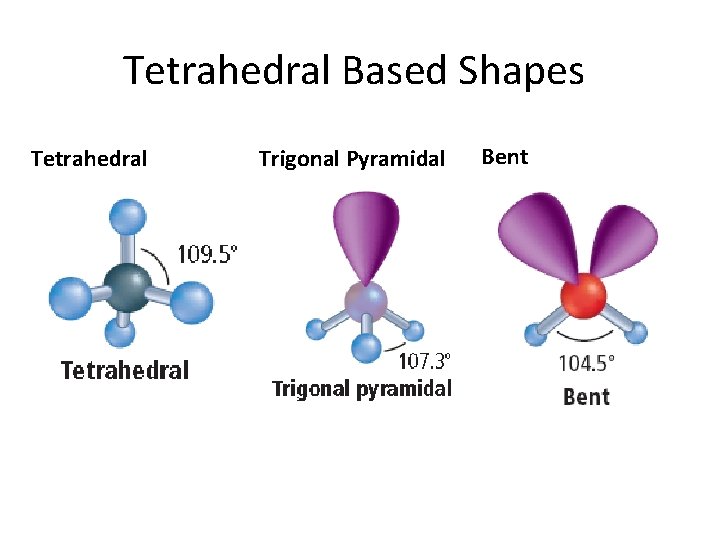
Tetrahedral Based Shapes Tetrahedral Trigonal Pyramidal Bent

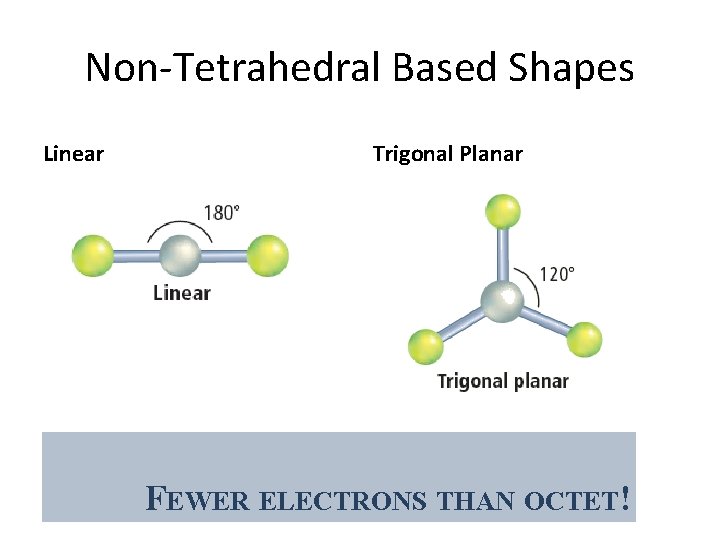
Non-Tetrahedral Based Shapes Linear Trigonal Planar FEWER ELECTRONS THAN OCTET!

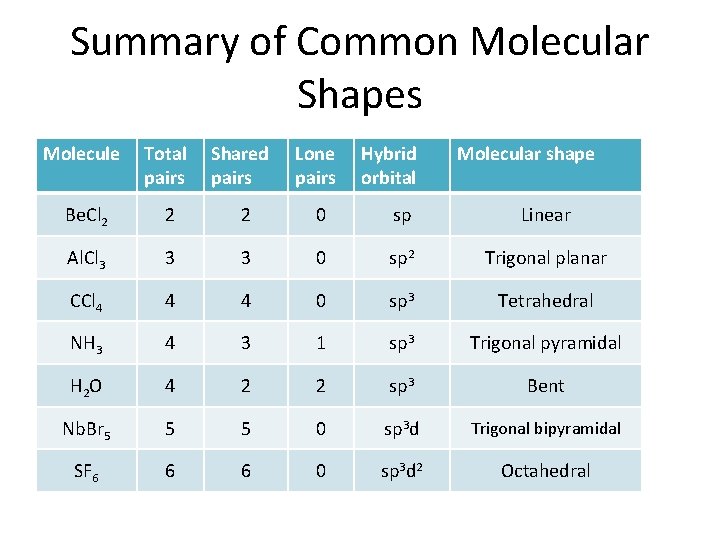
Summary of Common Molecular Shapes Molecule Total pairs Shared pairs Lone pairs Hybrid orbital Molecular shape Be. Cl 2 2 2 0 sp Linear Al. Cl 3 3 3 0 sp 2 Trigonal planar CCl 4 4 4 0 sp 3 Tetrahedral NH 3 4 3 1 sp 3 Trigonal pyramidal H 2 O 4 2 2 sp 3 Bent Nb. Br 5 5 5 0 sp 3 d Trigonal bipyramidal SF 6 6 6 0 sp 3 d 2 Octahedral

Molecular Shapes • CH 4 • PF 3 • OF 2 • NO 2 -

Chapter 9 Review

List the following GENERAL equations • Combustion • Synthesis • Decomposition • Double replacement • Single replacement

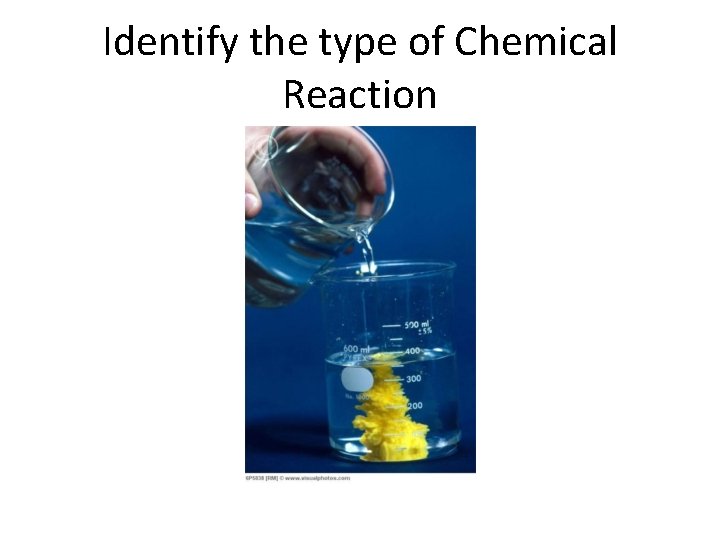
Identify the type of Chemical Reaction

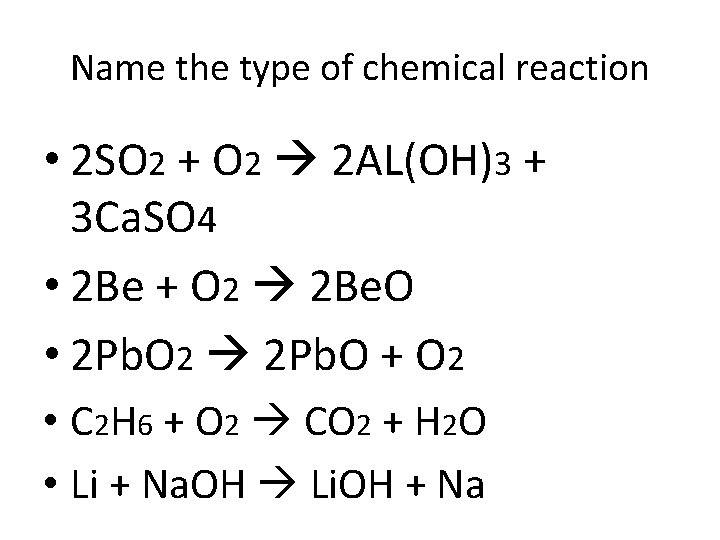
Name the type of chemical reaction • 2 SO 2 + O 2 2 AL(OH)3 + 3 Ca. SO 4 • 2 Be + O 2 2 Be. O • 2 Pb. O 2 2 Pb. O + O 2 • C 2 H 6 + O 2 CO 2 + H 2 O • Li + Na. OH Li. OH + Na

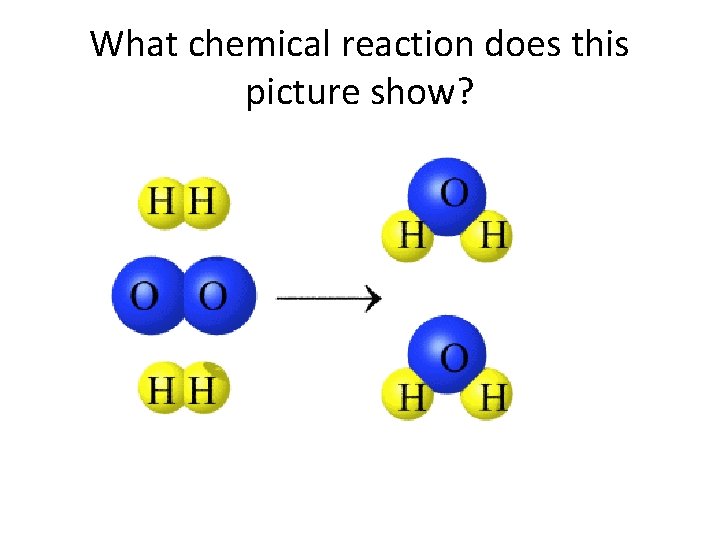
What chemical reaction does this picture show?

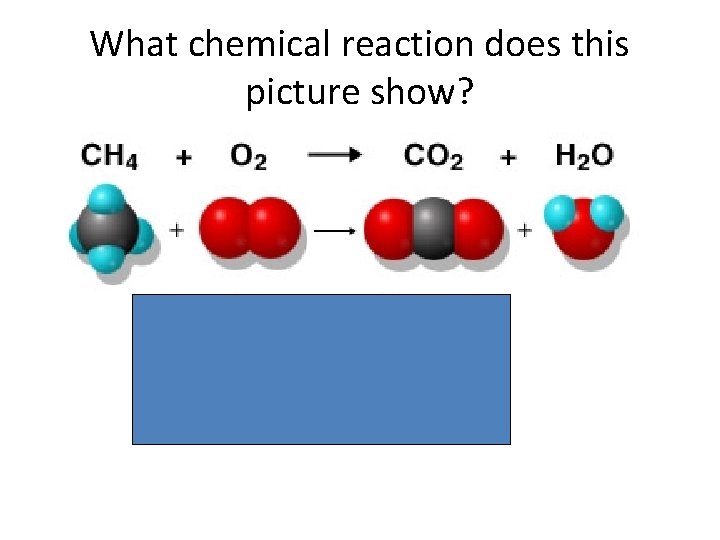
What chemical reaction does this picture show?

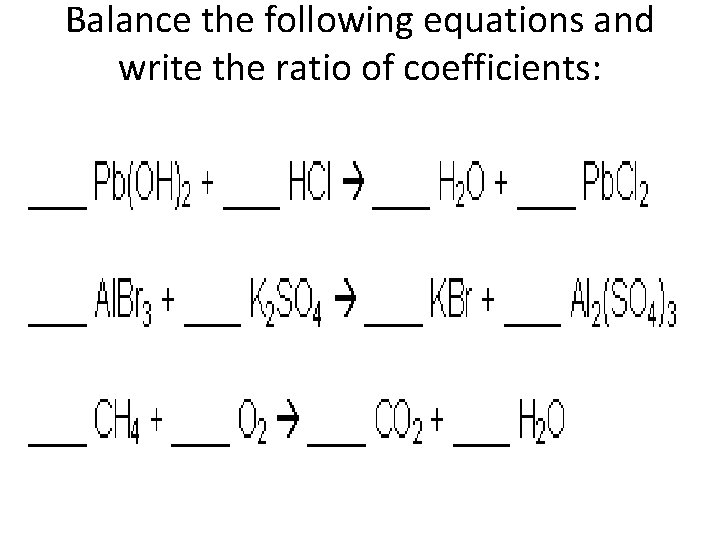
Balance the following equations and write the ratio of coefficients:

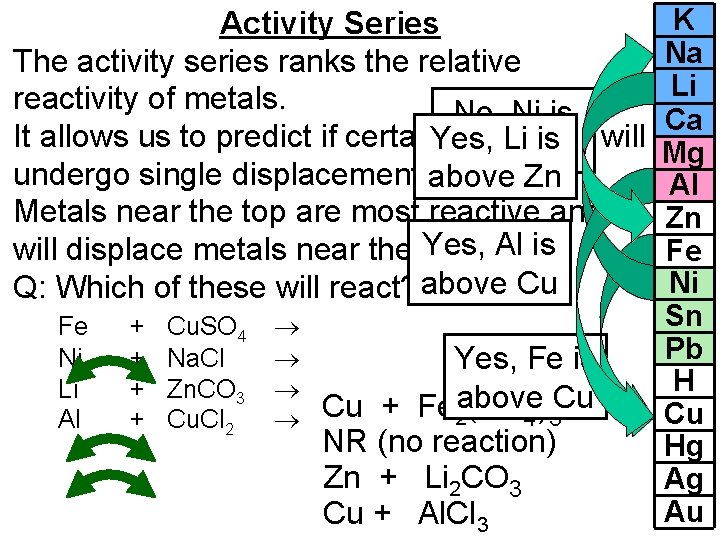
Activity Series The activity series ranks the relative reactivity of metals. No, Ni is It allows us to predict if certain. Yes, chemicals will Li is below Na undergo single displacement above reactions. Zn Metals near the top are most reactive and Yes, Al is will displace metals near the bottom. Q: Which of these will react? above Cu Fe Ni Li Al + + Cu. SO 4 Na. Cl Zn. CO 3 Cu. Cl 2 Yes, Fe is Cu + Fe 2 above (SO 4)3 Cu NR (no reaction) Zn + Li 2 CO 3 Cu + Al. Cl 3 K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au

Chapter 10 The Mole

Define the following: 1. Hydrate 2. Molecular formula 3. Empirical formula 4. Percent composition 5. Mole

Find the atomic mass for the following atoms: 9. C 10. H 11. Cl 12. O 13. Fe

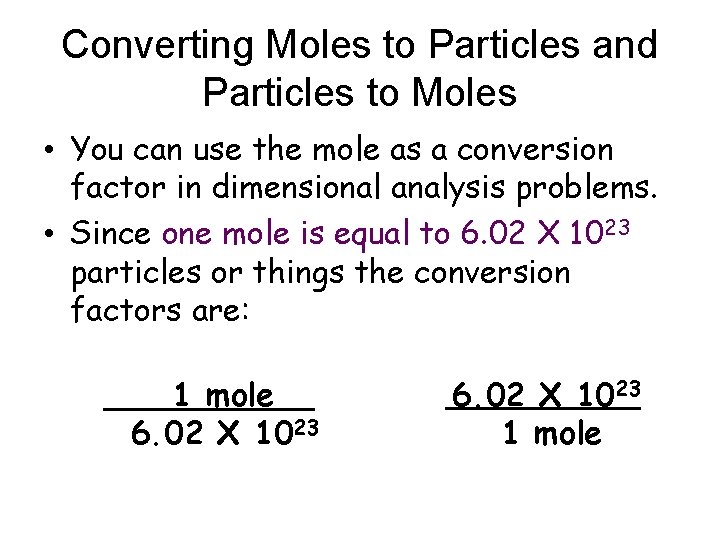
Converting Moles to Particles and Particles to Moles • You can use the mole as a conversion factor in dimensional analysis problems. • Since one mole is equal to 6. 02 X 1023 particles or things the conversion factors are: 1 mole__ 6. 02 X 1023 1 mole

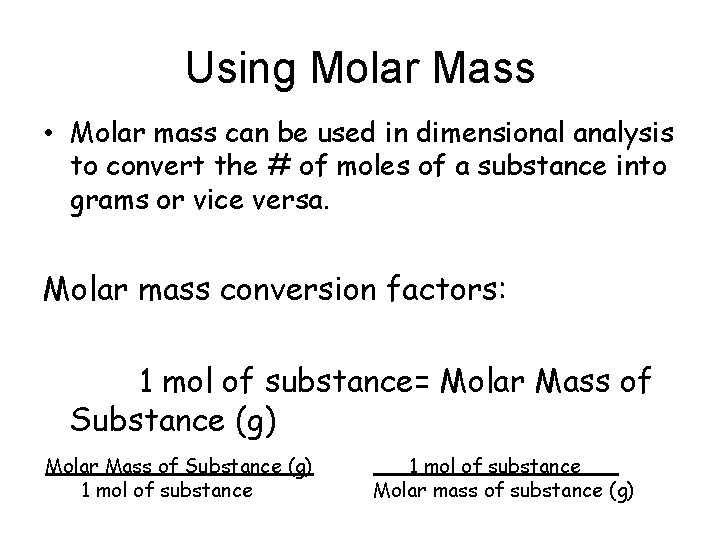
Using Molar Mass • Molar mass can be used in dimensional analysis to convert the # of moles of a substance into grams or vice versa. Molar mass conversion factors: 1 mol of substance= Molar Mass of Substance (g) 1 mol of substance___ Molar mass of substance (g)

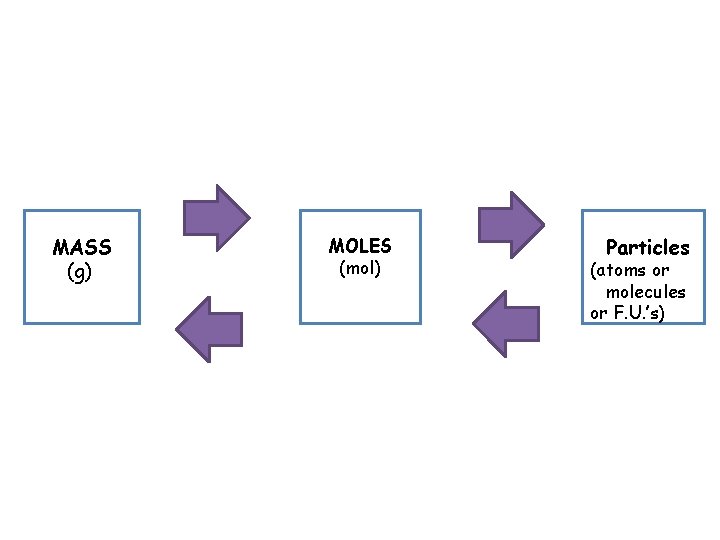
MASS (g) MOLES (mol) Particles (atoms or molecules or F. U. ’s)

Find the molar mass for the following compounds: 14. CH 2 O 15. Ca. SO 4 16. Na 3 PO 4

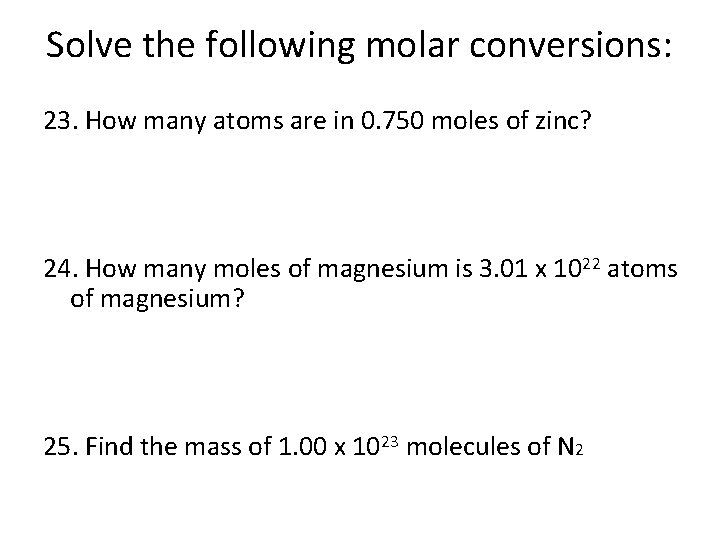
Solve the following molar conversions: 23. How many atoms are in 0. 750 moles of zinc? 24. How many moles of magnesium is 3. 01 x 1022 atoms of magnesium? 25. Find the mass of 1. 00 x 1023 molecules of N 2

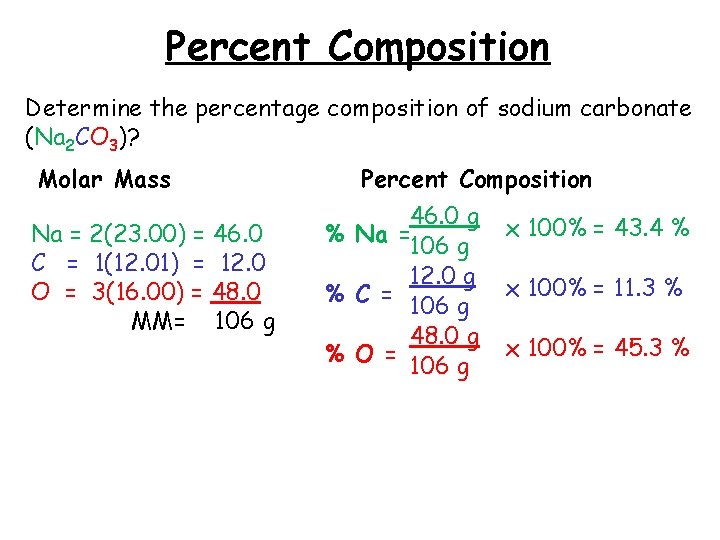
Percent Composition Determine the percentage composition of sodium carbonate (Na 2 CO 3)? Molar Mass Na = 2(23. 00) = 46. 0 C = 1(12. 01) = 12. 0 O = 3(16. 00) = 48. 0 MM= 106 g Percent Composition 46. 0 g % Na =106 g 12. 0 g % C = 106 g 48. 0 g % O = 106 g x 100% = 43. 4 % x 100% = 11. 3 % x 100% = 45. 3 %

Solve the following percent composition problems: 26. Mg(NO 3)2 27. (NH 4)2 S

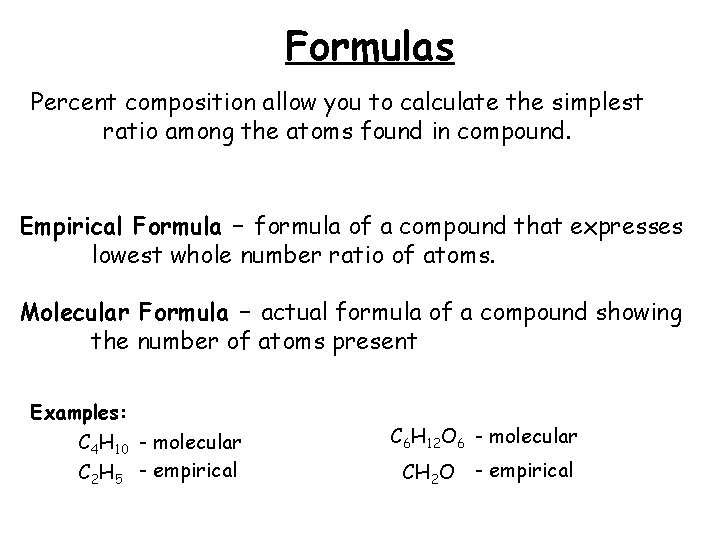
Formulas Percent composition allow you to calculate the simplest ratio among the atoms found in compound. Empirical Formula – formula of a compound that expresses lowest whole number ratio of atoms. Molecular Formula – actual formula of a compound showing the number of atoms present Examples: C 4 H 10 - molecular C 2 H 5 - empirical C 6 H 12 O 6 - molecular CH 2 O - empirical

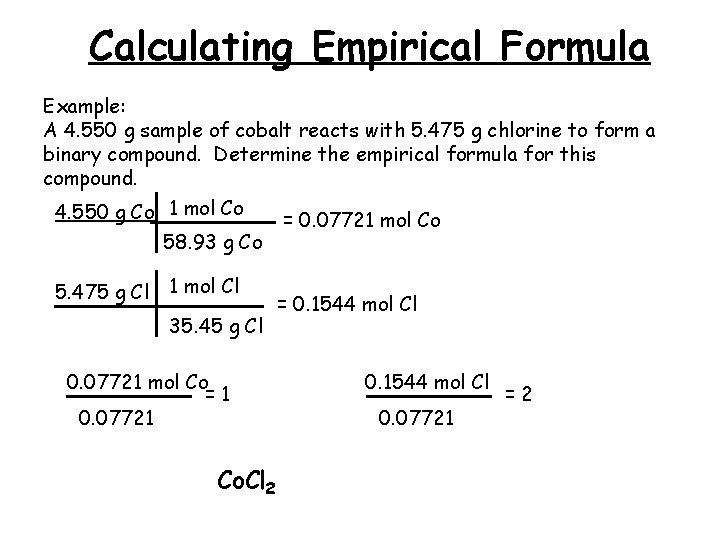
Calculating Empirical Formula Example: A 4. 550 g sample of cobalt reacts with 5. 475 g chlorine to form a binary compound. Determine the empirical formula for this compound. 4. 550 g Co 1 mol Co 58. 93 g Co 5. 475 g Cl 1 mol Cl 35. 45 g Cl 0. 07721 mol Co =1 0. 07721 Co. Cl 2 = 0. 07721 mol Co = 0. 1544 mol Cl 0. 07721 =2

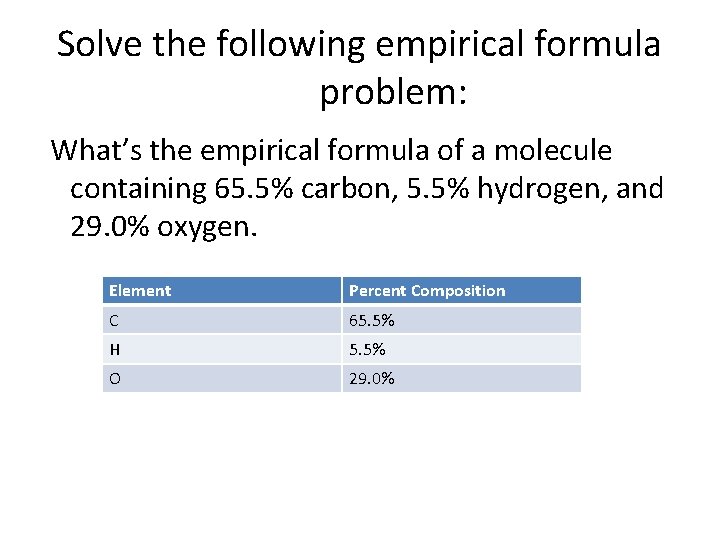
Solve the following empirical formula problem: What’s the empirical formula of a molecule containing 65. 5% carbon, 5. 5% hydrogen, and 29. 0% oxygen. Element Percent Composition C 65. 5% H 5. 5% O 29. 0%

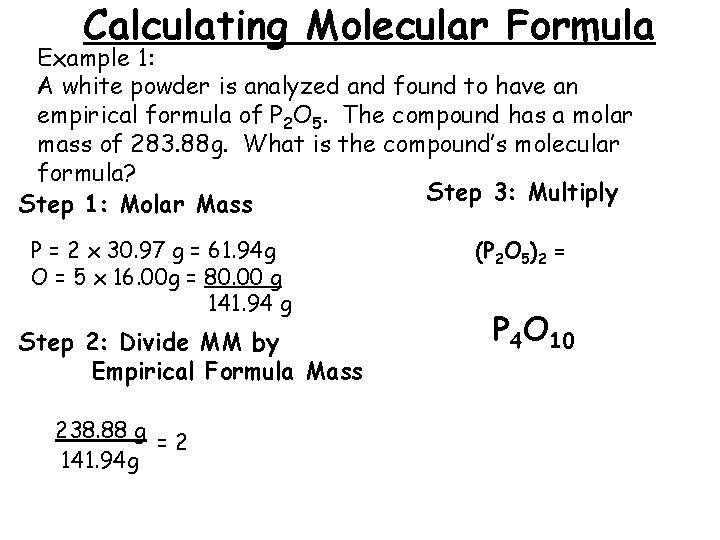
Calculating Molecular Formula Example 1: A white powder is analyzed and found to have an empirical formula of P 2 O 5. The compound has a molar mass of 283. 88 g. What is the compound’s molecular formula? Step 3: Multiply Step 1: Molar Mass P = 2 x 30. 97 g = 61. 94 g O = 5 x 16. 00 g = 80. 00 g 141. 94 g Step 2: Divide MM by Empirical Formula Mass 238. 88 g =2 141. 94 g (P 2 O 5)2 = P 4 O 10

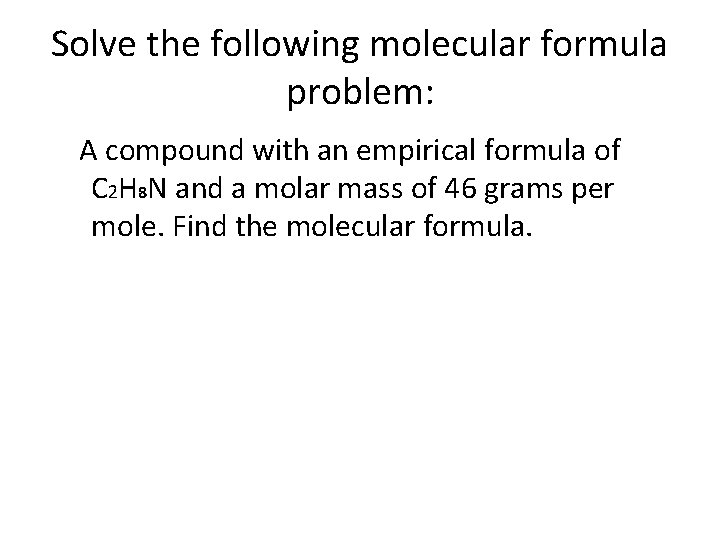
Solve the following molecular formula problem: A compound with an empirical formula of C 2 H 8 N and a molar mass of 46 grams per mole. Find the molecular formula.

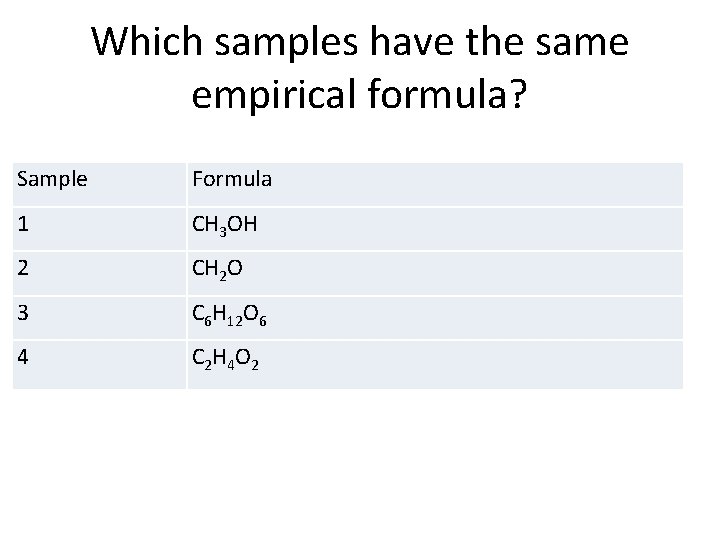
Which samples have the same empirical formula? Sample Formula 1 CH 3 OH 2 CH 2 O 3 C 6 H 12 O 6 4 C 2 H 4 O 2

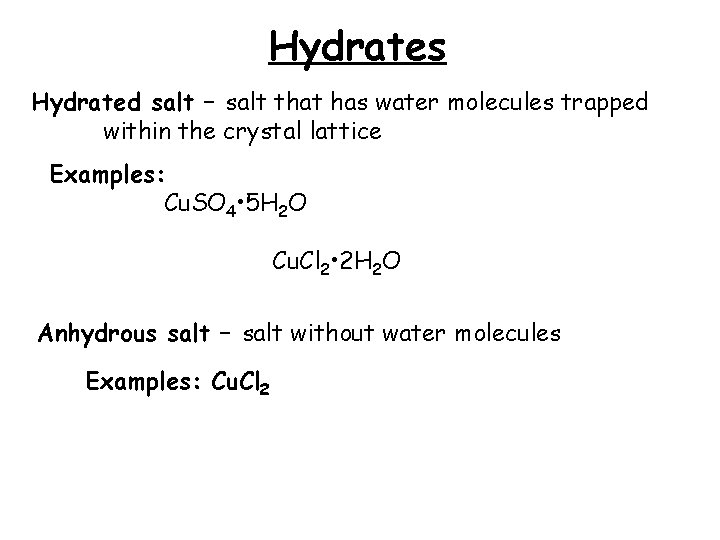
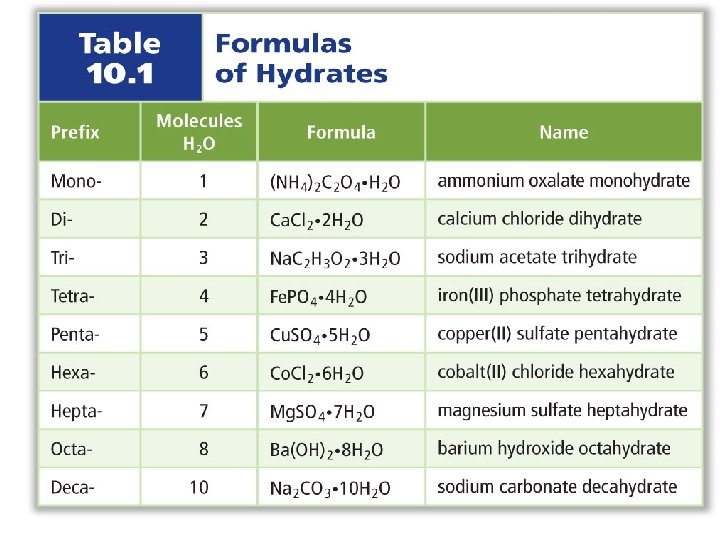
Hydrates Hydrated salt – salt that has water molecules trapped within the crystal lattice Examples: Cu. SO 4 • 5 H 2 O Cu. Cl 2 • 2 H 2 O Anhydrous salt – salt without water molecules Examples: Cu. Cl 2


Chapter 11 Review

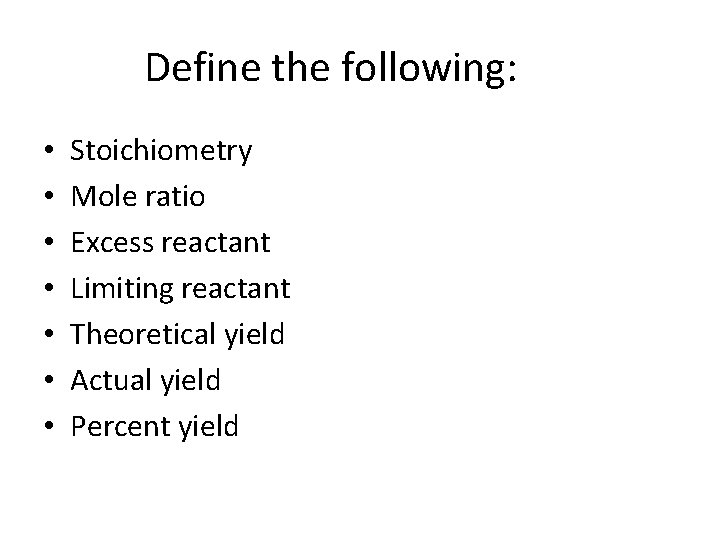
Define the following: • • Stoichiometry Mole ratio Excess reactant Limiting reactant Theoretical yield Actual yield Percent yield

Find the following molar masses: • • • O 2 O Al. PO 4 Na. Cl C 6 H 5 Cl Cu. O

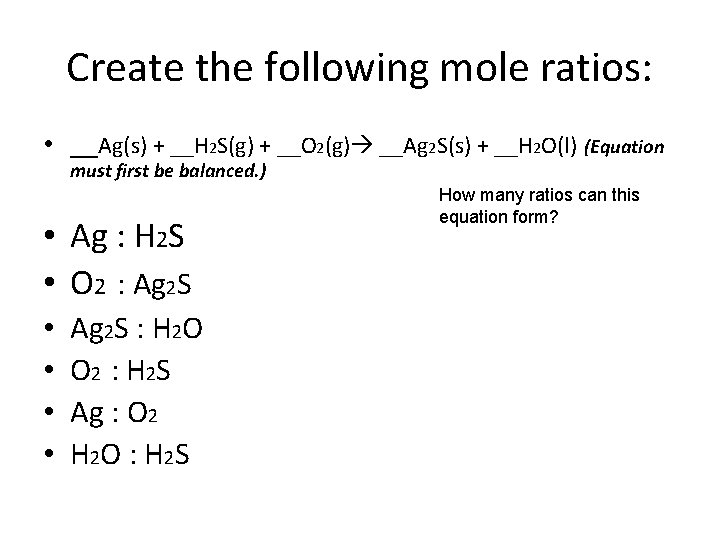
Create the following mole ratios: • __Ag(s) + __H 2 S(g) + __O 2(g) __Ag 2 S(s) + __H 2 O(l) (Equation must first be balanced. ) • Ag : H 2 S • O 2 : Ag 2 S • • Ag 2 S : H 2 O O 2 : H 2 S Ag : O 2 H 2 O : H 2 S How many ratios can this equation form?

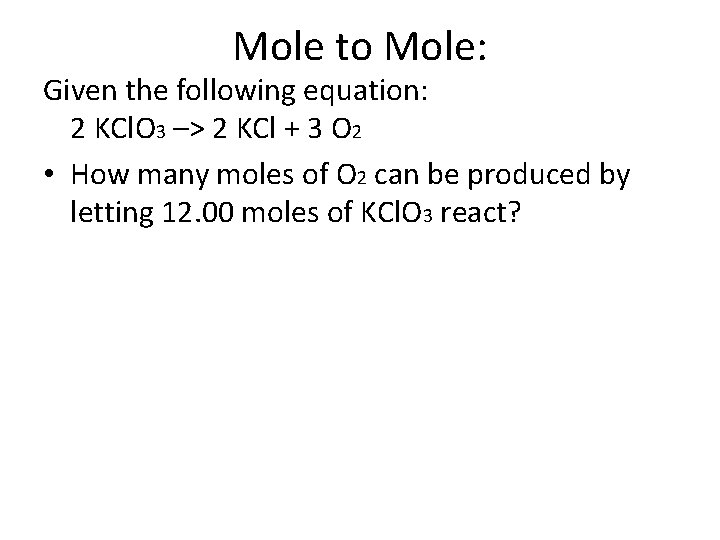
Mole to Mole: Given the following equation: 2 KCl. O 3 –> 2 KCl + 3 O 2 • How many moles of O 2 can be produced by letting 12. 00 moles of KCl. O 3 react?

Mole to Mass: Given the following equation: 2 KCl. O 3 –> 2 KCl + 3 O 2 • How many grams of O 2 can be produced by letting 3 moles of KCl. O 3 react?
Mass to Mass: Given the following equation: 2 KCl. O 3 –> 2 KCl + 3 O 2 • How many grams of O 2 can be produced by letting 34. 7 g of KCl. O 3 react?

Limiting Reactant Given the following equation: Al 2(SO 3)3 + 6 Na. OH 3 Na 2 SO 3 + 2 Al(OH)3 • If 10. 0 g of Al 2(SO 3)3 is reacted with 10. 0 g of Na. OH, determine the limiting reactant for how many grams of Al(OH)3 is formed.

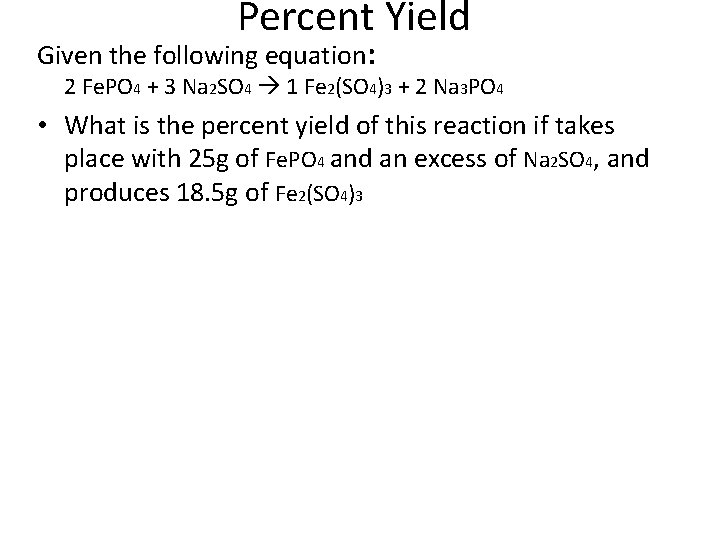
Percent Yield Given the following equation: 2 Fe. PO 4 + 3 Na 2 SO 4 1 Fe 2(SO 4)3 + 2 Na 3 PO 4 • What is the percent yield of this reaction if takes place with 25 g of Fe. PO 4 and an excess of Na 2 SO 4, and produces 18. 5 g of Fe 2(SO 4)3

Chapter 14 Review

Define the following: • • • Suspension Colloid Brownian motion Tyndall effect Souble Insoluble Miscible Immiccible Condentration Molartity Dilution

Name the type of heterogeneous mixture: • • Milk Fog Hairspray Muddy Water Blood Orange juice Gelatin

What is the percent by volume when 50 m. L of ethanol is diluted to 140 m. L with water?
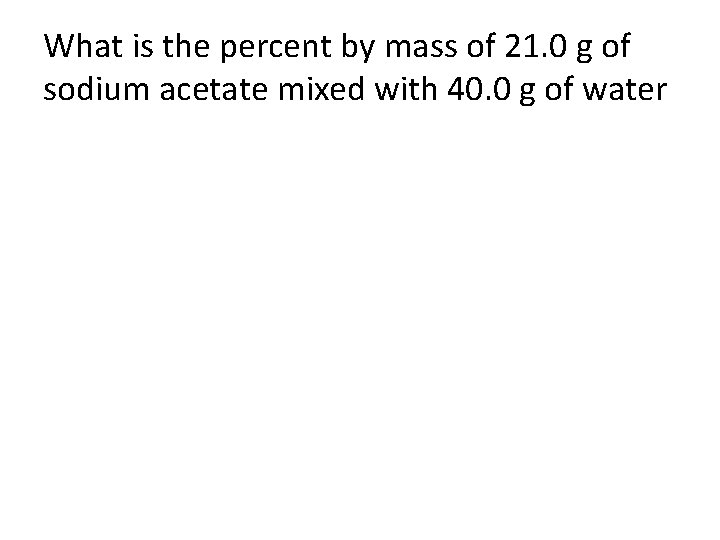
What is the percent by mass of 21. 0 g of sodium acetate mixed with 40. 0 g of water

What mass of lithium chloride is found in 85 g of a 25% by mass solution
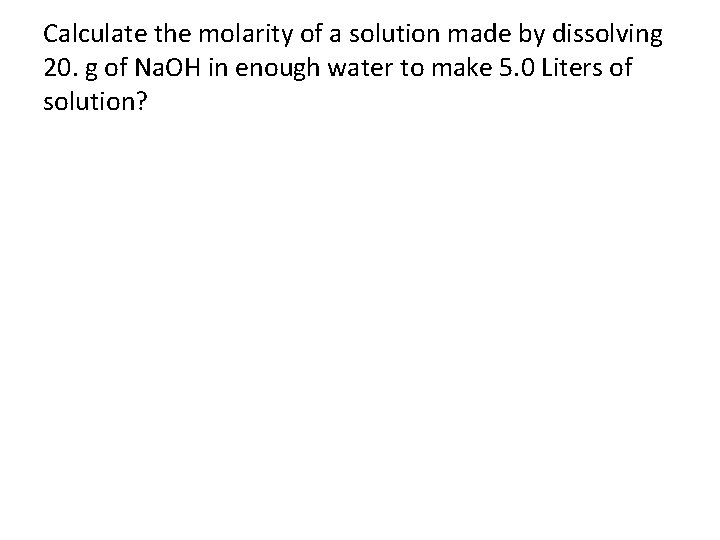
Calculate the molarity of a solution made by dissolving 20. g of Na. OH in enough water to make 5. 0 Liters of solution?

Full strength hydrochloric acid is 11. 6 M. How many liters of this concentrated solution is required to make 1. 0 L of a 1. 0 M solution?