Expert Guidance on Current and Future Therapeutic Strategies




![FORTE: Premaintenance Response Rates Response Rate[1, 2] 80 60 ≥ VGPR 89% ≥ VGPR FORTE: Premaintenance Response Rates Response Rate[1, 2] 80 60 ≥ VGPR 89% ≥ VGPR](https://slidetodoc.com/presentation_image_h2/5bca495cea78cf5c91f89a5d60480e4b/image-29.jpg)

![Myelosuppression and Infection § Myeloma and some treatment regimens associated with myelosuppression[1] ‒ Increased Myelosuppression and Infection § Myeloma and some treatment regimens associated with myelosuppression[1] ‒ Increased](https://slidetodoc.com/presentation_image_h2/5bca495cea78cf5c91f89a5d60480e4b/image-39.jpg)





- Slides: 86

Expert Guidance on Current and Future Therapeutic Strategies for Multiple Myeloma This program is supported by educational grants from Abb. Vie, Amgen, Celgene Corporation, Janssen Biotech, Inc. , administered by Janssen Scientific Affairs, LLC and Oncopeptides.

About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details

Faculty Charise Gleason, MSN, NP-BC, AOCNP Advanced Practice Provider Chief Winship Cancer Institute of Emory University Adjunct Faculty Nell Hodgson Woodruff School of Nursing Emory University Atlanta, Georgia Sagar Lonial, MD Professor and Chair Department of Hematology and Medical Oncology Chief Medical Officer Winship Cancer Institute of Emory University Atlanta, Georgia Kathryn Maples, Pharm. D, BCOP Clinical Pharmacy Specialist, Multiple Myeloma Department of Pharmacy Emory Healthcare Winship Cancer Institute Atlanta, Georgia

Faculty Disclosures Charise Gleason, MSN, NP-BC, AOCNP, has disclosed that she has received consulting fees from Celgene, Karyopharm, and Takeda. Sagar Lonial, MD, has disclosed that he has received consulting fees from Amgen, Bristol-Myers Squibb, Celgene, Glaxo. Smith. Kline, Janssen, Karyopharm, Merck, and Novartis. Kathryn Maples, Pharm. D, BCOP, has no relevant conflicts of interest to report.

Smoldering Myeloma

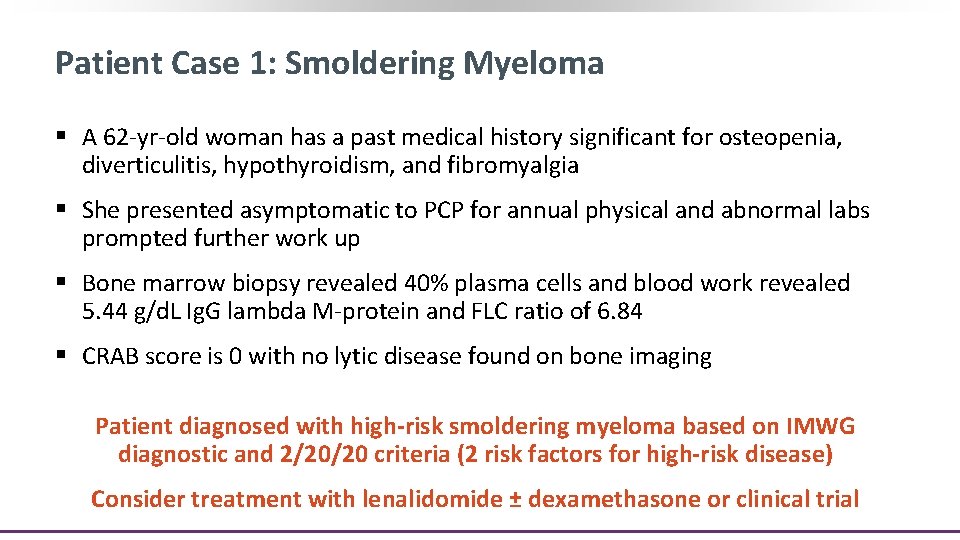
Patient Case 1: Smoldering Myeloma § A 62 -yr-old woman has a past medical history significant for osteopenia, diverticulitis, hypothyroidism, and fibromyalgia § She presented asymptomatic to PCP for annual physical and abnormal labs prompted further work up § Bone marrow biopsy revealed 40% plasma cells and blood work revealed 5. 44 g/d. L Ig. G lambda M-protein and FLC ratio of 6. 84 § CRAB score is 0 with no lytic disease found on bone imaging Patient diagnosed with high-risk smoldering myeloma based on IMWG diagnostic and 2/20/20 criteria (2 risk factors for high-risk disease) Consider treatment with lenalidomide ± dexamethasone or clinical trial

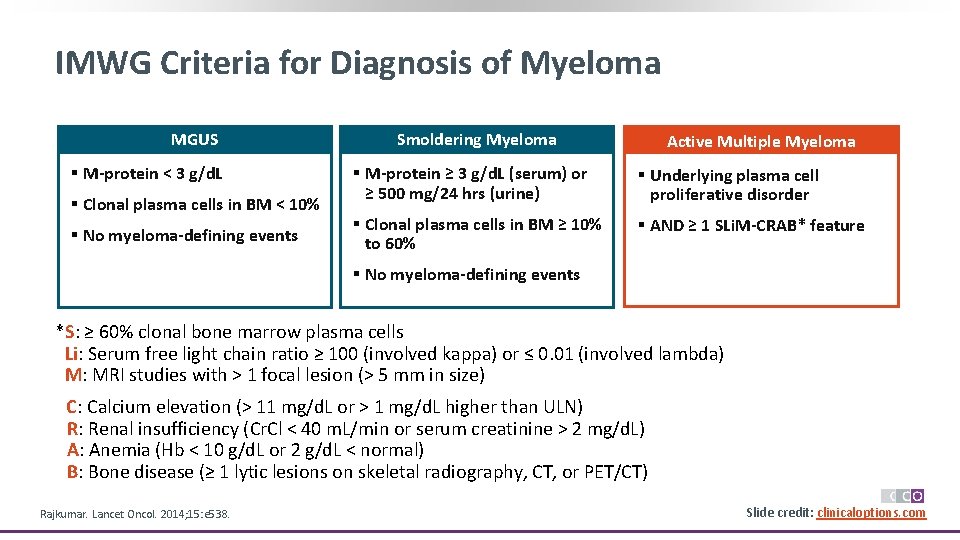
IMWG Criteria for Diagnosis of Myeloma MGUS § M-protein < 3 g/d. L § Clonal plasma cells in BM < 10% § No myeloma-defining events Smoldering Myeloma Active Multiple Myeloma § M-protein ≥ 3 g/d. L (serum) or ≥ 500 mg/24 hrs (urine) § Underlying plasma cell proliferative disorder § Clonal plasma cells in BM ≥ 10% to 60% § AND ≥ 1 SLi. M-CRAB* feature § No myeloma-defining events *S: ≥ 60% clonal bone marrow plasma cells Li: Serum free light chain ratio ≥ 100 (involved kappa) or ≤ 0. 01 (involved lambda) M: MRI studies with > 1 focal lesion (> 5 mm in size) C: Calcium elevation (> 11 mg/d. L or > 1 mg/d. L higher than ULN) R: Renal insufficiency (Cr. Cl < 40 m. L/min or serum creatinine > 2 mg/d. L) A: Anemia (Hb < 10 g/d. L or 2 g/d. L < normal) B: Bone disease (≥ 1 lytic lesions on skeletal radiography, CT, or PET/CT) Rajkumar. Lancet Oncol. 2014; 15: e 538. Slide credit: clinicaloptions. com

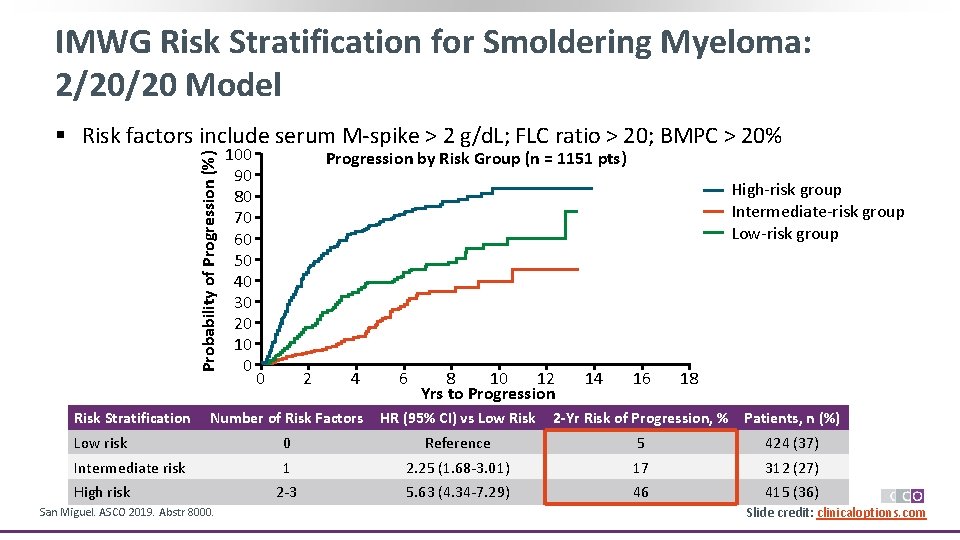
IMWG Risk Stratification for Smoldering Myeloma: 2/20/20 Model Probability of Progression (%) § Risk factors include serum M-spike > 2 g/d. L; FLC ratio > 20; BMPC > 20% Risk Stratification 100 90 80 70 60 50 40 30 20 10 0 Progression by Risk Group (n = 1151 pts) High-risk group Intermediate-risk group Low-risk group 0 2 4 6 8 10 12 Yrs to Progression 14 16 18 Number of Risk Factors HR (95% CI) vs Low Risk Low risk 0 Reference 5 424 (37) Intermediate risk 1 2. 25 (1. 68 -3. 01) 17 312 (27) 2 -3 5. 63 (4. 34 -7. 29) 46 415 (36) High risk San Miguel. ASCO 2019. Abstr 8000. 2 -Yr Risk of Progression, % Patients, n (%) Slide credit: clinicaloptions. com

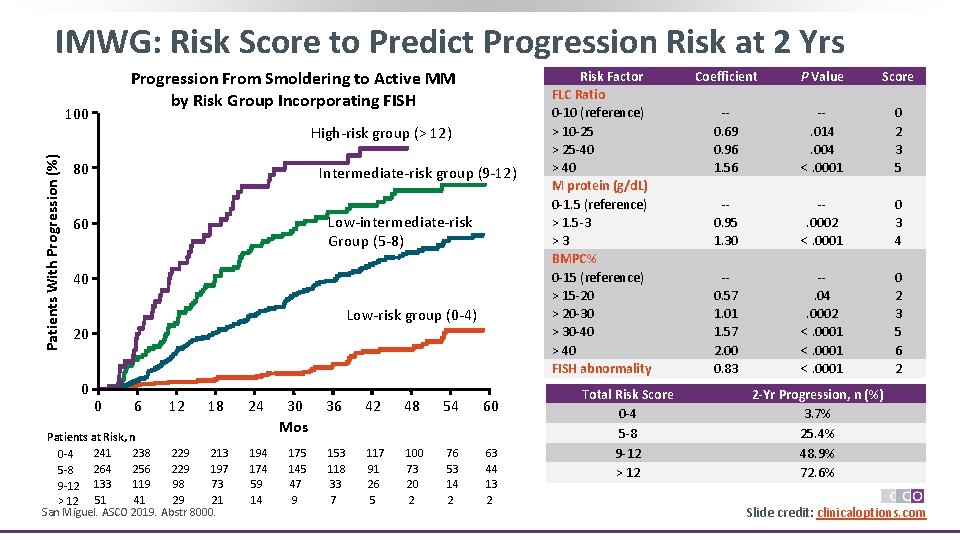
IMWG: Risk Score to Predict Progression Risk at 2 Yrs Progression From Smoldering to Active MM by Risk Group Incorporating FISH Patients With Progression (%) 100 High-risk group (> 12) 80 Intermediate-risk group (9 -12) Low-intermediate-risk Group (5 -8) 60 40 Low-risk group (0 -4) 20 0 0 6 12 18 Patients at Risk, n 241 238 229 213 0 -4 264 256 229 197 5 -8 119 98 73 9 -12 133 51 41 29 21 > 12 San Miguel. ASCO 2019. Abstr 8000. 24 194 174 59 14 30 Mos 175 145 47 9 36 42 48 54 60 153 118 33 7 117 91 26 5 100 73 20 2 76 53 14 2 63 44 13 2 Risk Factor FLC Ratio 0 -10 (reference) > 10 -25 > 25 -40 > 40 M protein (g/d. L) 0 -1. 5 (reference) > 1. 5 -3 >3 BMPC% 0 -15 (reference) > 15 -20 > 20 -30 > 30 -40 > 40 FISH abnormality Total Risk Score 0 -4 5 -8 9 -12 > 12 Coefficient P Value Score -0. 69 0. 96 1. 56 -. 014. 004 <. 0001 0 2 3 5 -0. 95 1. 30 -. 0002 <. 0001 0 3 4 -0. 57 1. 01 1. 57 2. 00 0. 83 -. 04. 0002 <. 0001 0 2 3 5 6 2 2 -Yr Progression, n (%) 3. 7% 25. 4% 48. 9% 72. 6% Slide credit: clinicaloptions. com

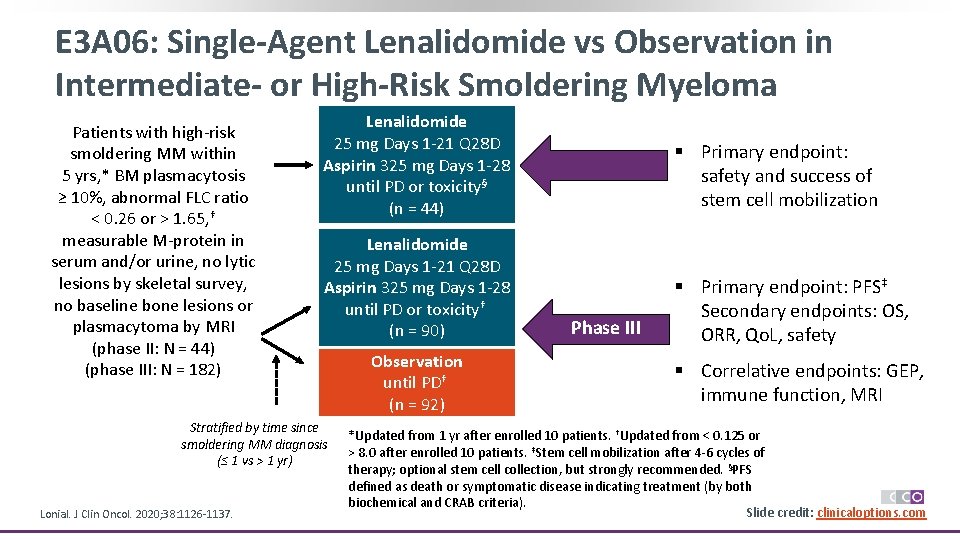
E 3 A 06: Single-Agent Lenalidomide vs Observation in Intermediate- or High-Risk Smoldering Myeloma Patients with high-risk smoldering MM within 5 yrs, * BM plasmacytosis ≥ 10%, abnormal FLC ratio < 0. 26 or > 1. 65, † measurable M-protein in serum and/or urine, no lytic lesions by skeletal survey, no baseline bone lesions or plasmacytoma by MRI (phase II: N = 44) (phase III: N = 182) Lenalidomide Phase II 25 mg Days 1 -21 Q 28 D Aspirin 325 mg Days 1 -28 until PD or toxicity§ (n = 44) § Primary endpoint: safety and success of stem cell mobilization Lenalidomide 25 mg Days 1 -21 Q 28 D Aspirin 325 mg Days 1 -28 until PD or toxicity† (n = 90) § Primary endpoint: PFS‡ Secondary endpoints: OS, ORR, Qo. L, safety Stratified by time since smoldering MM diagnosis (≤ 1 vs > 1 yr) Lonial. J Clin Oncol. 2020; 38: 1126 -1137. Observation until PD† (n = 92) Phase III § Correlative endpoints: GEP, immune function, MRI *Updated from 1 yr after enrolled 10 patients. †Updated from < 0. 125 or > 8. 0 after enrolled 10 patients. ‡Stem cell mobilization after 4 -6 cycles of therapy; optional stem cell collection, but strongly recommended. §PFS defined as death or symptomatic disease indicating treatment (by both biochemical and CRAB criteria). Slide credit: clinicaloptions. com

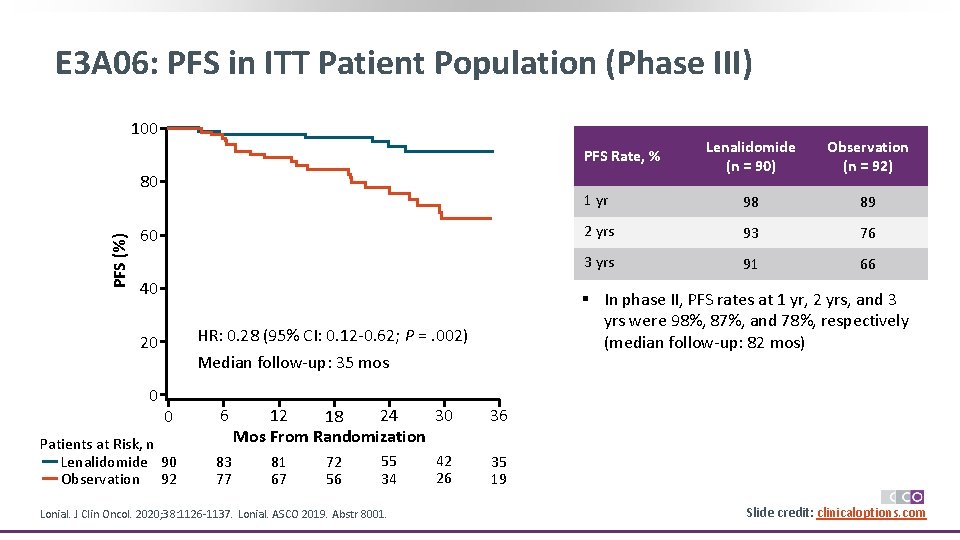
E 3 A 06: PFS in ITT Patient Population (Phase III) 100 Lenalidomide (n = 90) Observation (n = 92) 1 yr 98 89 2 yrs 93 76 3 yrs 91 66 PFS Rate, % PFS (%) 80 60 40 HR: 0. 28 (95% CI: 0. 12 -0. 62; P =. 002) 20 0 § In phase II, PFS rates at 1 yr, 2 yrs, and 3 yrs were 98%, 87%, and 78%, respectively (median follow-up: 82 mos) Median follow-up: 35 mos 0 6 Patients at Risk, n Lenalidomide 90 Observation 92 83 77 30 24 12 18 Mos From Randomization 36 42 26 35 19 81 67 72 56 55 34 Lonial. J Clin Oncol. 2020; 38: 1126 -1137. Lonial. ASCO 2019. Abstr 8001. Slide credit: clinicaloptions. com

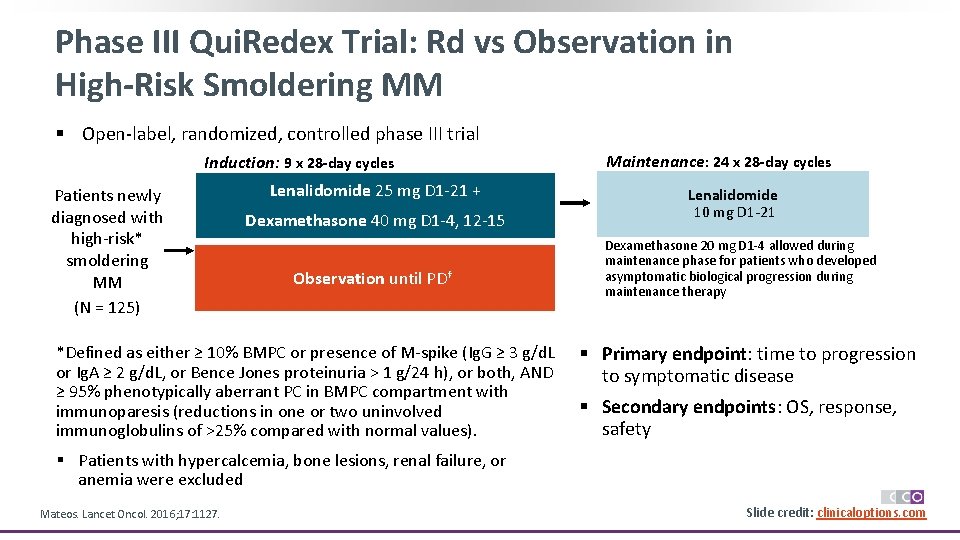
Phase III Qui. Redex Trial: Rd vs Observation in High-Risk Smoldering MM § Open-label, randomized, controlled phase III trial Induction: 9 x 28 -day cycles Patients newly diagnosed with high-risk* smoldering MM (N = 125) Lenalidomide 25 mg D 1 -21 + Dexamethasone 40 mg D 1 -4, 12 -15 Observation until PD† *Defined as either ≥ 10% BMPC or presence of M-spike (Ig. G ≥ 3 g/d. L or Ig. A ≥ 2 g/d. L, or Bence Jones proteinuria > 1 g/24 h), or both, AND ≥ 95% phenotypically aberrant PC in BMPC compartment with immunoparesis (reductions in one or two uninvolved immunoglobulins of >25% compared with normal values). Maintenance: 24 x 28 -day cycles Lenalidomide 10 mg D 1 -21 Dexamethasone 20 mg D 1 -4 allowed during maintenance phase for patients who developed asymptomatic biological progression during maintenance therapy § Primary endpoint: time to progression to symptomatic disease § Secondary endpoints: OS, response, safety § Patients with hypercalcemia, bone lesions, renal failure, or anemia were excluded Mateos. Lancet Oncol. 2016; 17: 1127. Slide credit: clinicaloptions. com

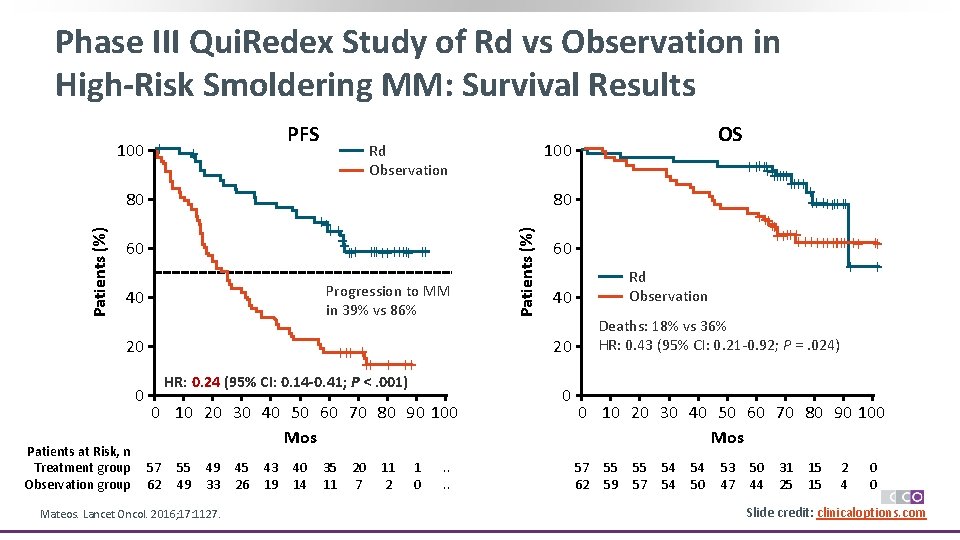
Phase III Qui. Redex Study of Rd vs Observation in High-Risk Smoldering MM: Survival Results PFS + Patients (%) 80 Progression to MM in 39% vs 86% 40 20 0 Patients at Risk, n Treatment group Observation group HR: 0. 24 (95% CI: 0. 14 -0. 41; P <. 001) 0 10 20 30 40 50 60 70 80 90 100 Mos 55 49 49 33 Mateos. Lancet Oncol. 2016; 17: 1127. 45 26 43 19 40 14 35 11 20 7 11 2 1 0 60 Rd Observation 40 Deaths: 18% vs 36% HR: 0. 43 (95% CI: 0. 21 -0. 92; P =. 024) 20 +++ + ++ 57 62 ++++++ ++ ++++++++++ + 80 ++++ ++ +++++ ++ 60 OS 100 Rd Observation Patients (%) 100 + . . 0 0 10 20 30 40 50 60 70 80 90 100 Mos 57 62 55 59 55 57 54 54 54 50 53 47 50 44 31 25 15 15 2 4 0 0 Slide credit: clinicaloptions. com

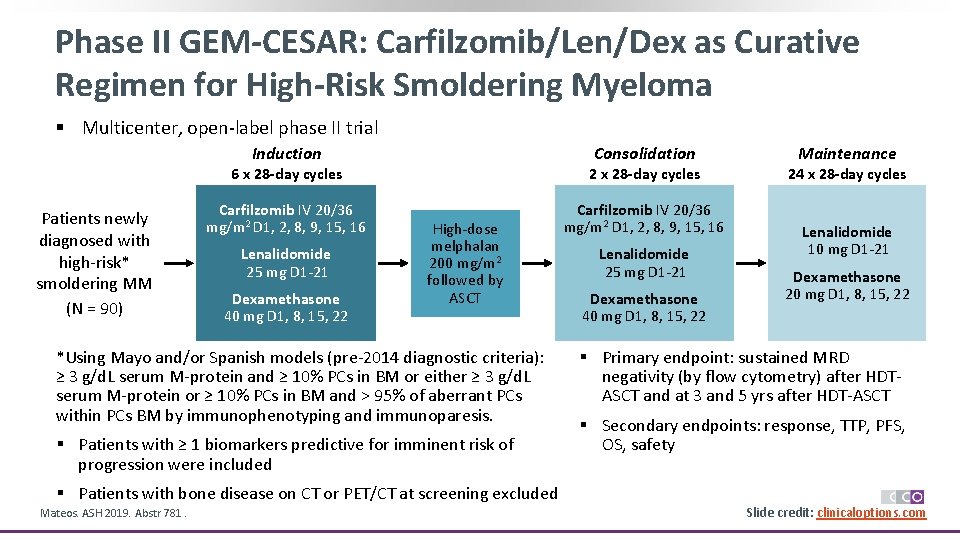
Phase II GEM-CESAR: Carfilzomib/Len/Dex as Curative Regimen for High-Risk Smoldering Myeloma § Multicenter, open-label phase II trial Consolidation Induction Patients newly diagnosed with high-risk* smoldering MM (N = 90) 6 x 28 -day cycles 2 x 28 -day cycles Carfilzomib IV 20/36 mg/m 2 D 1, 2, 8, 9, 15, 16 Lenalidomide 25 mg D 1 -21 Dexamethasone 40 mg D 1, 8, 15, 22 High-dose melphalan 200 mg/m 2 followed by ASCT *Using Mayo and/or Spanish models (pre-2014 diagnostic criteria): ≥ 3 g/d. L serum M-protein and ≥ 10% PCs in BM or either ≥ 3 g/d. L serum M-protein or ≥ 10% PCs in BM and > 95% of aberrant PCs within PCs BM by immunophenotyping and immunoparesis. § Patients with ≥ 1 biomarkers predictive for imminent risk of progression were included § Patients with bone disease on CT or PET/CT at screening excluded Mateos. ASH 2019. Abstr 781. Lenalidomide 25 mg D 1 -21 Dexamethasone 40 mg D 1, 8, 15, 22 Maintenance 24 x 28 -day cycles Lenalidomide 10 mg D 1 -21 Dexamethasone 20 mg D 1, 8, 15, 22 § Primary endpoint: sustained MRD negativity (by flow cytometry) after HDTASCT and at 3 and 5 yrs after HDT-ASCT § Secondary endpoints: response, TTP, PFS, OS, safety Slide credit: clinicaloptions. com

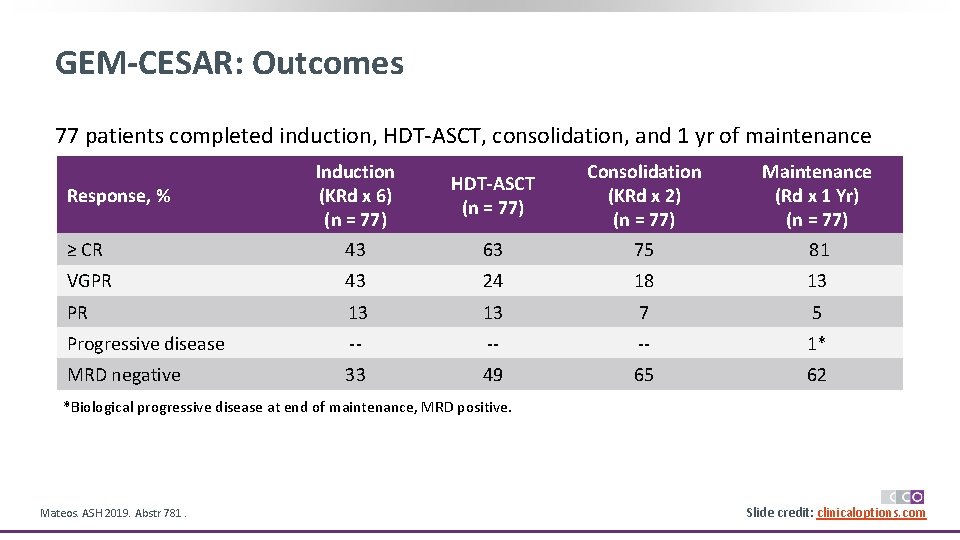
GEM-CESAR: Outcomes 77 patients completed induction, HDT-ASCT, consolidation, and 1 yr of maintenance Induction (KRd x 6) (n = 77) HDT-ASCT (n = 77) Consolidation (KRd x 2) (n = 77) Maintenance (Rd x 1 Yr) (n = 77) ≥ CR 43 63 75 81 VGPR 43 24 18 13 PR 13 13 7 5 Progressive disease -- -- -- 1* MRD negative 33 49 65 62 Response, % *Biological progressive disease at end of maintenance, MRD positive. Mateos. ASH 2019. Abstr 781. Slide credit: clinicaloptions. com

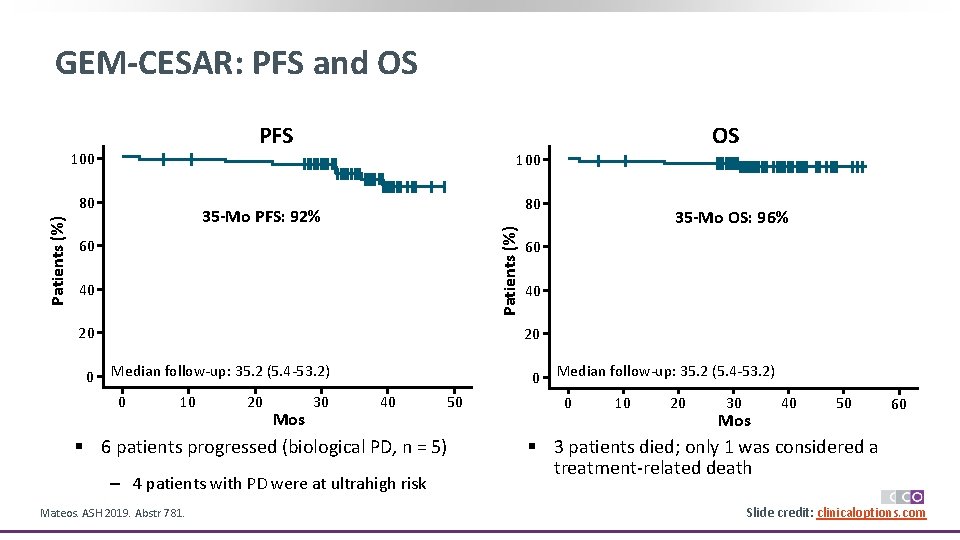
GEM-CESAR: PFS and OS PFS 100 80 80 35 -Mo PFS: 92% Patients (%) OS 60 40 20 0 35 -Mo OS: 96% 60 40 20 Median follow-up: 35. 2 (5. 4 -53. 2) 0 10 20 Mos 30 40 50 § 6 patients progressed (biological PD, n = 5) ‒ 4 patients with PD were at ultrahigh risk Mateos. ASH 2019. Abstr 781. Median follow-up: 35. 2 (5. 4 -53. 2) 0 10 20 30 40 Mos 50 60 § 3 patients died; only 1 was considered a treatment-related death Slide credit: clinicaloptions. com

Summary § Based on current diagnostic criteria, smoldering MM is defined as having M-protein ≥ 3 g/d. L (serum) and ≥ 10% to 60% clonal plasma cells in BM § Identify patients with high-risk smoldering myeloma using IMWG 2/20/20 criteria, with ≥ 2 of the following risk features: 1. M-spike > 2 g/d. L 2. FLC ratio > 20 3. BMPC > 20% § Consider treatment with lenalidomide with or without dexamethasone for patients with high-risk smoldering MM ‒ Clinical trials are ongoing to assess efficacy and safety of more aggressive treatment approaches for high-risk smoldering MM

Active Myeloma

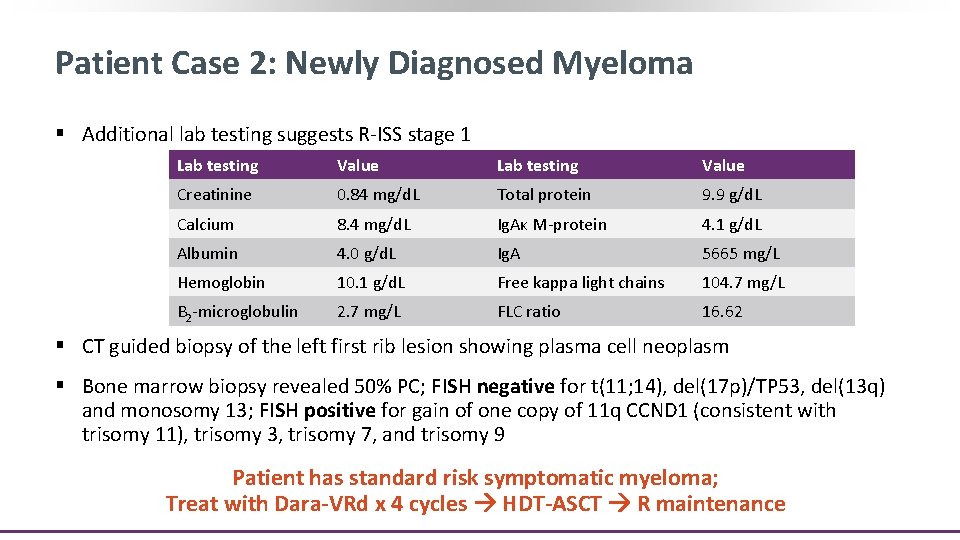
Patient Case 2: Newly Diagnosed Myeloma § A 48 -yr-old, previously healthy woman started noticing acute onset and gradually worsening neck pain approximately 4 mos ago ‒ Approximately 2 mos ago, she noted that the pain increased in severity and started having a burning sensation ‒ She denied any fever, chills, night sweats, or weight loss § MRI of neck revealed a C 3 pathologic fracture with a left chest wall mass § CT neck/chest/spine and MRI of spine revealed numerous enhancing lesions in cervical, thoracic, and lumbar vertebral spine as well as a mass on the left first rib

Patient Case 2: Newly Diagnosed Myeloma § Additional lab testing suggests R-ISS stage 1 Lab testing Value Creatinine 0. 84 mg/d. L Total protein 9. 9 g/d. L Calcium 8. 4 mg/d. L Ig. Aκ M-protein 4. 1 g/d. L Albumin 4. 0 g/d. L Ig. A 5665 mg/L Hemoglobin 10. 1 g/d. L Free kappa light chains 104. 7 mg/L Β 2 -microglobulin 2. 7 mg/L FLC ratio 16. 62 § CT guided biopsy of the left first rib lesion showing plasma cell neoplasm § Bone marrow biopsy revealed 50% PC; FISH negative for t(11; 14), del(17 p)/TP 53, del(13 q) and monosomy 13; FISH positive for gain of one copy of 11 q CCND 1 (consistent with trisomy 11), trisomy 3, trisomy 7, and trisomy 9 Patient has standard risk symptomatic myeloma; Treat with Dara-VRd x 4 cycles HDT-ASCT R maintenance

Enter your patient case online: clinicaloptions. com/Myeloma. Tool

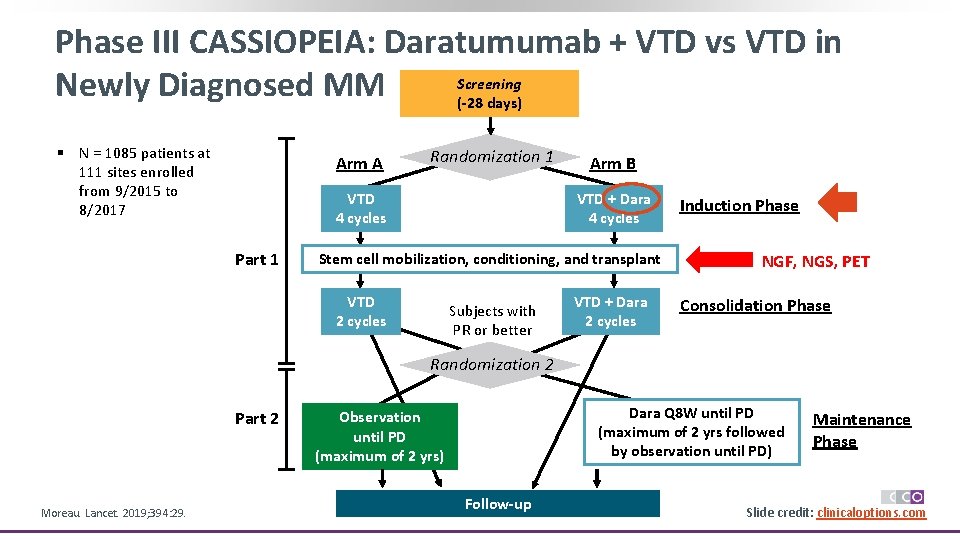
Phase III CASSIOPEIA: Daratumumab + VTD vs VTD in Screening Newly Diagnosed MM (-28 days) § N = 1085 patients at 111 sites enrolled from 9/2015 to 8/2017 Arm A Randomization 1 VTD 4 cycles Part 1 Arm B VTD + Dara 4 cycles Stem cell mobilization, conditioning, and transplant VTD 2 cycles Subjects with PR or better VTD + Dara 2 cycles Induction Phase NGF, NGS, PET Consolidation Phase Randomization 2 Part 2 Moreau. Lancet. 2019; 394: 29. Dara Q 8 W until PD (maximum of 2 yrs followed by observation until PD) Observation until PD (maximum of 2 yrs) Follow-up Maintenance Phase Slide credit: clinicaloptions. com

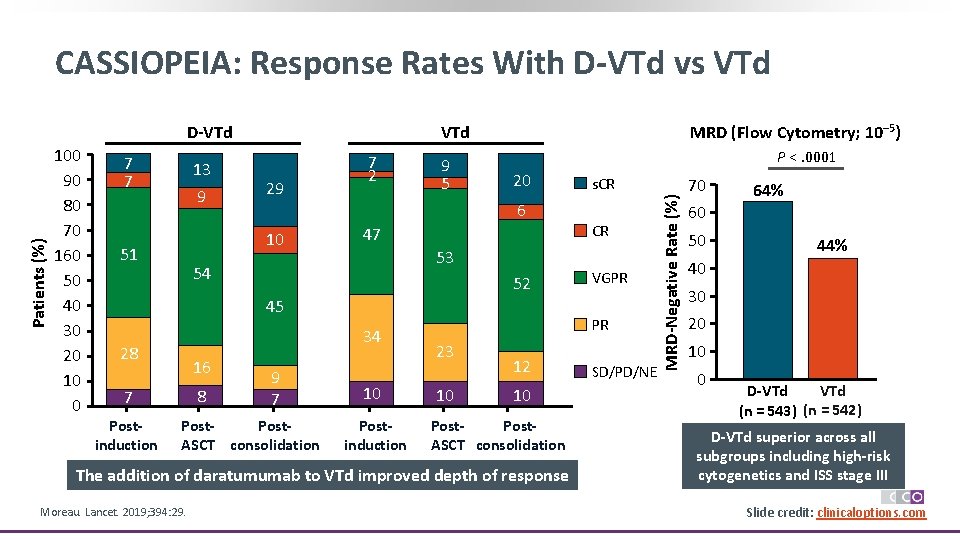
CASSIOPEIA: Response Rates With D-VTd vs VTd 100 90 80 70 160 50 40 30 20 10 0 7 7 13 9 29 7 2 9 5 P <. 0001 20 6 10 51 MRD (Flow Cytometry; 10– 5) VTd 47 52 45 34 16 8 7 Postinduction 9 7 Post. ASCT consolidation 10 Postinduction VGPR PR 23 10 12 10 Post. ASCT consolidation The addition of daratumumab to VTd improved depth of response Moreau. Lancet. 2019; 394: 29. CR 53 54 28 s. CR SD/PD/NE MRD-Negative Rate (%) Patients (%) D-VTd 70 64% 60 50 44% 40 30 20 10 0 VTd D-VTd (n = 543) (n = 542) D-VTd superior across all subgroups including high-risk cytogenetics and ISS stage III Slide credit: clinicaloptions. com

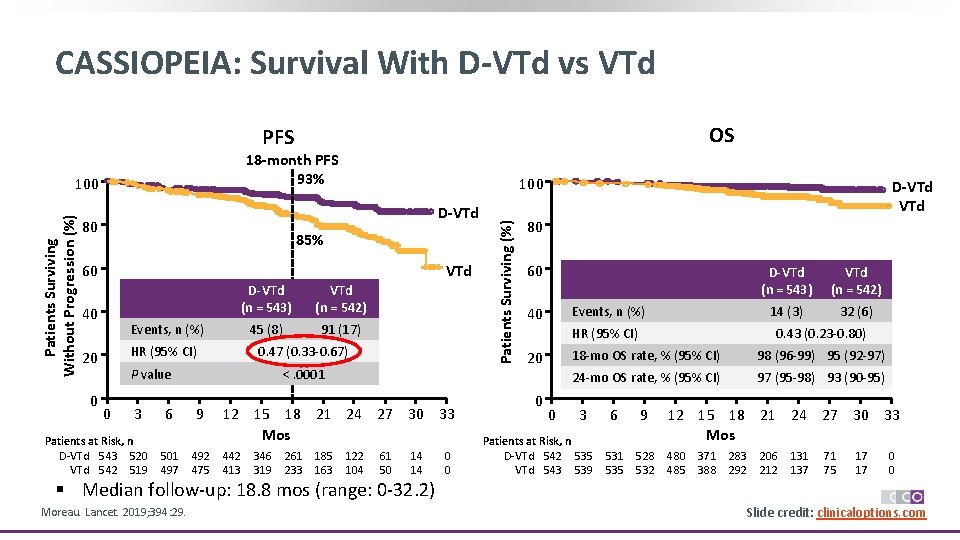
CASSIOPEIA: Survival With D-VTd vs VTd OS PFS 18 -month PFS 93% 100 D-VTd 80 85% 60 VTd 40 VTd (n = 542) 45 (8) 91 (17) Events, n (%) HR (95% CI) 20 0 D-VTd (n = 543) 0. 47 (0. 33 -0. 67) P value 0 3 Patients at Risk, n D-VTd 543 520 VTd 542 519 6 501 497 <. 0001 9 492 475 12 15 18 21 24 27 30 33 Mos 442 413 346 319 261 233 185 163 122 104 61 50 14 14 0 0 Patients Surviving (%) Patients Surviving Without Progression (%) 100 D-VTd 80 60 Events, n (%) 40 HR (95% CI) 20 0 0 D-VTd (n = 543) VTd (n = 542) 14 (3) 32 (6) 0. 43 (0. 23 -0. 80) 18 -mo OS rate, % (95% CI) 98 (96 -99) 95 (92 -97) 24 -mo OS rate, % (95% CI) 97 (95 -98) 93 (90 -95) 3 Patients at Risk, n D-VTd 542 535 VTd 543 539 6 9 531 535 528 532 12 15 18 21 24 27 30 33 Mos 480 485 371 388 283 292 206 212 131 137 71 75 17 17 0 0 § Median follow-up: 18. 8 mos (range: 0 -32. 2) Moreau. Lancet. 2019; 394: 29. Slide credit: clinicaloptions. com

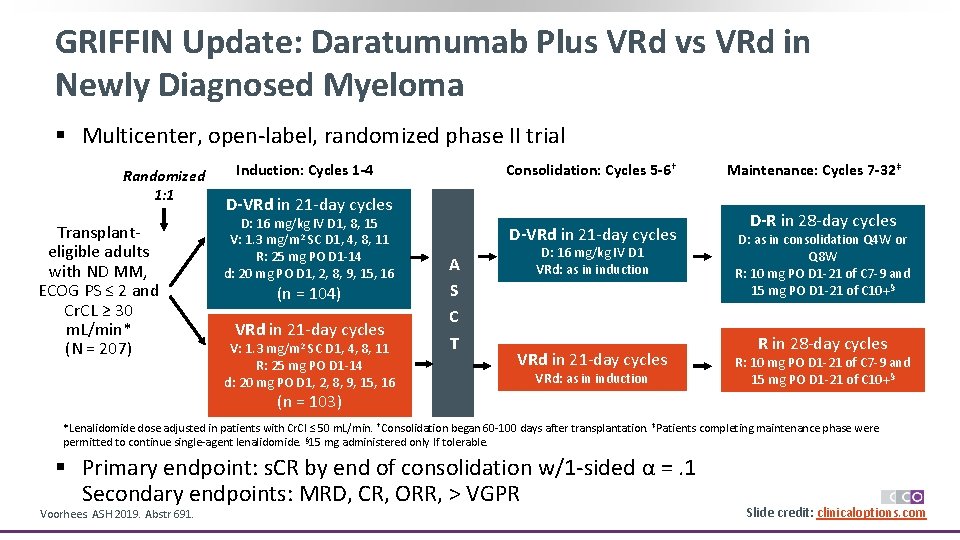
GRIFFIN Update: Daratumumab Plus VRd vs VRd in Newly Diagnosed Myeloma § Multicenter, open-label, randomized phase II trial Randomized 1: 1 Transplanteligible adults with ND MM, ECOG PS ≤ 2 and Cr. CL ≥ 30 m. L/min* (N = 207) Induction: Cycles 1 -4 Consolidation: Cycles 5 -6† D-VRd in 21 -day cycles D: 16 mg/kg IV D 1, 8, 15 V: 1. 3 mg/m 2 SC D 1, 4, 8, 11 R: 25 mg PO D 1 -14 d: 20 mg PO D 1, 2, 8, 9, 15, 16 (n = 104) VRd in 21 -day cycles V: 1. 3 mg/m 2 SC D 1, 4, 8, 11 R: 25 mg PO D 1 -14 d: 20 mg PO D 1, 2, 8, 9, 15, 16 D-VRd in 21 -day cycles A S C T D: 16 mg/kg IV D 1 VRd: as in induction VRd in 21 -day cycles VRd: as in induction Maintenance: Cycles 7 -32‡ D-R in 28 -day cycles D: as in consolidation Q 4 W or Q 8 W R: 10 mg PO D 1 -21 of C 7 -9 and 15 mg PO D 1 -21 of C 10+§ R in 28 -day cycles R: 10 mg PO D 1 -21 of C 7 -9 and 15 mg PO D 1 -21 of C 10+§ (n = 103) *Lenalidomide dose adjusted in patients with Cr. Cl ≤ 50 m. L/min. †Consolidation began 60 -100 days after transplantation. ‡Patients completing maintenance phase were permitted to continue single-agent lenalidomide. § 15 mg administered only If tolerable. § Primary endpoint: s. CR by end of consolidation w/1 -sided α =. 1 Secondary endpoints: MRD, CR, ORR, > VGPR Voorhees. ASH 2019. Abstr 691. Slide credit: clinicaloptions. com

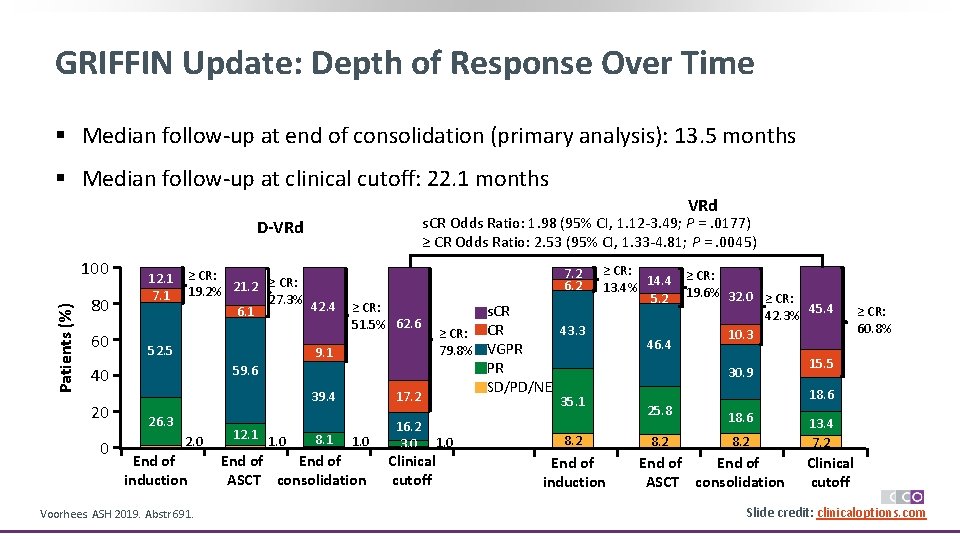
GRIFFIN Update: Depth of Response Over Time § Median follow-up at end of consolidation (primary analysis): 13. 5 months § Median follow-up at clinical cutoff: 22. 1 months VRd s. CR Odds Ratio: 1. 98 (95% CI, 1. 12 -3. 49; P =. 0177) ≥ CR Odds Ratio: 2. 53 (95% CI, 1. 33 -4. 81; P =. 0045) D-VRd Patients (%) 100 80 60 12. 1 7. 1 ≥ CR: 19. 2% 21. 2 27. 3% 42. 4 6. 1 52. 5 9. 1 0 ≥ CR: 51. 5% 62. 6 59. 6 40 20 7. 2 6. 2 26. 3 2. 0 End of induction Voorhees. ASH 2019. Abstr 691. 12. 1 1. 0 39. 4 17. 2 8. 1 16. 2 3. 0 1. 0 End of ASCT consolidation Clinical cutoff s. CR 43. 3 ≥ CR: CR 79. 8% VGPR PR SD/PD/NE ≥ CR: 14. 4 ≥ CR: 13. 4% 5. 2 19. 6% 32. 0 ≥ CR: 45. 4 42. 3% 10. 3 46. 4 15. 5 30. 9 35. 1 1. 0 8. 2 End of induction ≥ CR: 60. 8% 18. 6 25. 8 18. 6 8. 2 End of ASCT consolidation 13. 4 7. 2 Clinical cutoff Slide credit: clinicaloptions. com

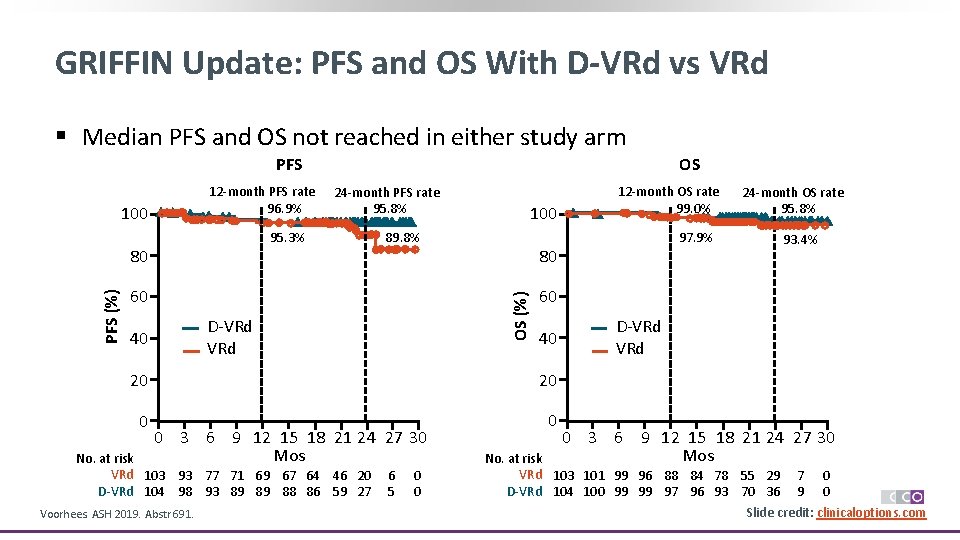
GRIFFIN Update: PFS and OS With D-VRd vs VRd § Median PFS and OS not reached in either study arm PFS 12 -month PFS rate 96. 9% 100 24 -month PFS rate 95. 8% 95. 3% 80 89. 8% 60 D-VRd 40 100 40 20 0 0 No. at risk VRd 103 93 77 71 69 67 64 46 20 D-VRd 104 98 93 89 89 88 86 59 27 Voorhees. ASH 2019. Abstr 691. 6 5 0 0 97. 9% 24 -month OS rate 95. 8% 93. 4% 60 20 0 3 6 9 12 15 18 21 24 27 30 Mos 12 -month OS rate 99. 0% 80 OS (%) PFS (%) OS D-VRd 0 3 6 9 12 15 18 21 24 27 30 Mos No. at risk VRd 103 101 99 96 88 84 78 55 29 7 0 D-VRd 104 100 99 99 97 96 93 70 36 9 0 Slide credit: clinicaloptions. com

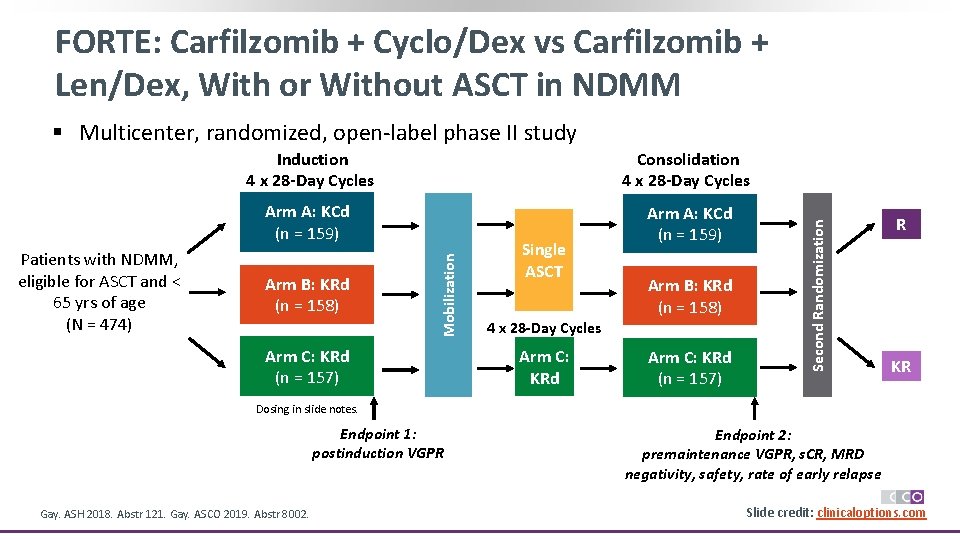
FORTE: Carfilzomib + Cyclo/Dex vs Carfilzomib + Len/Dex, With or Without ASCT in NDMM § Multicenter, randomized, open-label phase II study Arm A: KCd (n = 159) Arm B: KRd (n = 158) Arm C: KRd (n = 157) Single ASCT Arm B: KRd (n = 158) 4 x 28 -Day Cycles Arm C: KRd (n = 157) Second Randomization Consolidation 4 x 28 -Day Cycles Mobilization Patients with NDMM, eligible for ASCT and < 65 yrs of age (N = 474) Induction 4 x 28 -Day Cycles R KR Dosing in slide notes. Endpoint 1: postinduction VGPR Gay. ASH 2018. Abstr 121. Gay. ASCO 2019. Abstr 8002. Endpoint 2: premaintenance VGPR, s. CR, MRD negativity, safety, rate of early relapse Slide credit: clinicaloptions. com
![FORTE Premaintenance Response Rates Response Rate1 2 80 60 VGPR 89 VGPR FORTE: Premaintenance Response Rates Response Rate[1, 2] 80 60 ≥ VGPR 89% ≥ VGPR](https://slidetodoc.com/presentation_image_h2/5bca495cea78cf5c91f89a5d60480e4b/image-29.jpg)
FORTE: Premaintenance Response Rates Response Rate[1, 2] 80 60 ≥ VGPR 89% ≥ VGPR 76% 29% 40 15% 20 32% 0 KCd-ASCT (n = 159) 29% 16% 44% KRd-ASCT (n = 158) ≥ VGPR 87% 26% 18% 43% KRd 12 (n = 157) s. CR *Unconfirmed patients missing immunofixation/s. FLC analysis. Gay F, et al. ASH 2018. Abstract 121. Gay. ASCO 2019. Abstr 8002. 100 Response Rate (%) 100 80 60 Response Rate with KRd by Risk[2] R-ISS II/III ≥ VGPR: 92% ≥ VGPR 79% 32% 14% 15% 0 46% 49% KRd-ASCT (n = 48) KRd 12 (n = 39) CR confirmed/unconfirmed* 30% 29% 17% 19% 38% KRd-ASCT (n = 92) KRd 12 (n = 94) 15% 40 20 ≥ VGPR 86% VGPR Slide credit: clinicaloptions. com

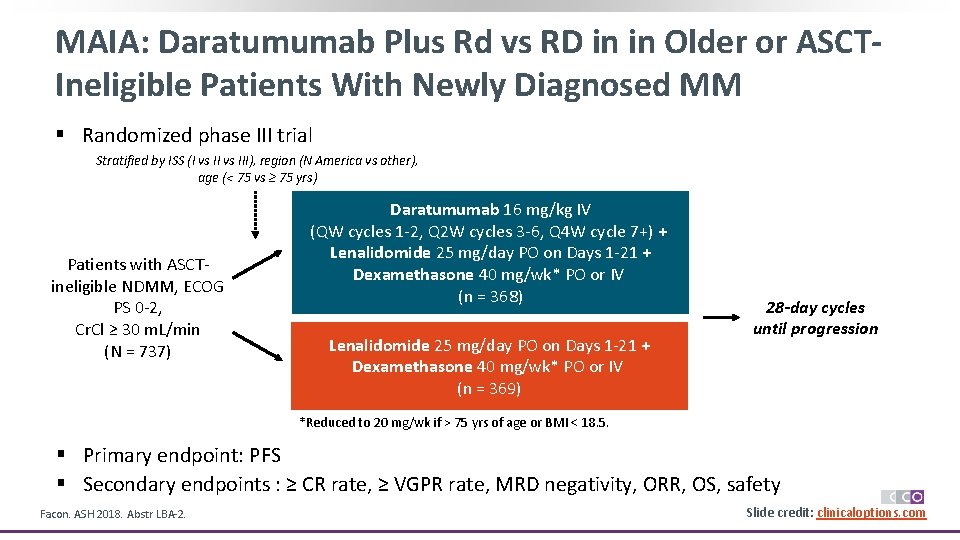
MAIA: Daratumumab Plus Rd vs RD in in Older or ASCTIneligible Patients With Newly Diagnosed MM § Randomized phase III trial Stratified by ISS (I vs III), region (N America vs other), age (< 75 vs ≥ 75 yrs) Patients with ASCTineligible NDMM, ECOG PS 0 -2, Cr. Cl ≥ 30 m. L/min (N = 737) Daratumumab 16 mg/kg IV (QW cycles 1 -2, Q 2 W cycles 3 -6, Q 4 W cycle 7+) + Lenalidomide 25 mg/day PO on Days 1 -21 + Dexamethasone 40 mg/wk* PO or IV (n = 368) Lenalidomide 25 mg/day PO on Days 1 -21 + Dexamethasone 40 mg/wk* PO or IV (n = 369) 28 -day cycles until progression *Reduced to 20 mg/wk if > 75 yrs of age or BMI < 18. 5. § Primary endpoint: PFS § Secondary endpoints : ≥ CR rate, ≥ VGPR rate, MRD negativity, ORR, OS, safety Facon. ASH 2018. Abstr LBA-2. Slide credit: clinicaloptions. com

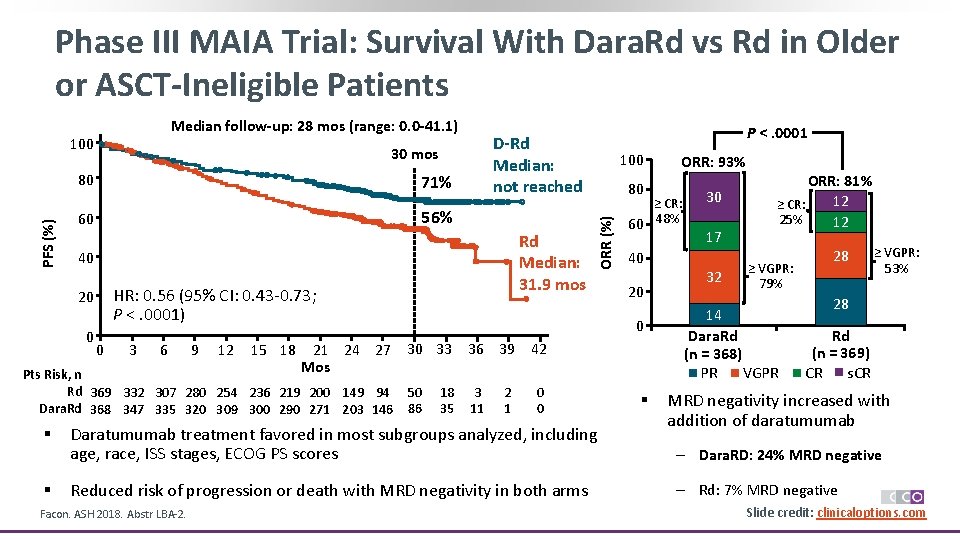
Phase III MAIA Trial: Survival With Dara. Rd vs Rd in Older or ASCT-Ineligible Patients Median follow-up: 28 mos (range: 0. 0 -41. 1) 30 mos PFS (%) 80 71% 56% 60 0 HR: 0. 56 (95% CI: 0. 43 -0. 73; P <. 0001) 0 3 6 9 12 15 18 § 100 80 50 86 18 35 2 1 0 0 Daratumumab treatment favored in most subgroups analyzed, including age, race, ISS stages, ECOG PS scores Reduced risk of progression or death with MRD negativity in both arms Facon. ASH 2018. Abstr LBA-2. 40 20 36 39 42 3 11 ORR: 93% ≥ CR: 60 48% 0 21 24 27 30 33 Mos Pts Risk, n Rd 369 332 307 280 254 236 219 200 149 94 Dara. Rd 368 347 335 320 309 300 290 271 203 146 § Rd Median: 31. 9 mos 40 20 P <. 0001 D-Rd Median: not reached ORR (%) 100 § 30 17 32 ORR: 81% 12 ≥ CR: 25% 12 ≥ VGPR: 79% 14 Dara. Rd (n = 368) PR VGPR 28 ≥ VGPR: 53% 28 Rd (n = 369) s. CR CR MRD negativity increased with addition of daratumumab ‒ Dara. RD: 24% MRD negative ‒ Rd: 7% MRD negative Slide credit: clinicaloptions. com

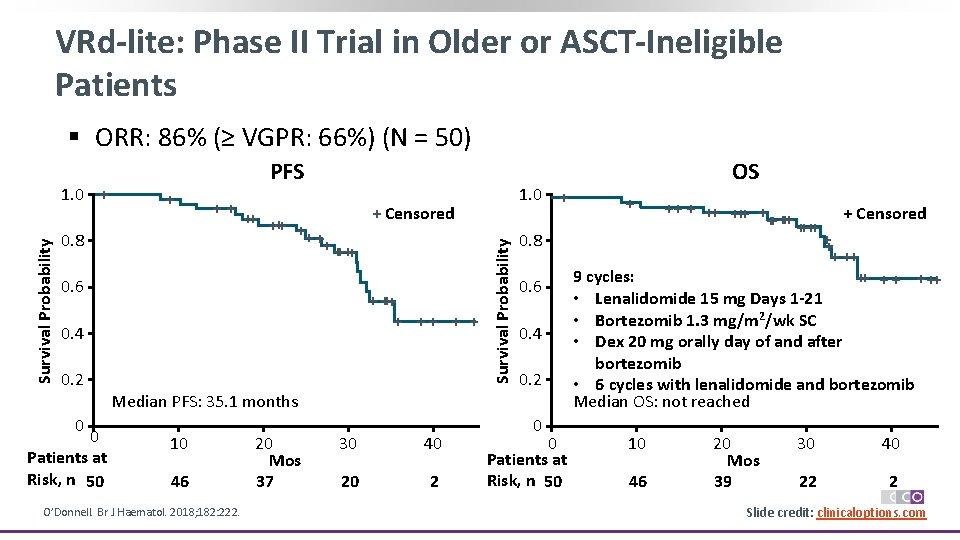
VRd-lite: Phase II Trial in Older or ASCT-Ineligible Patients § ORR: 86% (≥ VGPR: 66%) (N = 50) + ++ 0. 8 + Censored ++ ++ + + ++++ 0. 6 + +++ + + 0. 4 0. 2 1. 0 + Survival Probability 1. 0 + PFS 0. 8 0. 6 0. 4 0. 2 Median PFS: 35. 1 months 0 0 Patients at Risk, n 50 10 46 O’Donnell. Br J Haematol. 2018; 182: 222. 20 Mos 37 30 40 20 2 0 0 Patients at Risk, n 50 OS + ++ +++ + + ++++ ++ ++ + Censored ++ + ++ 9 cycles: ++ + ++ • Lenalidomide 15 mg Days 1 -21 • Bortezomib 1. 3 mg/m 2/wk SC • Dex 20 mg orally day of and after bortezomib • 6 cycles with lenalidomide and bortezomib Median OS: not reached 10 46 20 Mos 39 30 40 22 2 Slide credit: clinicaloptions. com

Considerations for Managing Patients With Myeloma

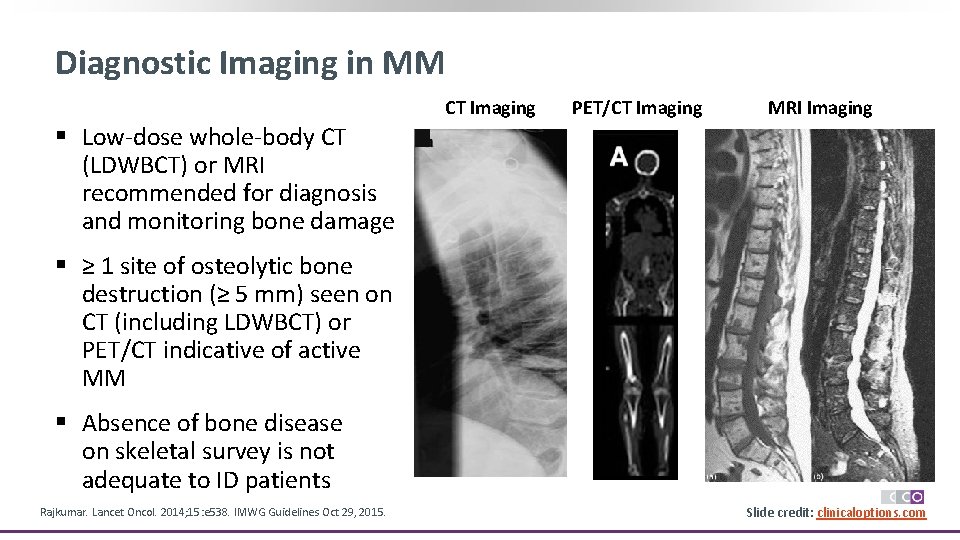
Diagnostic Imaging in MM CT Imaging PET/CT Imaging MRI Imaging § Low-dose whole-body CT (LDWBCT) or MRI recommended for diagnosis and monitoring bone damage § ≥ 1 site of osteolytic bone destruction (≥ 5 mm) seen on CT (including LDWBCT) or PET/CT indicative of active MM § Absence of bone disease on skeletal survey is not adequate to ID patients Rajkumar. Lancet Oncol. 2014; 15: e 538. IMWG Guidelines Oct 29, 2015. Slide credit: clinicaloptions. com

How Do (Should) We Use MRD in the Clinic Today? Flow Cytometry Next-Gen Sequencing Imaging § Absence of clonal plasma cells in bone marrow aspirate using next-gen flow cytometry § Absence of clonal plasma cells in BM aspirate with < 2 identical DNA sequence reads § Disappearance of areas of tracer uptake at baseline PET/CT or decrease to < normal surrounding tissue § Sensitivity: 10 -4 to 10 -6 § Sensitivity: high (? ) § Need for specialized, validated equipment § Sent to lab for evaluation (clono. SEQ) § Can be used as monitoring along with other assays § Clono. SEQ FDA approved for MRD testing in acute lymphoblastic leukemia and myeloma § BUT there are currently no data on altering length of induction therapy, need for ASCT and/or consolidation, or maintenance based on MRD results Slide credit: clinicaloptions. com

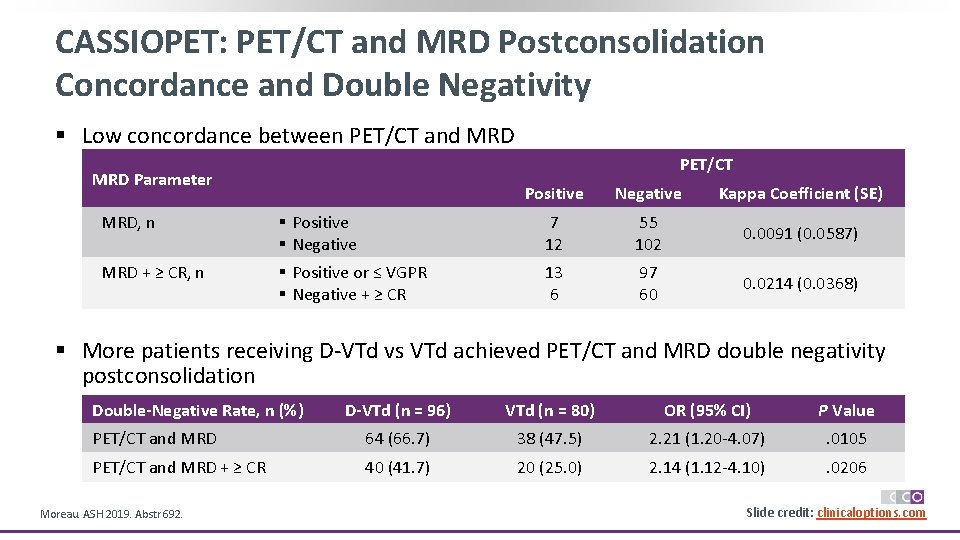
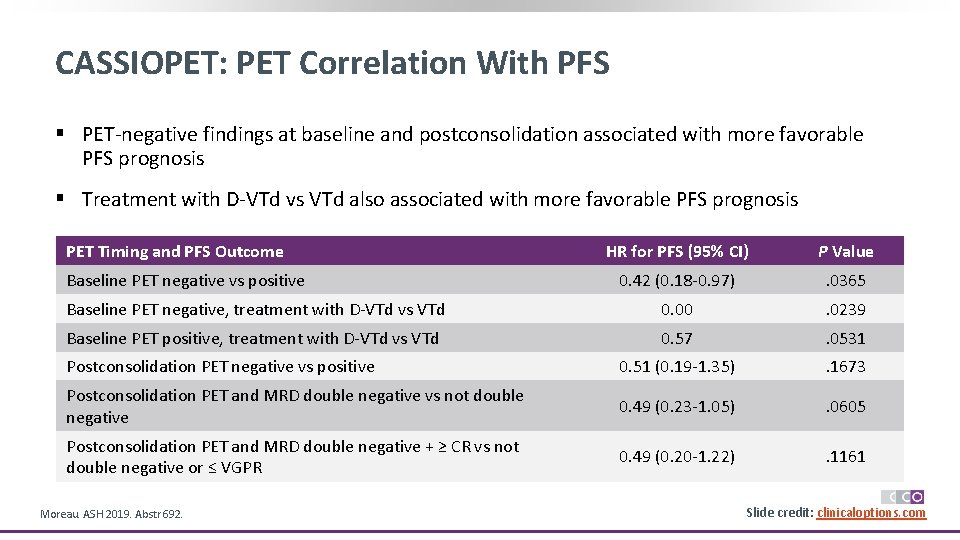
CASSIOPET: PET/CT and MRD Postconsolidation Concordance and Double Negativity § Low concordance between PET/CT and MRD PET/CT MRD Parameter Positive Negative Kappa Coefficient (SE) MRD, n § Positive § Negative 7 12 55 102 0. 0091 (0. 0587) MRD + ≥ CR, n § Positive or ≤ VGPR § Negative + ≥ CR 13 6 97 60 0. 0214 (0. 0368) § More patients receiving D-VTd vs VTd achieved PET/CT and MRD double negativity postconsolidation Double-Negative Rate, n (%) D-VTd (n = 96) VTd (n = 80) OR (95% CI) P Value PET/CT and MRD 64 (66. 7) 38 (47. 5) 2. 21 (1. 20 -4. 07) . 0105 PET/CT and MRD + ≥ CR 40 (41. 7) 20 (25. 0) 2. 14 (1. 12 -4. 10) . 0206 Moreau. ASH 2019. Abstr 692. Slide credit: clinicaloptions. com

CASSIOPET: PET Correlation With PFS § PET-negative findings at baseline and postconsolidation associated with more favorable PFS prognosis § Treatment with D-VTd vs VTd also associated with more favorable PFS prognosis PET Timing and PFS Outcome HR for PFS (95% CI) P Value 0. 42 (0. 18 -0. 97) . 0365 Baseline PET negative, treatment with D-VTd vs VTd 0. 00 . 0239 Baseline PET positive, treatment with D-VTd vs VTd 0. 57 . 0531 Postconsolidation PET negative vs positive 0. 51 (0. 19 -1. 35) . 1673 Postconsolidation PET and MRD double negative vs not double negative 0. 49 (0. 23 -1. 05) . 0605 Postconsolidation PET and MRD double negative + ≥ CR vs not double negative or ≤ VGPR 0. 49 (0. 20 -1. 22) . 1161 Baseline PET negative vs positive Moreau. ASH 2019. Abstr 692. Slide credit: clinicaloptions. com

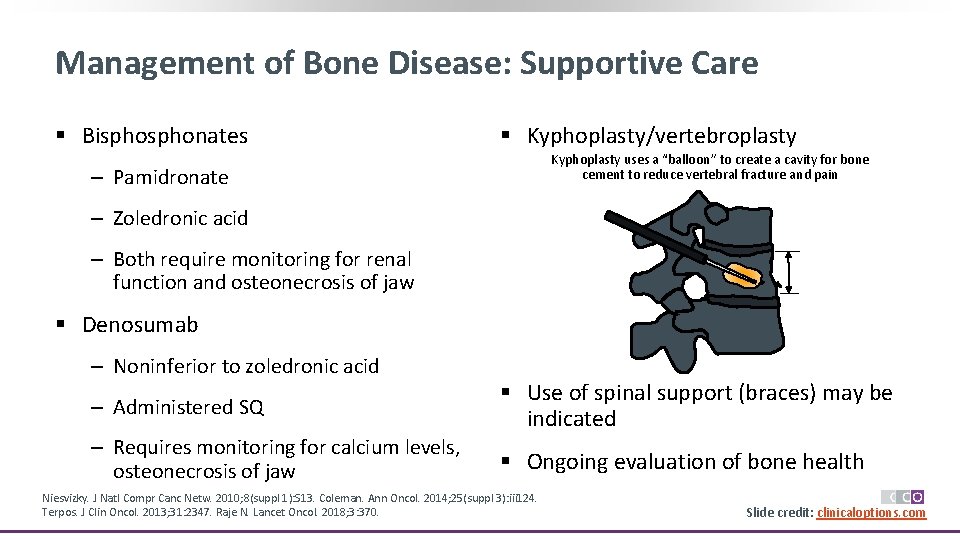
Management of Bone Disease: Supportive Care § Bisphonates § Kyphoplasty/vertebroplasty Kyphoplasty uses a “balloon” to create a cavity for bone cement to reduce vertebral fracture and pain ‒ Pamidronate ‒ Zoledronic acid ‒ Both require monitoring for renal function and osteonecrosis of jaw § Denosumab ‒ Noninferior to zoledronic acid ‒ Administered SQ § Use of spinal support (braces) may be indicated ‒ Requires monitoring for calcium levels, osteonecrosis of jaw § Ongoing evaluation of bone health Niesvizky. J Natl Compr Canc Netw. 2010; 8(suppl 1): S 13. Coleman. Ann Oncol. 2014; 25(suppl 3): iii 124. Terpos. J Clin Oncol. 2013; 31: 2347. Raje N. Lancet Oncol. 2018; 3: 370. Slide credit: clinicaloptions. com
![Myelosuppression and Infection Myeloma and some treatment regimens associated with myelosuppression1 Increased Myelosuppression and Infection § Myeloma and some treatment regimens associated with myelosuppression[1] ‒ Increased](https://slidetodoc.com/presentation_image_h2/5bca495cea78cf5c91f89a5d60480e4b/image-39.jpg)
Myelosuppression and Infection § Myeloma and some treatment regimens associated with myelosuppression[1] ‒ Increased risk of infection, potential for decreased Qo. L and treatment interruption ‒ Dose modification guidelines available in package inserts § Infection prophylaxis[2] ‒ Patients should remain up to date on appropriate vaccinations ‒ VZV prophylaxis when receiving PIs or daratumumab ‒ IVIG/prophylactic antibiotics controversial; use only when warranted ‒ Patient education emphasizing importance of alerting treating clinicians of potential infection can reduce unnecessary antibiotics 1. Miceli. Clin J Oncol Nurs. 2008; 12(3 suppl): 13. 2. Delforge. Blood. 2017; 129: 2359. Slide credit: clinicaloptions. com

Use of Vaccinations for Patients With Myeloma § Inactivated vaccines are safe for patients with myeloma § All patients with myeloma should receive annual flu vaccine § Consider pneumococcal vaccine every 5 yrs § Vaccines post ASCT 4 -6 Mos After Flu 6 -12 Mos After Polio Diphtheria-tetanus-pertussis Pneumococcal (PCV 13, then PPV 23 2 -3 mos later) ‒ Pneumococcal conjugate vaccine 13 (PCV 13) H influenzae type B (Hib) ‒ Then pneumococcal polysaccharide vaccine 23 (PPV 23) 2 -3 mos later Meningococcal group B Meningococcal group C (often Hib/Men. C combined, then Men. ACWY 1 mo later) Slide credit: clinicaloptions. com

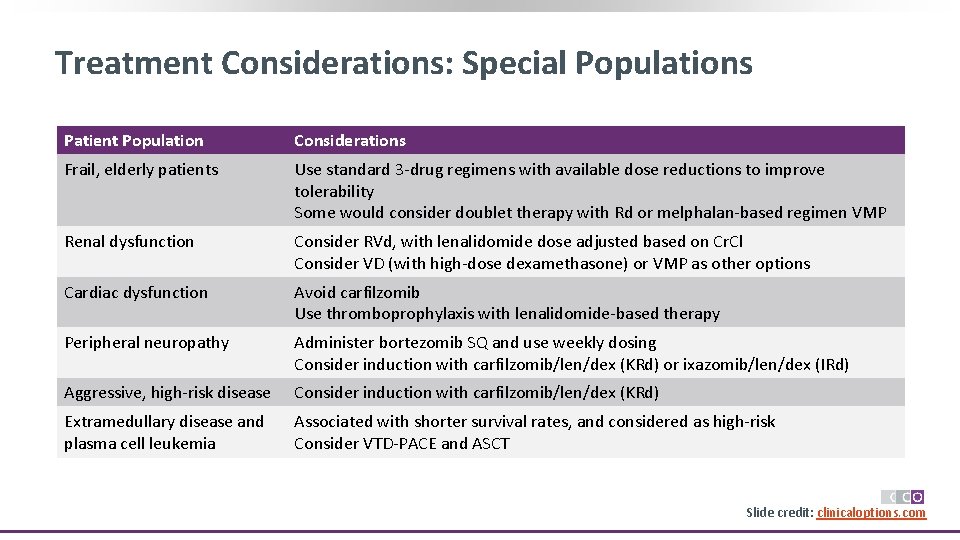
Treatment Considerations: Special Populations Patient Population Considerations Frail, elderly patients Use standard 3 -drug regimens with available dose reductions to improve tolerability Some would consider doublet therapy with Rd or melphalan-based regimen VMP Renal dysfunction Consider RVd, with lenalidomide dose adjusted based on Cr. Cl Consider VD (with high-dose dexamethasone) or VMP as other options Cardiac dysfunction Avoid carfilzomib Use thromboprophylaxis with lenalidomide-based therapy Peripheral neuropathy Administer bortezomib SQ and use weekly dosing Consider induction with carfilzomib/len/dex (KRd) or ixazomib/len/dex (IRd) Aggressive, high-risk disease Consider induction with carfilzomib/len/dex (KRd) Extramedullary disease and plasma cell leukemia Associated with shorter survival rates, and considered as high-risk Consider VTD-PACE and ASCT Slide credit: clinicaloptions. com

Summary: Newly Diagnosed Active MM § Standard induction therapy for patients with newly diagnosed active MM undergoing ASCT: PI/IMi. D/dexamethasone ± daratumumab ‒ Recommend VRd ± daratumumab followed by ASCT and R maintenance therapy (± daratumumab) for patients with standard-risk cytogenetics ‒ Consider KRd followed by ASCT and IMi. D/PI-based maintenance therapy for patients with high-risk cytogenetics § Standard therapy for patients with newly diagnosed active MM who are not eligible for ASCT: consider Dara-Rd or VRd-Lite (or KRd for patients who can tolerate it) § Treatment selection and supportive care should be individualized based on patient comorbidities/fitness and disease characteristics

Relapsed/Refractory Myeloma

Patient Case 3: Early Relapsed Myeloma § A 52 -year-old man was diagnosed with standard-risk MM § He was treated with VRd x 4 cycles followed by ASCT and started on lenalidomide maintenance § The patient relapsed within 1 -yr of his transplant while receiving lenalidomide maintenance therapy Patient has early relapse after ASCT and len maintenance Consider treatment with Dara. Pd, Dara. Kd, CAR-T trial, etc.

Enter your patient case online: clinicaloptions. com/Myeloma. Tool

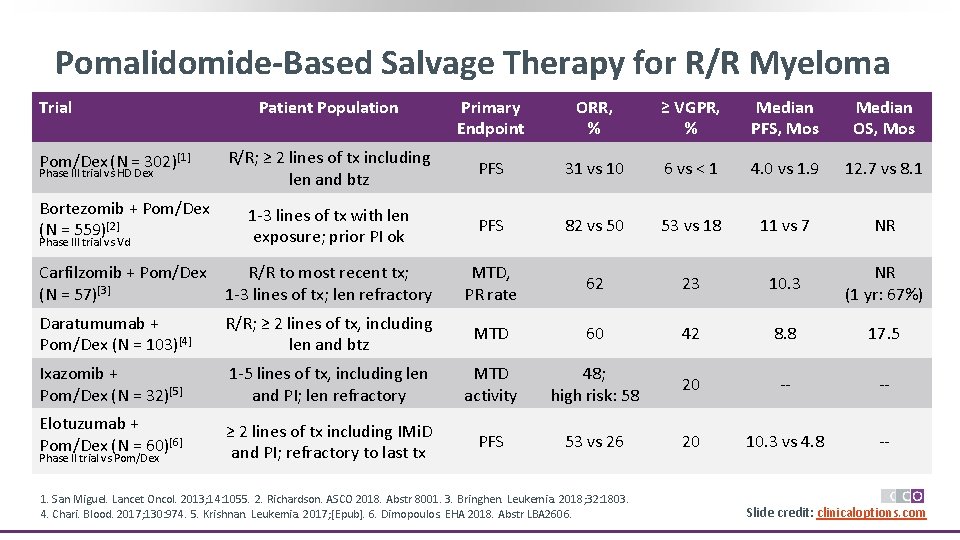
Pomalidomide-Based Salvage Therapy for R/R Myeloma Primary Endpoint ORR, % ≥ VGPR, % Median PFS, Mos Median OS, Mos R/R; ≥ 2 lines of tx including len and btz PFS 31 vs 10 6 vs < 1 4. 0 vs 1. 9 12. 7 vs 8. 1 Bortezomib + Pom/Dex (N = 559)[2] 1 -3 lines of tx with len exposure; prior PI ok PFS 82 vs 50 53 vs 18 11 vs 7 NR Carfilzomib + Pom/Dex (N = 57)[3] R/R to most recent tx; 1 -3 lines of tx; len refractory MTD, PR rate 62 23 10. 3 NR (1 yr: 67%) Daratumumab + Pom/Dex (N = 103)[4] R/R; ≥ 2 lines of tx, including len and btz MTD 60 42 8. 8 17. 5 Ixazomib + Pom/Dex (N = 32)[5] 1 -5 lines of tx, including len and PI; len refractory MTD activity 48; high risk: 58 20 -- -- Elotuzumab + Pom/Dex (N = 60)[6] ≥ 2 lines of tx including IMi. D and PI; refractory to last tx PFS 53 vs 26 20 10. 3 vs 4. 8 -- Trial Pom/Dex (N = 302)[1] Phase III trial vs HD Dex Phase III trial vs Vd Phase II trial vs Pom/Dex Patient Population 1. San Miguel. Lancet Oncol. 2013; 14: 1055. 2. Richardson. ASCO 2018. Abstr 8001. 3. Bringhen. Leukemia. 2018; 32: 1803. 4. Chari. Blood. 2017; 130: 974. 5. Krishnan. Leukemia. 2017; [Epub]. 6. Dimopoulos. EHA 2018. Abstr LBA 2606. Slide credit: clinicaloptions. com

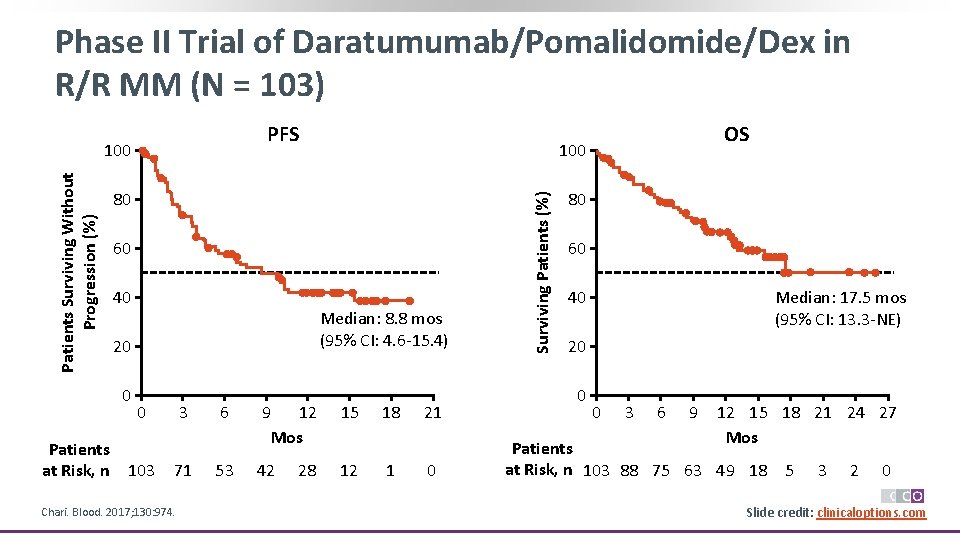
Phase II Trial of Daratumumab/Pomalidomide/Dex in R/R MM (N = 103) PFS 80 60 40 Median: 8. 8 mos (95% CI: 4. 6 -15. 4) 20 0 Patients at Risk, n 100 0 103 3 71 Chari. Blood. 2017; 130: 974. 6 53 9 12 Mos 15 42 12 28 18 1 21 0 Surviving Patients (%) Patients Surviving Without Progression (%) 100 OS 80 60 40 Median: 17. 5 mos (95% CI: 13. 3 -NE) 20 0 0 3 6 9 12 15 18 21 24 27 Mos Patients at Risk, n 103 88 75 63 49 18 5 3 2 0 Slide credit: clinicaloptions. com

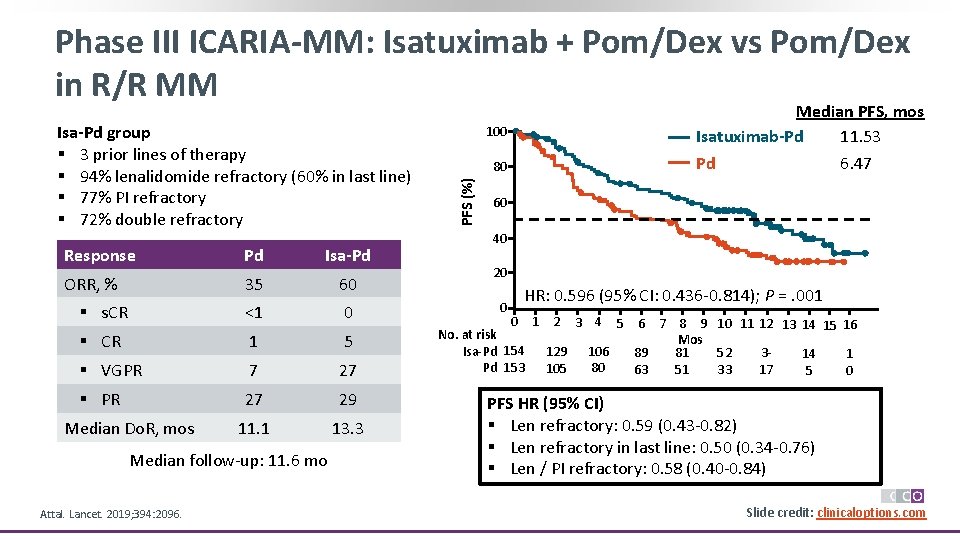
Phase III ICARIA-MM: Isatuximab + Pom/Dex vs Pom/Dex in R/R MM Response Pd Isa-Pd ORR, % 35 60 § s. CR <1 0 § CR 1 5 § VGPR 7 27 § PR 27 29 11. 1 13. 3 Median Do. R, mos Median follow-up: 11. 6 mo Attal. Lancet. 2019; 394: 2096. 100 80 PFS (%) Isa-Pd group § 3 prior lines of therapy § 94% lenalidomide refractory (60% in last line) § 77% PI refractory § 72% double refractory Median PFS, mos Isatuximab-Pd 11. 53 Pd 6. 47 60 40 20 0 HR: 0. 596 (95% CI: 0. 436 -0. 814); P =. 001 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 No. at risk Mos Isa-Pd 154 129 106 89 81 352 14 1 80 Pd 153 105 63 51 17 33 5 0 PFS HR (95% CI) § Len refractory: 0. 59 (0. 43 -0. 82) § Len refractory in last line: 0. 50 (0. 34 -0. 76) § Len / PI refractory: 0. 58 (0. 40 -0. 84) Slide credit: clinicaloptions. com

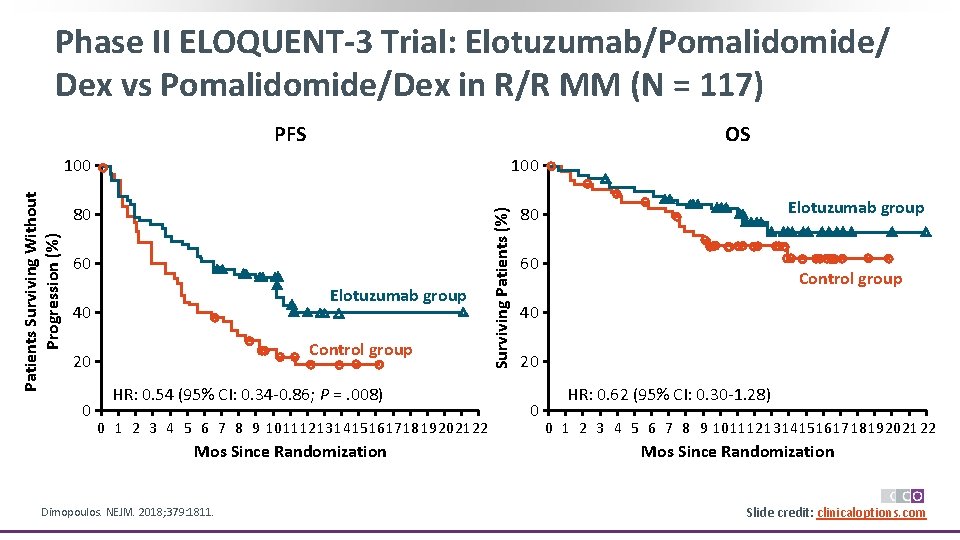
Phase II ELOQUENT-3 Trial: Elotuzumab/Pomalidomide/ Dex vs Pomalidomide/Dex in R/R MM (N = 117) OS 100 80 80 60 Elotuzumab group 40 Control group 20 0 HR: 0. 54 (95% CI: 0. 34 -0. 86; P =. 008) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 1415 1617 18 19 2021 22 Mos Since Randomization Dimopoulos. NEJM. 2018; 379: 1811. Surviving Patients (%) Patients Surviving Without Progression (%) PFS Elotuzumab group 60 Control group 40 20 0 HR: 0. 62 (95% CI: 0. 30 -1. 28) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 1415 1617 18 19 2021 22 Mos Since Randomization Slide credit: clinicaloptions. com

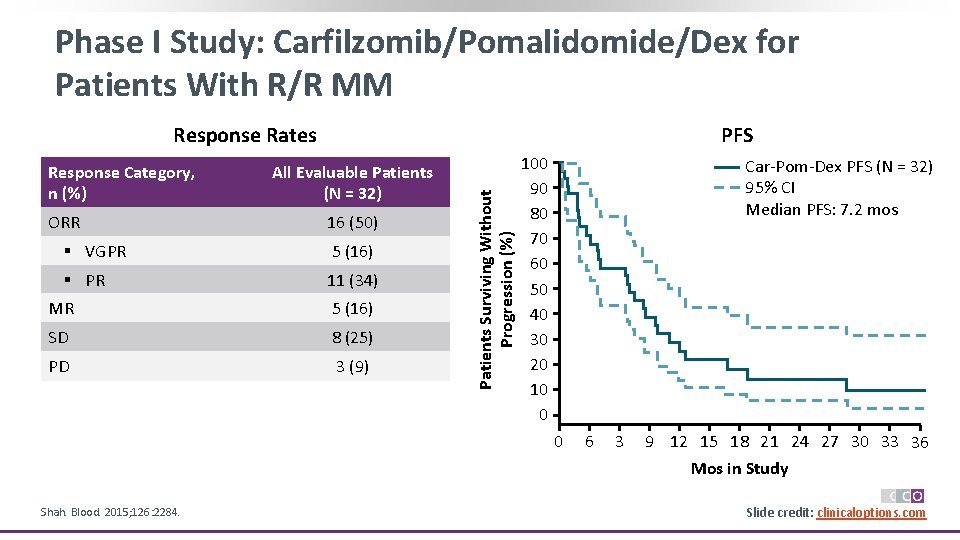
Phase I Study: Carfilzomib/Pomalidomide/Dex for Patients With R/R MM Response Category, n (%) ORR PFS All Evaluable Patients (N = 32) 16 (50) § VGPR 5 (16) § PR 11 (34) MR 5 (16) SD 8 (25) PD 3 (9) Patients Surviving Without Progression (%) Response Rates 100 90 80 70 60 50 40 30 20 10 0 Car-Pom-Dex PFS (N = 32) 95% CI Median PFS: 7. 2 mos 0 Shah. Blood. 2015; 126: 2284. 6 3 9 12 15 18 21 24 27 30 33 36 Mos in Study Slide credit: clinicaloptions. com

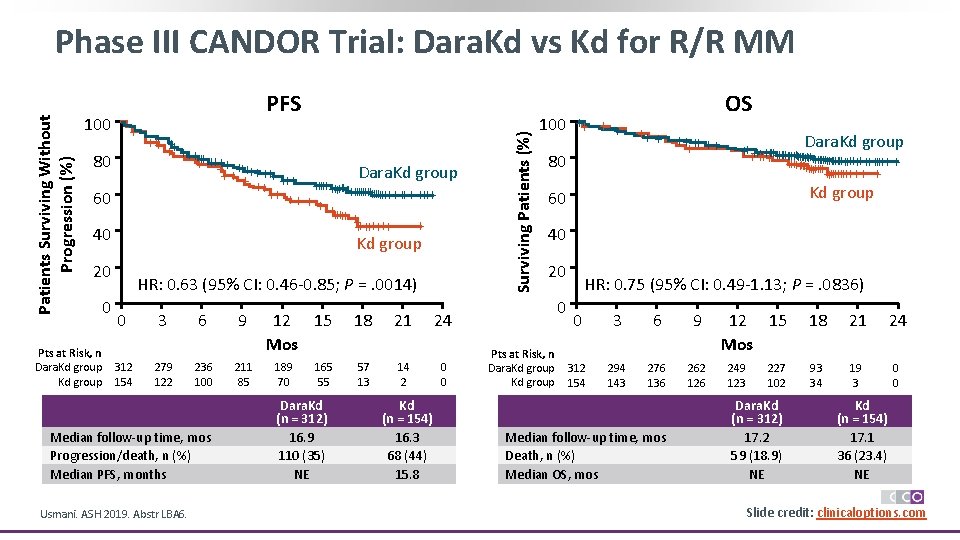
PFS 100 + + +++ ++++ Dara. Kd group ++ + ++++ ++++++ ++ ++ + + +++++++ + ++++++ + Kd group 80 60 40 20 Pts at Risk, n Dara. Kd group 0 HR: 0. 63 (95% CI: 0. 46 -0. 85; P =. 0014) 0 3 6 9 312 154 279 122 236 100 211 85 Median follow-up time, mos Progression/death, n (%) Median PFS, months Usmani. ASH 2019. Abstr LBA 6. 12 15 Mos 189 70 165 55 Dara. Kd (n = 312) 16. 9 110 (35) NE 18 21 24 57 13 14 2 0 0 Kd (n = 154) 16. 3 68 (44) 15. 8 Surviving Patients (%) Patients Surviving Without Progression (%) Phase III CANDOR Trial: Dara. Kd vs Kd for R/R MM 100 ++ + + ++++ ++ + 80 60 OS Dara. Kd group +++++++ + + +++ +++++++++ ++++ + ++++++++ + Kd group 40 20 0 Pts at Risk, n Dara. Kd group HR: 0. 75 (95% CI: 0. 49 -1. 13; P =. 0836) 0 3 6 9 312 154 294 143 276 136 262 126 Median follow-up time, mos Death, n (%) Median OS, mos 12 15 Mos 249 123 227 102 Dara. Kd (n = 312) 17. 2 59 (18. 9) NE 18 21 24 93 34 19 3 0 0 Kd (n = 154) 17. 1 36 (23. 4) NE Slide credit: clinicaloptions. com

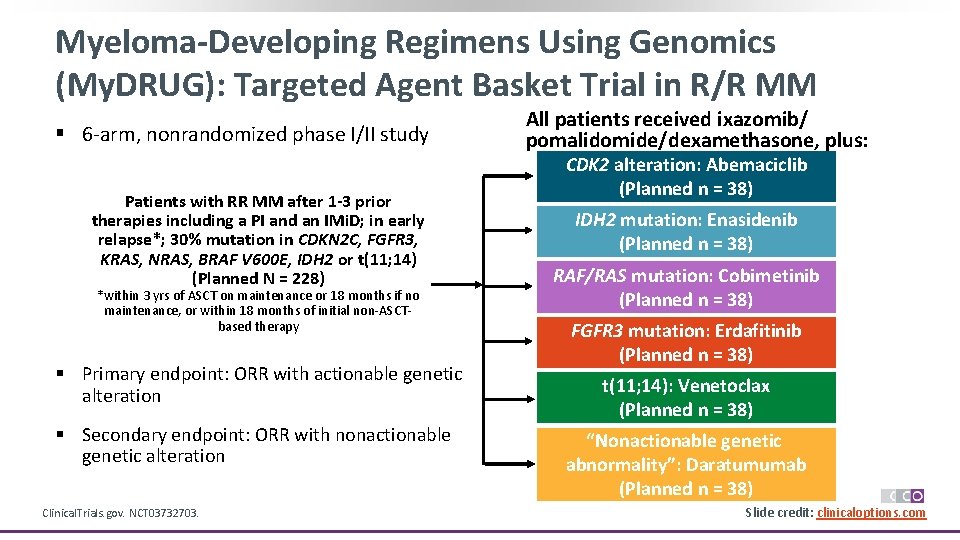
Myeloma-Developing Regimens Using Genomics (My. DRUG): Targeted Agent Basket Trial in R/R MM § 6 -arm, nonrandomized phase I/II study Patients with RR MM after 1 -3 prior Pts with PSR serous therapies including a PI and an IMi. D; in early OC and germline relapse*; 30% mutation in CDKN 2 C, FGFR 3, BRCA 1/2 mutation, 2 KRAS, NRAS, BRAF≥V 600 E, IDH 2 or t(11; 14) prior lines of platinum(Planned N = 228) based therapy, CR *within 3 yrs of ASCT on or maintenance or 18 months if no maintenance, or within PR on most recent 18 months of initial non-ASCTtherapy based therapy (N = 295) § Primary endpoint: ORR with actionable genetic alteration § Secondary endpoint: ORR with nonactionable genetic alteration Clinical. Trials. gov. NCT 03732703. All patients received ixazomib/ pomalidomide/dexamethasone, plus: CDK 2 alteration: Abemaciclib (Planned n = 38) IDH 2 mutation: Enasidenib (Planned n = 38) RAF/RAS mutation: Cobimetinib (Planned n = 38) FGFR 3 mutation: Erdafitinib (Planned n = 38) *n = 195 received treatment. t(11; 14): Venetoclax (Planned n = 38) “Nonactionable genetic abnormality”: Daratumumab (Planned n = 38) Slide credit: clinicaloptions. com

Patient Case 4: Heavily Pretreated R/R Myeloma § A 71 -yr-old man with history of R/R MM; Prior lines of treatment include: 1. VRd x 4 cycles, Mel 200 mg/m 2 followed by ASCT and Len maintenance 2. Carfilzomib/pomalidomide/dex upon first relapse 3. Venetoclax/dex upon progression (due to t(11; 14) on bone marrow) 4. Daratumumab/pomalidomide/dex upon progression 5. Carfilzomib/cyclophosphamide/dex (carfilzomib changed to ixazomib due to cardiac complications) § Now presents with progression of disease Treatment options include selinexor, alkylator-based therapies, or clinical trials

Enter your patient case online: clinicaloptions. com/Myeloma. Tool

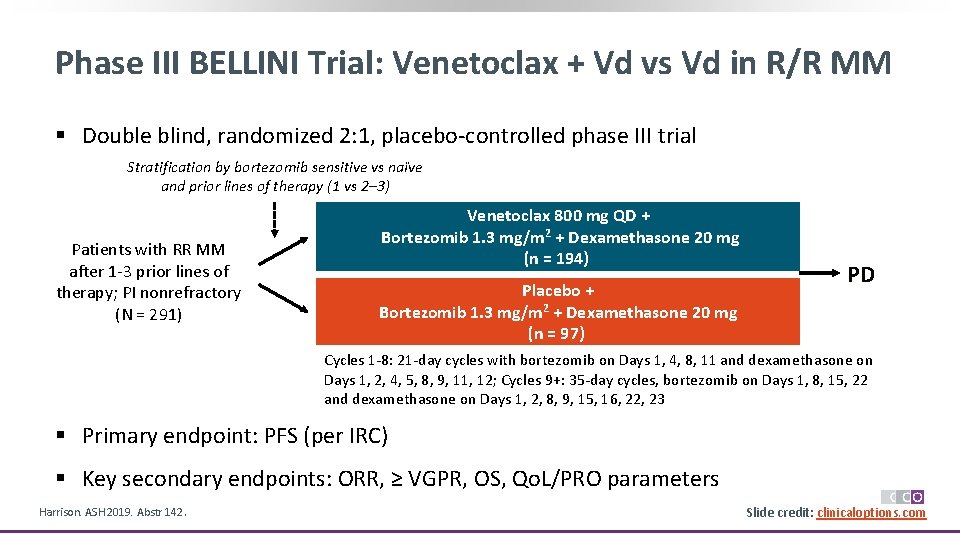
Phase III BELLINI Trial: Venetoclax + Vd vs Vd in R/R MM § Double blind, randomized 2: 1, placebo-controlled phase III trial Stratification by bortezomib sensitive vs naïve and prior lines of therapy (1 vs 2– 3) Patients with RR MM after 1 -3 prior lines of therapy; PI nonrefractory (N = 291) Venetoclax 800 mg QD + Bortezomib 1. 3 mg/m 2 + Dexamethasone 20 mg (n = 194) Placebo + Bortezomib 1. 3 mg/m 2 + Dexamethasone 20 mg (n = 97) PD Cycles 1 -8: 21 -day cycles with bortezomib on Days 1, 4, 8, 11 and dexamethasone on Days 1, 2, 4, 5, 8, 9, 11, 12; Cycles 9+: 35 -day cycles, bortezomib on Days 1, 8, 15, 22 and dexamethasone on Days 1, 2, 8, 9, 15, 16, 22, 23 § Primary endpoint: PFS (per IRC) § Key secondary endpoints: ORR, ≥ VGPR, OS, Qo. L/PRO parameters Harrison. ASH 2019. Abstr 142. Slide credit: clinicaloptions. com

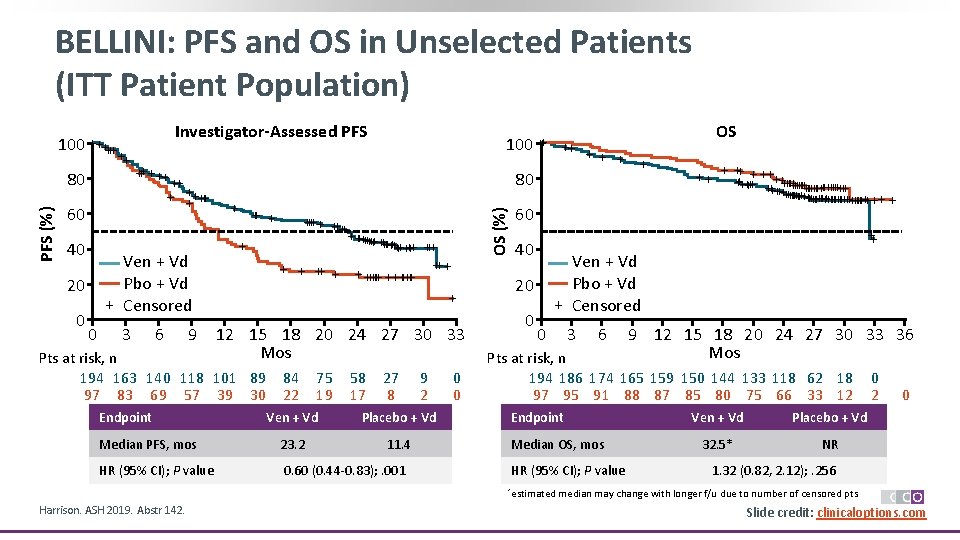
BELLINI: PFS and OS in Unselected Patients (ITT Patient Population) PFS (%) 80 Investigator-Assessed PFS ++ +++++ 60 40 Ven + Vd Pbo + Vd + Censored 20 0 0 100 ++ 3 6 9 + + +++ +++++++ +++ ++ + + +++++++ + 12 15 18 20 24 27 30 33 Mos Pts at risk, n 194 163 140 118 101 89 97 83 69 57 39 30 Endpoint ++ + 80 OS (%) 100 +++++ + 84 22 75 19 58 17 27 8 9 2 Ven + Vd Placebo + Vd Median PFS, mos 23. 2 11. 4 HR (95% CI); P value 0. 60 (0. 44 -0. 83); . 001 0 0 + 60 40 + ++ +++++++++++++ ++++++++++++++ + + Ven + Vd Pbo + Vd + Censored 20 0 ++ ++ 0 3 6 9 12 15 18 20 24 27 30 33 36 Mos Pts at risk, n 194 186 174 165 159 150 144 133 118 62 18 97 95 91 88 87 85 80 75 66 33 12 Endpoint Median OS, mos HR (95% CI); P value *estimated Harrison. ASH 2019. Abstr 142. OS Ven + Vd Placebo + Vd 32. 5* NR 0 2 0 1. 32 (0. 82, 2. 12); . 256 median may change with longer f/u due to number of censored pts. Slide credit: clinicaloptions. com

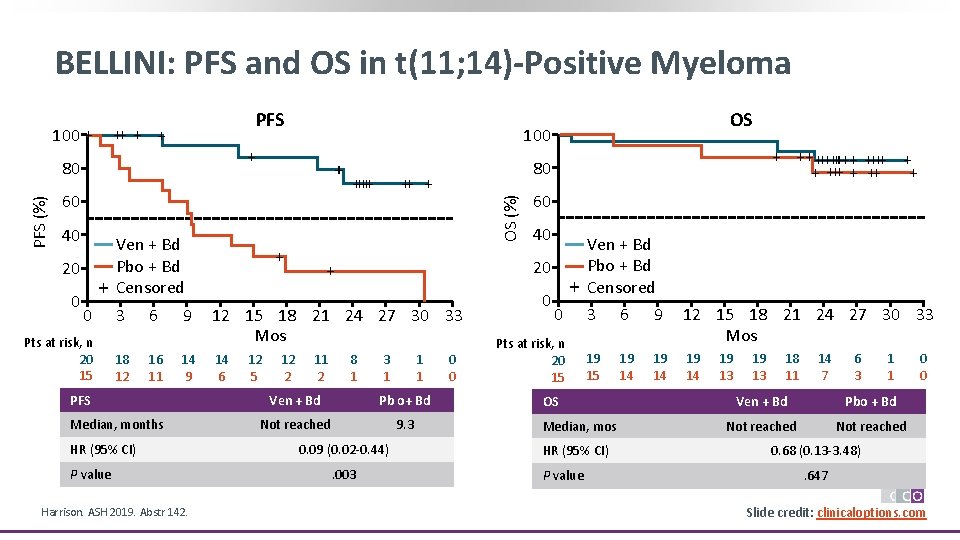
BELLINI: PFS and OS in t(11; 14)-Positive Myeloma 100 + PFS ++ + 60 40 20 0 + 0 Pts at risk, n 20 15 Ven + Bd Pbo + Bd Censored 3 18 12 6 16 11 9 14 9 PFS Median, months HR (95% CI) P value Harrison. ASH 2019. Abstr 142. +++++ + 12 5 12 2 11 2 60 40 20 + 12 15 18 21 24 27 30 33 Mos 14 6 + +++++ + ++ + 80 OS (%) PFS (%) 100 + 80 OS 8 1 3 1 1 1 Ven + Bd Pb o+ Bd Not reached 9. 3 0. 09 (0. 02 -0. 44). 003 0 0 0 + 0 Pts at risk, n 20 15 Ven + Bd Pbo + Bd Censored 3 6 9 12 15 18 21 24 27 30 33 Mos 19 15 19 14 OS Median, mos HR (95% CI) P value 19 13 18 11 14 7 6 3 1 1 Ven + Bd Pbo + Bd Not reached 0 0 0. 68 (0. 13 -3. 48). 647 Slide credit: clinicaloptions. com

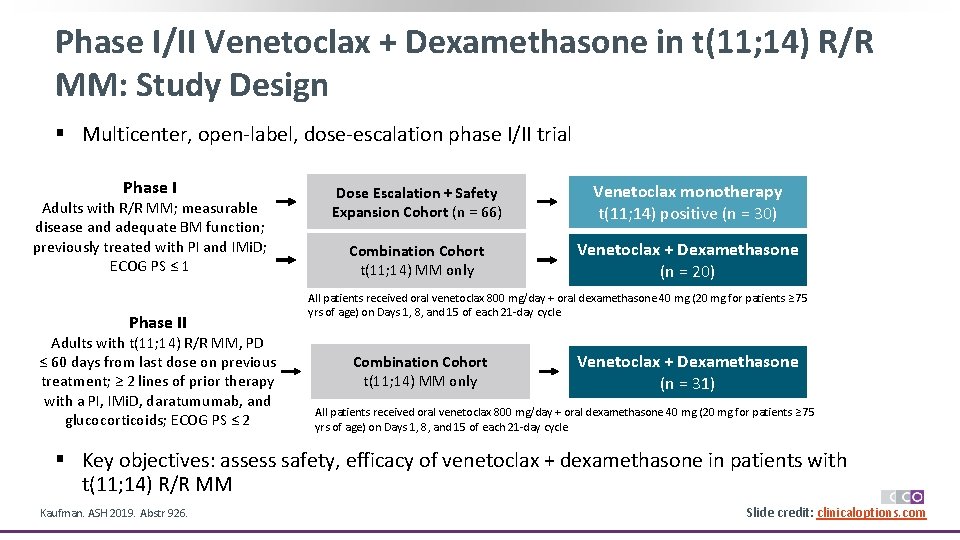
Phase I/II Venetoclax + Dexamethasone in t(11; 14) R/R MM: Study Design § Multicenter, open-label, dose-escalation phase I/II trial Phase I Adults with R/R MM; measurable disease and adequate BM function; previously treated with PI and IMi. D; ECOG PS ≤ 1 Phase II Adults with t(11; 14) R/R MM, PD ≤ 60 days from last dose on previous treatment; ≥ 2 lines of prior therapy with a PI, IMi. D, daratumumab, and glucocorticoids; ECOG PS ≤ 2 Dose Escalation + Safety Expansion Cohort (n = 66) Venetoclax monotherapy t(11; 14) positive (n = 30) Combination Cohort t(11; 14) MM only Venetoclax + Dexamethasone (n = 20) All patients received oral venetoclax 800 mg/day + oral dexamethasone 40 mg (20 mg for patients ≥ 75 yrs of age) on Days 1, 8, and 15 of each 21 -day cycle Combination Cohort t(11; 14) MM only Venetoclax + Dexamethasone (n = 31) All patients received oral venetoclax 800 mg/day + oral dexamethasone 40 mg (20 mg for patients ≥ 75 yrs of age) on Days 1, 8, and 15 of each 21 -day cycle § Key objectives: assess safety, efficacy of venetoclax + dexamethasone in patients with t(11; 14) R/R MM Kaufman. ASH 2019. Abstr 926. Slide credit: clinicaloptions. com

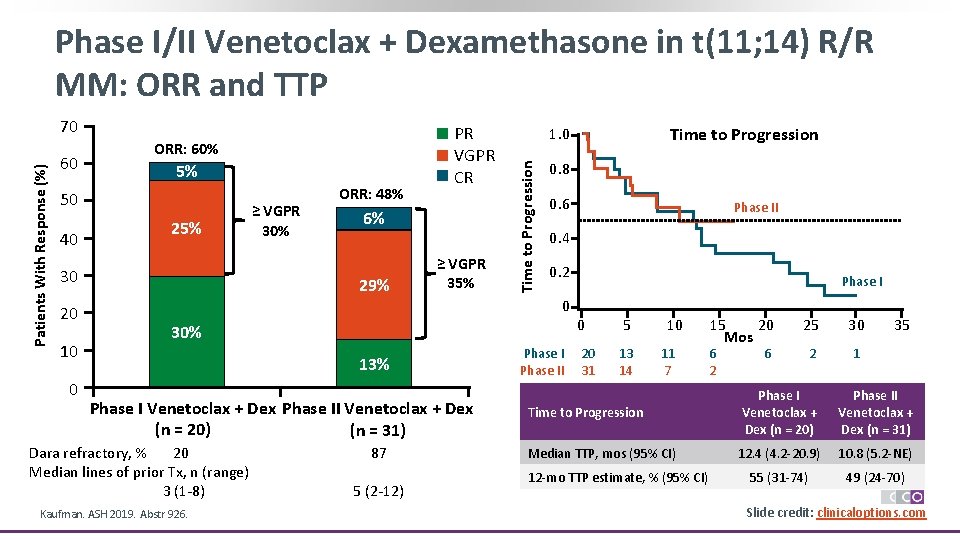
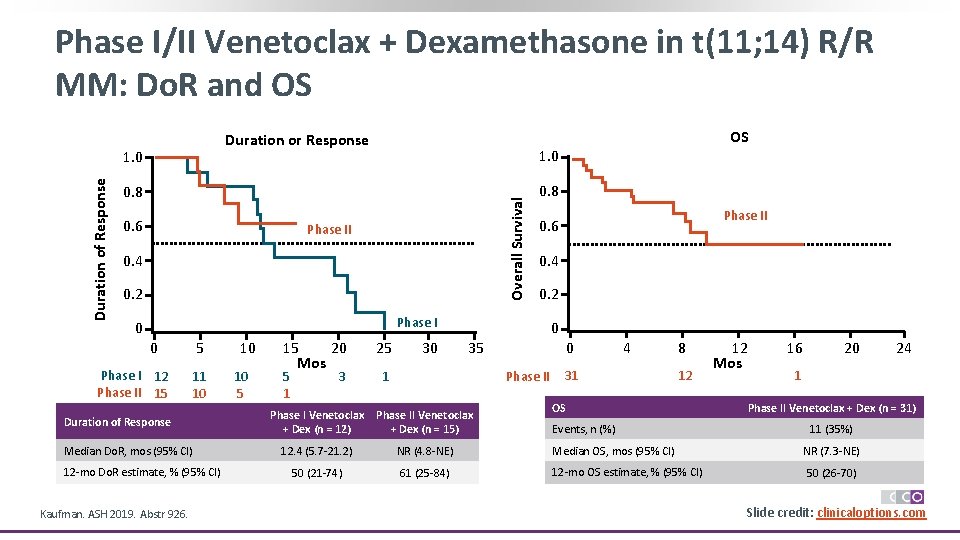
Phase I/II Venetoclax + Dexamethasone in t(11; 14) R/R MM: ORR and TTP 60 ORR: 60% 5% 50 40 25% 30 20 10 0 ≥ VGPR 30% ORR: 48% PR VGPR CR 6% 29% ≥ VGPR 35% 0. 8 0. 6 0. 2 Phase I 0 0 13% Phase I Venetoclax + Dex Phase II Venetoclax + Dex (n = 20) (n = 31) Kaufman. ASH 2019. Abstr 926. Phase II 0. 4 30% Dara refractory, % 20 Median lines of prior Tx, n (range) 3 (1 -8) Time to Progression 1. 0 Time to Progression Patients With Response (%) 70 87 5 (2 -12) Phase II 20 31 5 13 14 10 11 7 15 20 Mos 6 2 25 30 2 1 6 35 Time to Progression Phase I Venetoclax + Dex (n = 20) Phase II Venetoclax + Dex (n = 31) Median TTP, mos (95% CI) 12. 4 (4. 2 -20. 9) 10. 8 (5. 2 -NE) 55 (31 -74) 49 (24 -70) 12 -mo TTP estimate, % (95% CI) Slide credit: clinicaloptions. com

Phase I/II Venetoclax + Dexamethasone in t(11; 14) R/R MM: Do. R and OS Duration or Response 1. 0 0. 8 Overall Survival Duration of Response 1. 0 Phase 2 0. 6 Phase II 0. 4 0. 2 0. 8 Phase I 12 Phase II 15 5 11 10 Duration of Response Median Do. R, mos (95% CI) 12 -mo Do. R estimate, % (95% CI) Kaufman. ASH 2019. Abstr 926. 10 10 5 15 20 Mos 5 1 3 25 30 Phase II Phase 2 0. 6 0. 4 0. 2 Phase I 0 0 OS 0 35 1 0 Phase II 4 31 8 12 OS 12 Mos 16 20 1 Phase II Venetoclax + Dex (n = 31) Phase I Venetoclax + Dex (n = 12) Phase II Venetoclax + Dex (n = 15) 12. 4 (5. 7 -21. 2) NR (4. 8 -NE) Median OS, mos (95% CI) NR (7. 3 -NE) 50 (21 -74) 61 (25 -84) 12 -mo OS estimate, % (95% CI) 50 (26 -70) Events, n (%) 24 11 (35%) Slide credit: clinicaloptions. com

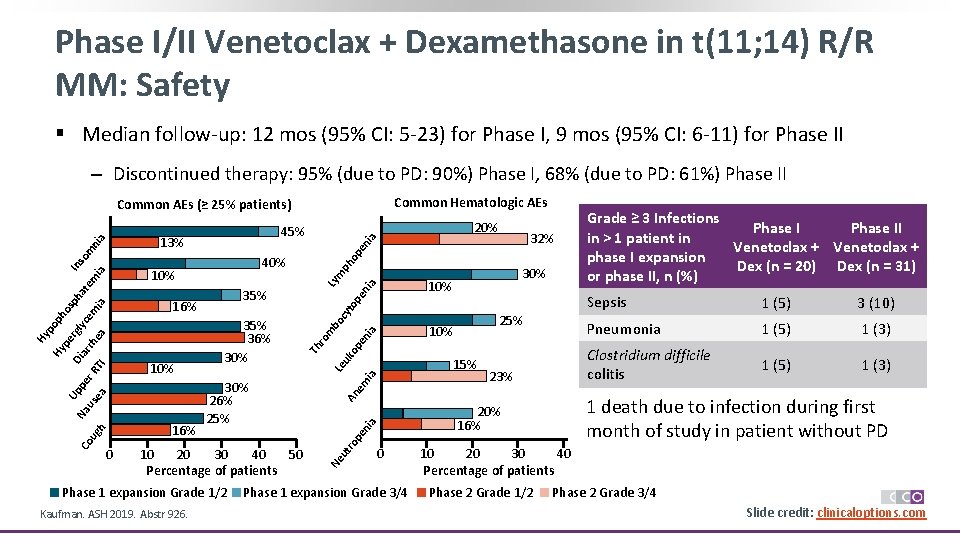
Phase I/II Venetoclax + Dexamethasone in t(11; 14) R/R MM: Safety § Median follow-up: 12 mos (95% CI: 5 -23) for Phase I, 9 mos (95% CI: 6 -11) for Phase II ‒ Discontinued therapy: 95% (due to PD: 90%) Phase I, 68% (due to PD: 61%) Phase II Common Hematologic AEs Common AEs (≥ 25% patients) ia en op m to pe ni a Ly ia uk op en m bo cy 25% 10% An em ia Le 23% a ni 0 Phase 1 expansion Grade 3/4 Grade ≥ 3 Infections Phase II in > 1 patient in Venetoclax + phase I expansion Dex (n = 20) Dex (n = 31) or phase II, n (%) Sepsis 1 (5) 3 (10) Pneumonia 1 (5) 1 (3) Clostridium difficile colitis 1 (5) 1 (3) 1 death due to infection during first month of study in patient without PD 20% 16% pe h ug Phase 1 expansion Grade 1/2 Kaufman. ASH 2019. Abstr 926. 30% 15% ut ro 10 20 30 40 50 Percentage of patients Ne ea us 30% 26% 25% ro ea TI r. R pe Na Co 0 35% 36% 30% Th ia em rh ar Di Up 35% 16% lyc rg pe Hy Hy po ph os ph at e m ia In 10% 16% 32% ph 40% so m ni a 13% 10% 20% 45% 10 20 30 40 Percentage of patients Phase 2 Grade 1/2 Phase 2 Grade 3/4 Slide credit: clinicaloptions. com

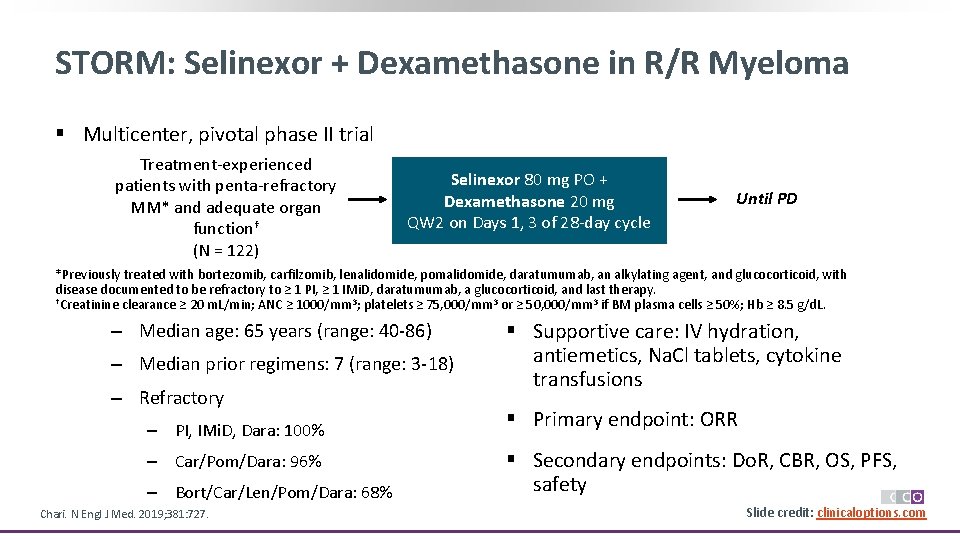
STORM: Selinexor + Dexamethasone in R/R Myeloma § Multicenter, pivotal phase II trial Treatment-experienced patients with penta-refractory MM* and adequate organ function† (N = 122) Selinexor 80 mg PO + Dexamethasone 20 mg QW 2 on Days 1, 3 of 28 -day cycle Until PD *Previously treated with bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, an alkylating agent, and glucocorticoid, with disease documented to be refractory to ≥ 1 PI, ≥ 1 IMi. D, daratumumab, a glucocorticoid, and last therapy. †Creatinine clearance ≥ 20 m. L/min; ANC ≥ 1000/mm 3; platelets ≥ 75, 000/mm 3 or ≥ 50, 000/mm 3 if BM plasma cells ≥ 50%; Hb ≥ 8. 5 g/d. L. ‒ Median age: 65 years (range: 40 -86) ‒ Median prior regimens: 7 (range: 3 -18) ‒ Refractory ‒ PI, IMi. D, Dara: 100% ‒ Car/Pom/Dara: 96% ‒ Bort/Car/Len/Pom/Dara: 68% Chari. N Engl J Med. 2019; 381: 727. § Supportive care: IV hydration, antiemetics, Na. Cl tablets, cytokine transfusions § Primary endpoint: ORR § Secondary endpoints: Do. R, CBR, OS, PFS, safety Slide credit: clinicaloptions. com

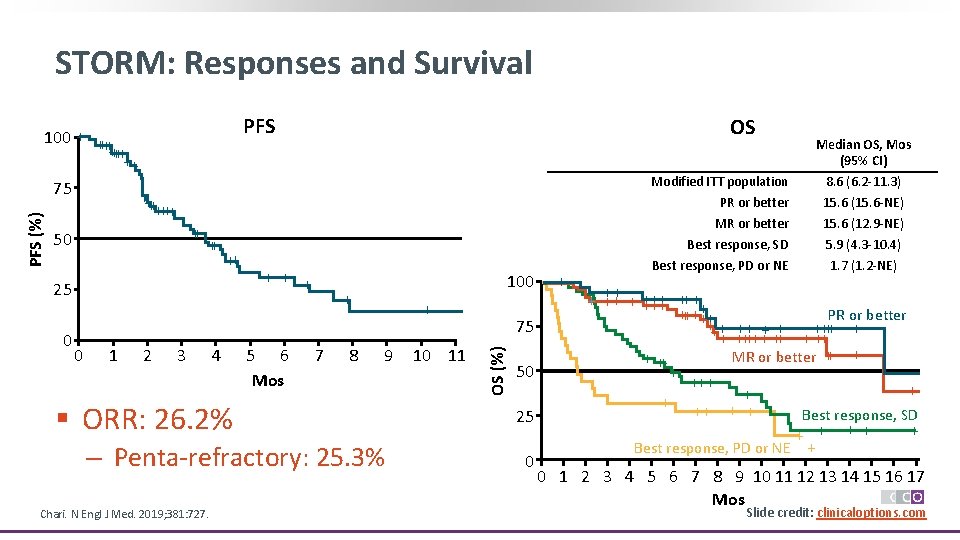
STORM: Responses and Survival PFS ++++++ ++ 50 ++ ++ 25 0 0 1 2 3 4 OS ++ + 5 6 Mos 100 + 7 8 + 9 § ORR: 26. 2% ‒ Penta-refractory: 25. 3% Chari. N Engl J Med. 2019; 381: 727. 10 11 75 OS (%) PFS (%) 100 + ++ ++++++ ++ 75 50 25 0 + Median OS, Mos (95% CI) Modified ITT population PR or better 8. 6 (6. 2 -11. 3) 15. 6 (15. 6 -NE) MR or better Best response, SD Best response, PD or NE 15. 6 (12. 9 -NE) 5. 9 (4. 3 -10. 4) 1. 7 (1. 2 -NE) + ++++ +++ PR or better +++++++ ++ + + ++++ + ++ MR or better ++ + +++ + + ++ + Best response, SD + + + Best response, PD or NE + 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Mos Slide credit: clinicaloptions. com

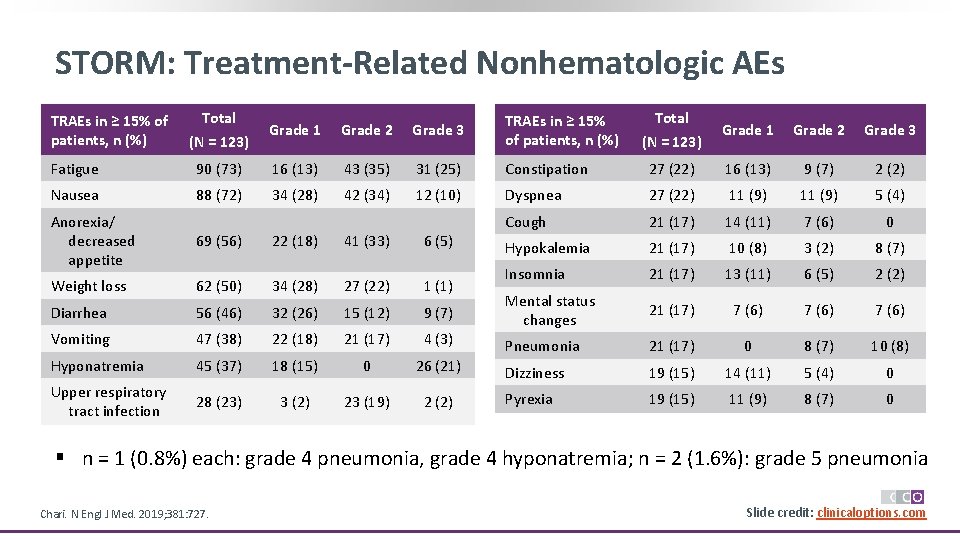
STORM: Treatment-Related Nonhematologic AEs TRAEs in ≥ 15% of patients, n (%) Total (N = 123) Grade 1 Grade 2 Grade 3 Fatigue 90 (73) 16 (13) 43 (35) 31 (25) Constipation 27 (22) 16 (13) 9 (7) 2 (2) Nausea 88 (72) 34 (28) 42 (34) 12 (10) Dyspnea 27 (22) 11 (9) 5 (4) Cough 21 (17) 14 (11) 7 (6) 0 Hypokalemia 21 (17) 10 (8) 3 (2) 8 (7) Insomnia 21 (17) 13 (11) 6 (5) 2 (2) Mental status changes 21 (17) 7 (6) Pneumonia 21 (17) 0 8 (7) 10 (8) Dizziness 19 (15) 14 (11) 5 (4) 0 Pyrexia 19 (15) 11 (9) 8 (7) 0 Anorexia/ decreased appetite 69 (56) 22 (18) 41 (33) 6 (5) Weight loss 62 (50) 34 (28) 27 (22) 1 (1) Diarrhea 56 (46) 32 (26) 15 (12) 9 (7) Vomiting 47 (38) 22 (18) 21 (17) 4 (3) Hyponatremia 45 (37) 18 (15) 0 26 (21) Upper respiratory tract infection 28 (23) 3 (2) 23 (19) 2 (2) § n = 1 (0. 8%) each: grade 4 pneumonia, grade 4 hyponatremia; n = 2 (1. 6%): grade 5 pneumonia Chari. N Engl J Med. 2019; 381: 727. Slide credit: clinicaloptions. com

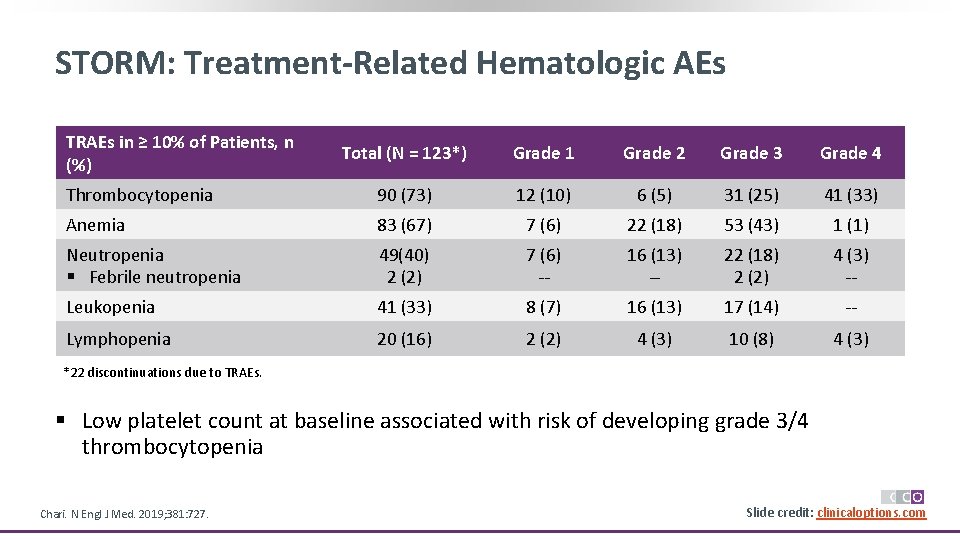
STORM: Treatment-Related Hematologic AEs TRAEs in ≥ 10% of Patients, n (%) Total (N = 123*) Grade 1 Grade 2 Grade 3 Grade 4 Thrombocytopenia 90 (73) 12 (10) 6 (5) 31 (25) 41 (33) Anemia 83 (67) 7 (6) 22 (18) 53 (43) 1 (1) Neutropenia § Febrile neutropenia 49(40) 2 (2) 7 (6) -- 16 (13) -- 22 (18) 2 (2) 4 (3) -- Leukopenia 41 (33) 8 (7) 16 (13) 17 (14) -- Lymphopenia 20 (16) 2 (2) 4 (3) 10 (8) 4 (3) *22 discontinuations due to TRAEs. § Low platelet count at baseline associated with risk of developing grade 3/4 thrombocytopenia Chari. N Engl J Med. 2019; 381: 727. Slide credit: clinicaloptions. com

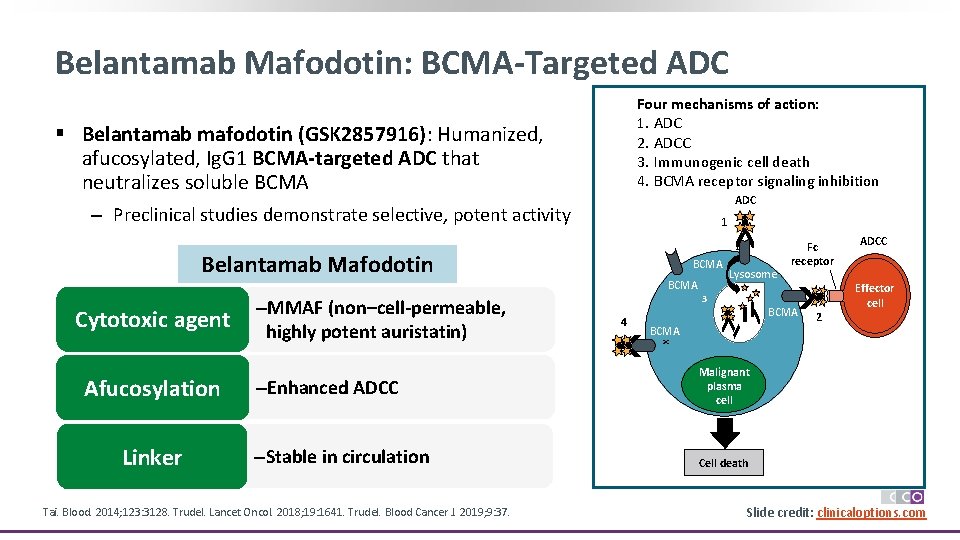
Belantamab Mafodotin: BCMA-Targeted ADC Four mechanisms of action: 1. ADC 2. ADCC 3. Immunogenic cell death 4. BCMA receptor signaling inhibition § Belantamab mafodotin (GSK 2857916): Humanized, afucosylated, Ig. G 1 BCMA-targeted ADC that neutralizes soluble BCMA ADC ‒ Preclinical studies demonstrate selective, potent activity 1 1 Belantamab Mafodotin Afucosylation Linker –MMAF (non–cell-permeable, highly potent auristatin) –Enhanced ADCC –Stable in circulation Tai. Blood. 2014; 123: 3128. Trudel. Lancet Oncol. 2018; 19: 1641. Trudel. Blood Cancer J. 2019; 9: 37. BCMA 4 3 Lysosome 3 BCMA 4 BCMA 2 ADCC Effector cell x Cytotoxic agent BCMA Fc receptor Malignant plasma cell Cell death Slide credit: clinicaloptions. com

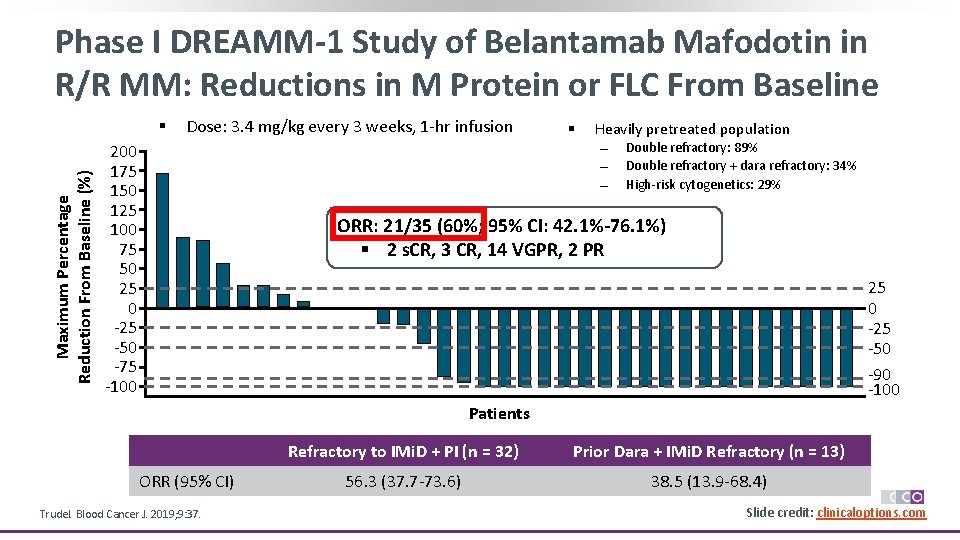
Phase I DREAMM-1 Study of Belantamab Mafodotin in R/R MM: Reductions in M Protein or FLC From Baseline Maximum Percentage Reduction From Baseline (%) § Dose: 3. 4 mg/kg every 3 weeks, 1 -hr infusion § Heavily pretreated population 200 175 150 125 100 75 50 25 0 -25 -50 -75 -100 Double refractory: 89% Double refractory + dara refractory: 34% High-risk cytogenetics: 29% ORR: 21/35 (60%; 95% CI: 42. 1%-76. 1%) § 2 s. CR, 3 CR, 14 VGPR, 2 PR 25 0 -25 -50 -90 -100 Patients ORR (95% CI) Trudel. Blood Cancer J. 2019; 9: 37. Refractory to IMi. D + PI (n = 32) Prior Dara + IMi. D Refractory (n = 13) 56. 3 (37. 7 -73. 6) 38. 5 (13. 9 -68. 4) Slide credit: clinicaloptions. com

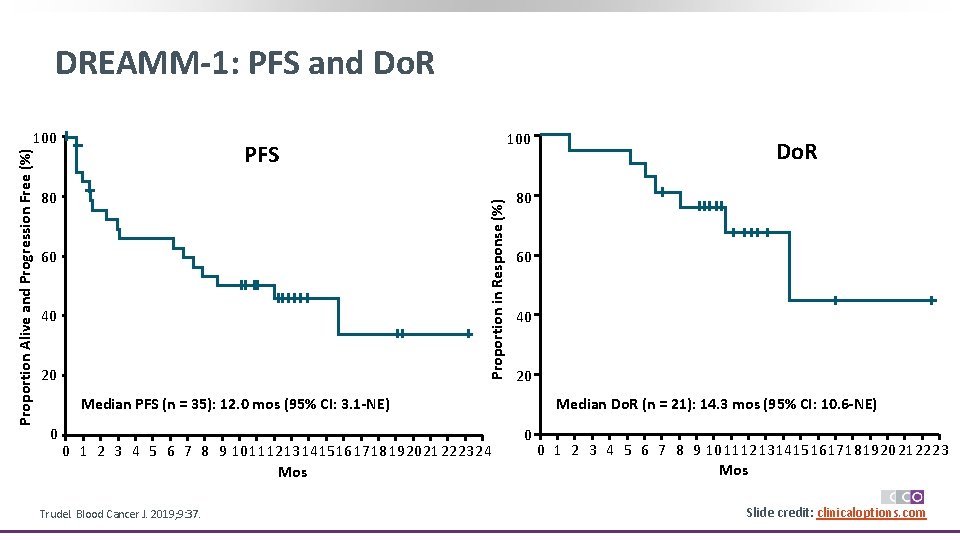
DREAMM-1: PFS and Do. R 100 PFS 80 Proportion in Response (%) Proportion Alive and Progression Free (%) 100 60 40 20 80 60 40 20 Median PFS (n = 35): 12. 0 mos (95% CI: 3. 1 -NE) 0 0 1 2 3 4 5 6 7 8 9 1011121314151617181920212223 24 Mos Trudel. Blood Cancer J. 2019; 9: 37. Do. R Median Do. R (n = 21): 14. 3 mos (95% CI: 10. 6 -NE) 0 0 1 2 3 4 5 6 7 8 9 1011121314151617181920212223 Mos Slide credit: clinicaloptions. com

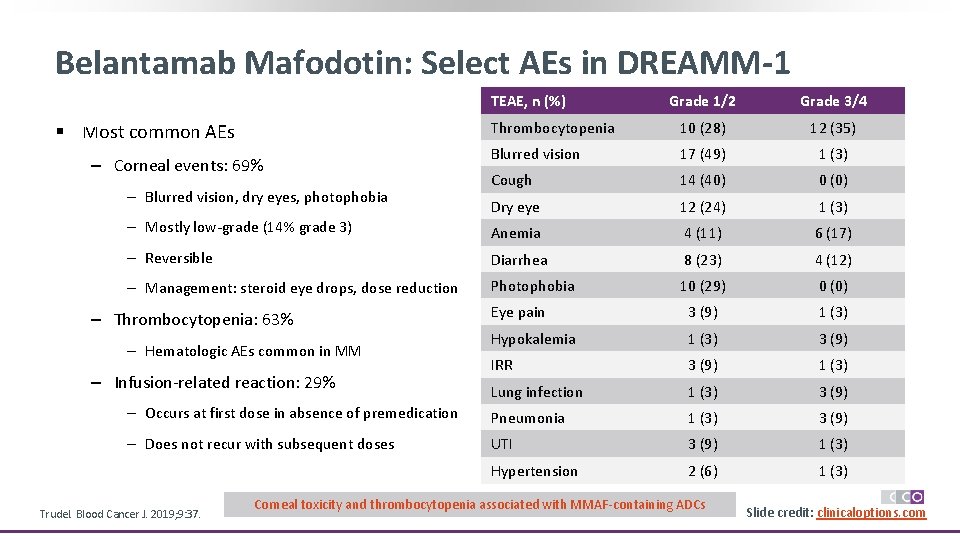
Belantamab Mafodotin: Select AEs in DREAMM-1 TEAE, n (%) Grade 1/2 Grade 3/4 Thrombocytopenia 10 (28) 12 (35) Blurred vision 17 (49) 1 (3) Cough 14 (40) 0 (0) Dry eye 12 (24) 1 (3) ‒ Mostly low-grade (14% grade 3) Anemia 4 (11) 6 (17) ‒ Reversible Diarrhea 8 (23) 4 (12) ‒ Management: steroid eye drops, dose reduction Photophobia 10 (29) 0 (0) Eye pain 3 (9) 1 (3) Hypokalemia 1 (3) 3 (9) IRR 3 (9) 1 (3) Lung infection 1 (3) 3 (9) ‒ Occurs at first dose in absence of premedication Pneumonia 1 (3) 3 (9) ‒ Does not recur with subsequent doses UTI 3 (9) 1 (3) Hypertension 2 (6) 1 (3) § Most common AEs ‒ Corneal events: 69% ‒ Blurred vision, dry eyes, photophobia ‒ Thrombocytopenia: 63% ‒ Hematologic AEs common in MM ‒ Infusion-related reaction: 29% Trudel. Blood Cancer J. 2019; 9: 37. Corneal toxicity and thrombocytopenia associated with MMAF-containing ADCs Slide credit: clinicaloptions. com

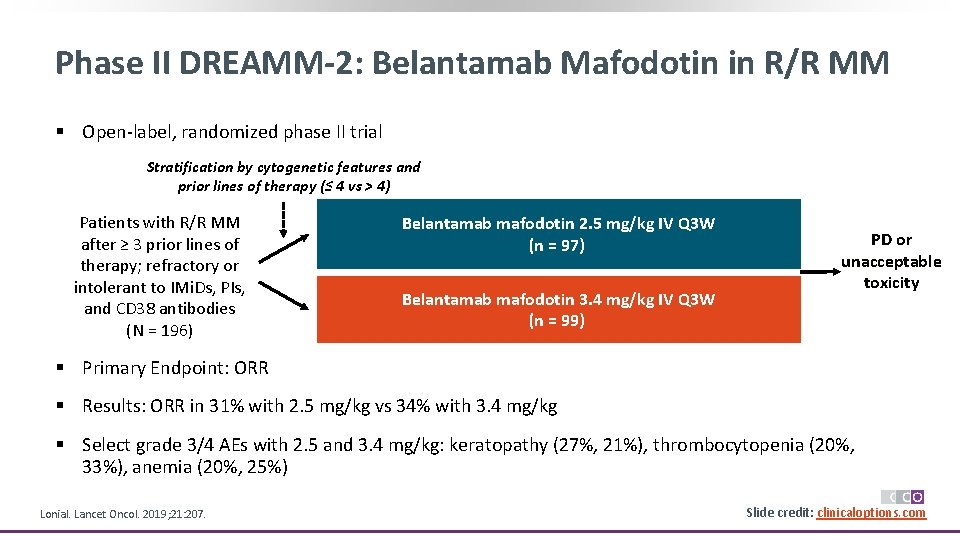
Phase II DREAMM-2: Belantamab Mafodotin in R/R MM § Open-label, randomized phase II trial Stratification by cytogenetic features and prior lines of therapy (≤ 4 vs > 4) Patients with R/R MM after ≥ 3 prior lines of therapy; refractory or intolerant to IMi. Ds, PIs, and CD 38 antibodies (N = 196) Belantamab mafodotin 2. 5 mg/kg IV Q 3 W (n = 97) Belantamab mafodotin 3. 4 mg/kg IV Q 3 W (n = 99) PD or unacceptable toxicity § Primary Endpoint: ORR § Results: ORR in 31% with 2. 5 mg/kg vs 34% with 3. 4 mg/kg § Select grade 3/4 AEs with 2. 5 and 3. 4 mg/kg: keratopathy (27%, 21%), thrombocytopenia (20%, 33%), anemia (20%, 25%) Lonial. Lancet Oncol. 2019; 21: 207. Slide credit: clinicaloptions. com

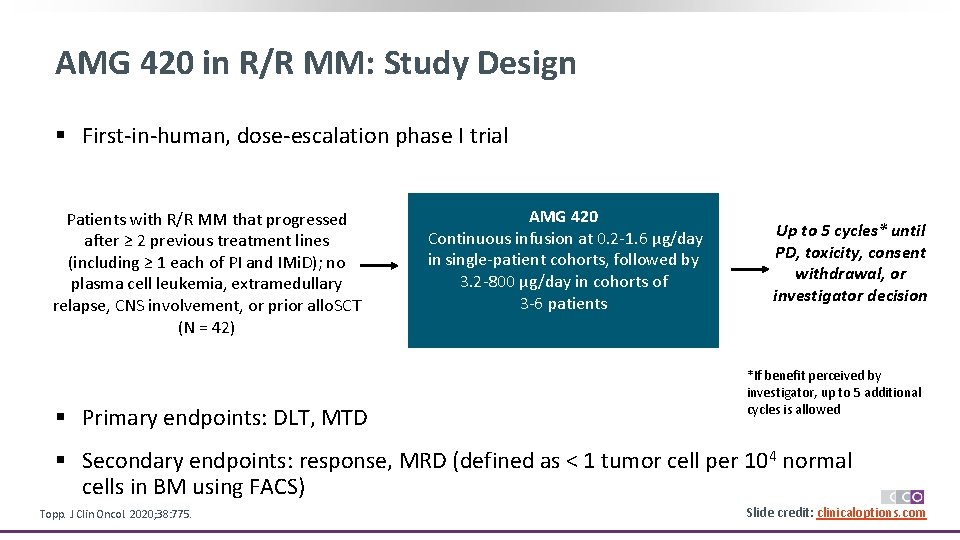
AMG 420 in R/R MM: Study Design § First-in-human, dose-escalation phase I trial Patients with R/R MM that progressed after ≥ 2 previous treatment lines (including ≥ 1 each of PI and IMi. D); no plasma cell leukemia, extramedullary relapse, CNS involvement, or prior allo. SCT (N = 42) § Primary endpoints: DLT, MTD AMG 420 Continuous infusion at 0. 2 -1. 6 µg/day in single-patient cohorts, followed by 3. 2 -800 µg/day in cohorts of 3 -6 patients Up to 5 cycles* until PD, toxicity, consent withdrawal, or investigator decision allowed *If benefit perceived by investigator, up to 5 additional cycles is allowed § Secondary endpoints: response, MRD (defined as < 1 tumor cell per 104 normal cells in BM using FACS) Topp. J Clin Oncol. 2020; 38: 775. Slide credit: clinicaloptions. com

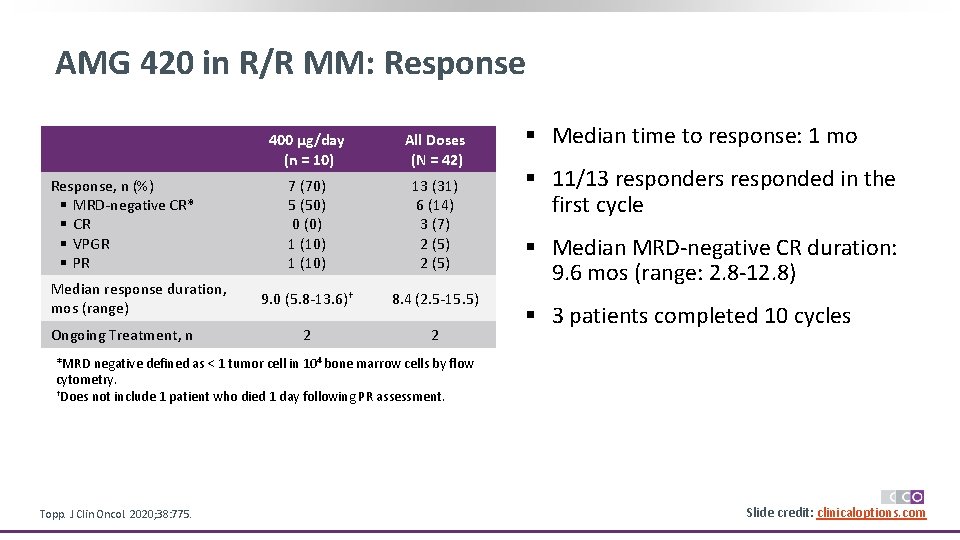
AMG 420 in R/R MM: Response, n (%) § MRD-negative CR* § CR § VPGR § PR Median response duration, mos (range) Ongoing Treatment, n 400 µg/day (n = 10) All Doses (N = 42) 7 (70) 5 (50) 0 (0) 1 (10) 13 (31) 6 (14) 3 (7) 2 (5) 9. 0 (5. 8 -13. 6)† 8. 4 (2. 5 -15. 5) 2 2 § Median time to response: 1 mo § 11/13 responders responded in the first cycle § Median MRD-negative CR duration: 9. 6 mos (range: 2. 8 -12. 8) § 3 patients completed 10 cycles *MRD negative defined as < 1 tumor cell in 104 bone marrow cells by flow cytometry. †Does not include 1 patient who died 1 day following PR assessment. Topp. J Clin Oncol. 2020; 38: 775. Slide credit: clinicaloptions. com

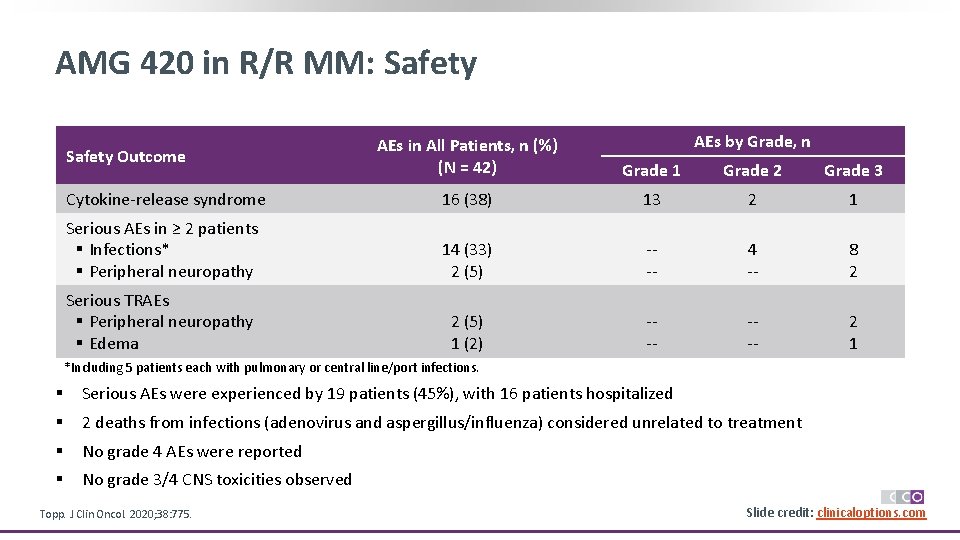
AMG 420 in R/R MM: Safety AEs by Grade, n AEs in All Patients, n (%) (N = 42) Grade 1 Grade 2 Grade 3 Cytokine-release syndrome 16 (38) 13 2 1 Serious AEs in ≥ 2 patients § Infections* § Peripheral neuropathy 14 (33) 2 (5) --- 4 -- 8 2 Serious TRAEs § Peripheral neuropathy § Edema 2 (5) 1 (2) --- 2 1 Safety Outcome *Including 5 patients each with pulmonary or central line/port infections. § Serious AEs were experienced by 19 patients (45%), with 16 patients hospitalized § 2 deaths from infections (adenovirus and aspergillus/influenza) considered unrelated to treatment § No grade 4 AEs were reported § No grade 3/4 CNS toxicities observed Topp. J Clin Oncol. 2020; 38: 775. Slide credit: clinicaloptions. com

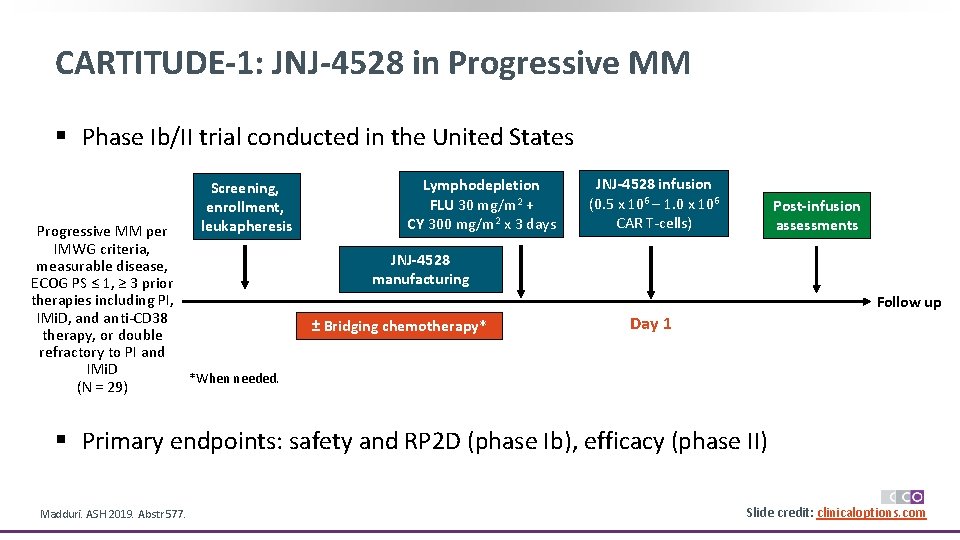
CARTITUDE-1: JNJ-4528 in Progressive MM § Phase Ib/II trial conducted in the United States Screening, enrollment, leukapheresis Progressive MM per IMWG criteria, measurable disease, ECOG PS ≤ 1, ≥ 3 prior therapies including PI, IMi. D, and anti-CD 38 therapy, or double refractory to PI and IMi. D *When needed. (N = 29) Lymphodepletion FLU 30 mg/m 2 + CY 300 mg/m 2 x 3 days JNJ-4528 infusion (0. 5 x 106 – 1. 0 x 106 CAR T-cells) Post-infusion assessments JNJ-4528 manufacturing Follow up ± Bridging chemotherapy* Day 1 § Primary endpoints: safety and RP 2 D (phase Ib), efficacy (phase II) Madduri. ASH 2019. Abstr 577. Slide credit: clinicaloptions. com

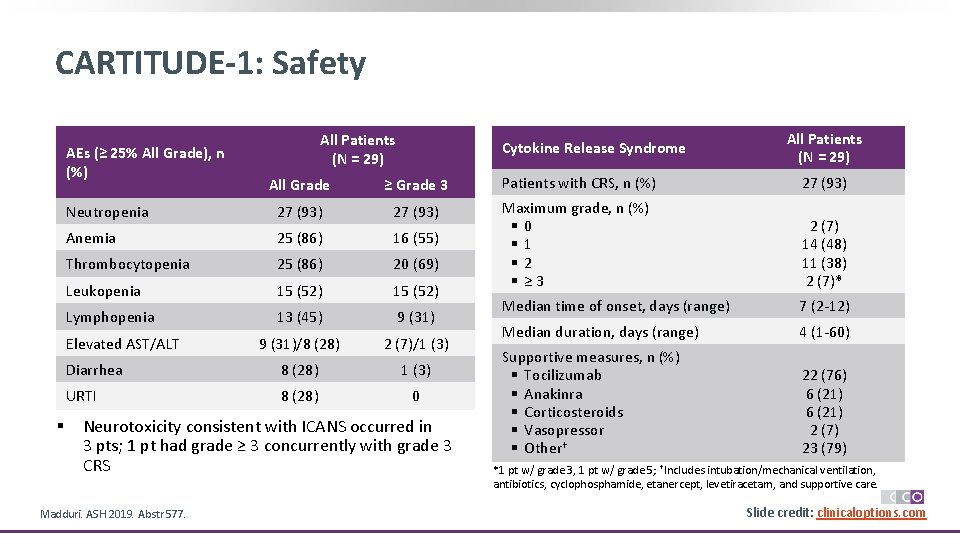
CARTITUDE-1: Safety AEs (≥ 25% All Grade), n (%) Cytokine Release Syndrome All Patients (N = 29) All Grade ≥ Grade 3 Patients with CRS, n (%) 27 (93) Neutropenia 27 (93) Anemia 25 (86) 16 (55) Thrombocytopenia 25 (86) 20 (69) Leukopenia 15 (52) Maximum grade, n (%) § 0 § 1 § 2 § ≥ 3 2 (7) 14 (48) 11 (38) 2 (7)* Lymphopenia 13 (45) 9 (31) Median time of onset, days (range) 7 (2 -12) 9 (31)/8 (28) 2 (7)/1 (3) Median duration, days (range) 4 (1 -60) Diarrhea 8 (28) 1 (3) URTI 8 (28) 0 Supportive measures, n (%) § Tocilizumab § Anakinra § Corticosteroids § Vasopressor § Other† 22 (76) 6 (21) 2 (7) 23 (79) Elevated AST/ALT § All Patients (N = 29) Neurotoxicity consistent with ICANS occurred in 3 pts; 1 pt had grade ≥ 3 concurrently with grade 3 CRS Madduri. ASH 2019. Abstr 577. *1 pt w/ grade 3, 1 pt w/ grade 5; †Includes intubation/mechanical ventilation, antibiotics, cyclophosphamide, etanercept, levetiracetam, and supportive care. Slide credit: clinicaloptions. com

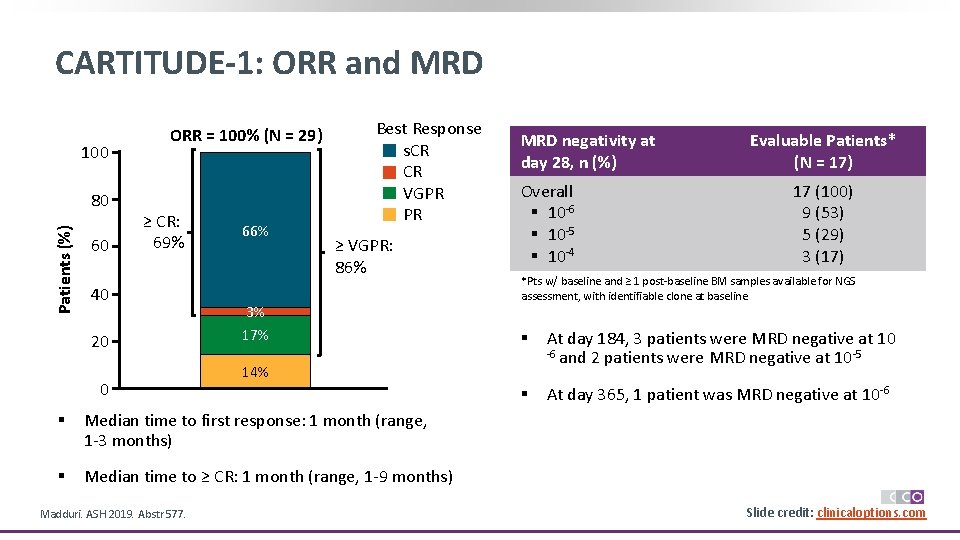
CARTITUDE-1: ORR and MRD 100 Patients (%) 80 60 ORR = 100% (N = 29) ≥ CR: 69% 40 20 0 66% Best Response s. CR CR VGPR PR ≥ VGPR: 86% 3% 17% Overall § 10 -6 § 10 -5 § 10 -4 Median time to first response: 1 month (range, 1 -3 months) § Median time to ≥ CR: 1 month (range, 1 -9 months) Evaluable Patients* (N = 17) 17 (100) 9 (53) 5 (29) 3 (17) *Pts w/ baseline and ≥ 1 post-baseline BM samples available for NGS assessment, with identifiable clone at baseline § At day 184, 3 patients were MRD negative at 10 -6 and 2 patients were MRD negative at 10 -5 § At day 365, 1 patient was MRD negative at 10 -6 14% § Madduri. ASH 2019. Abstr 577. MRD negativity at day 28, n (%) Slide credit: clinicaloptions. com

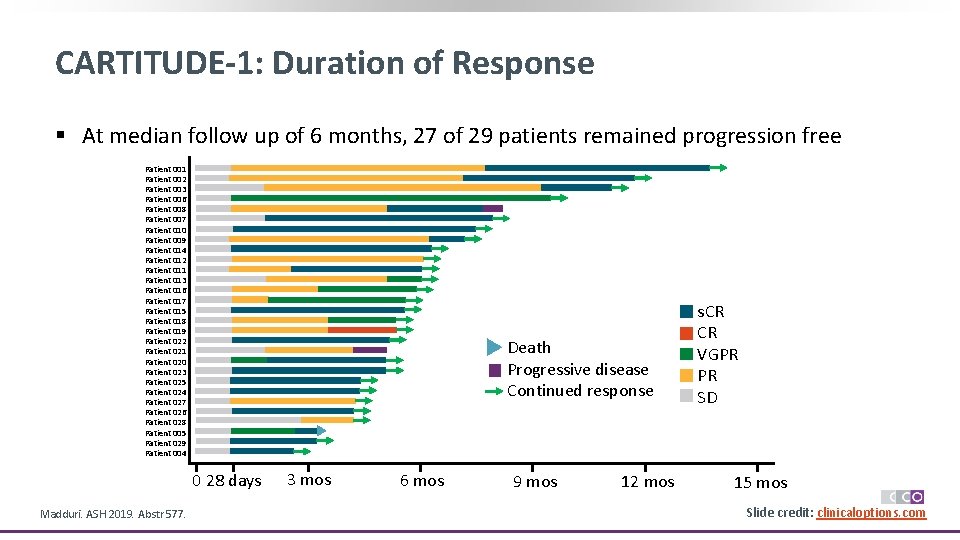
CARTITUDE-1: Duration of Response § At median follow up of 6 months, 27 of 29 patients remained progression free Patient 001 Patient 002 Patient 003 Patient 006 Patient 008 Patient 007 Patient 010 Patient 009 Patient 014 Patient 012 Patient 011 Patient 013 Patient 016 Patient 017 Patient 015 Patient 018 Patient 019 Patient 022 Patient 021 Patient 020 Patient 023 Patient 025 Patient 024 Patient 027 Patient 026 Patient 028 Patient 005 Patient 029 Patient 004 Death Progressive disease Continued response 0 28 days Madduri. ASH 2019. Abstr 577. 3 mos 6 mos 9 mos 12 mos s. CR CR VGPR PR SD 15 mos Slide credit: clinicaloptions. com

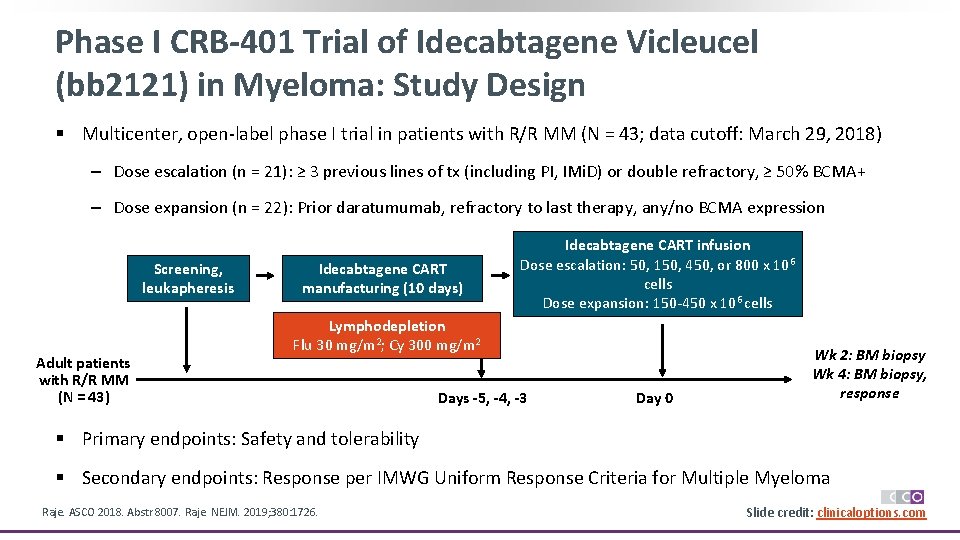
Phase I CRB-401 Trial of Idecabtagene Vicleucel (bb 2121) in Myeloma: Study Design § Multicenter, open-label phase I trial in patients with R/R MM (N = 43; data cutoff: March 29, 2018) ‒ Dose escalation (n = 21): ≥ 3 previous lines of tx (including PI, IMi. D) or double refractory, ≥ 50% BCMA+ ‒ Dose expansion (n = 22): Prior daratumumab, refractory to last therapy, any/no BCMA expression Screening, leukapheresis Adult patients with R/R MM (N = 43) Idecabtagene CART manufacturing (10 days) Idecabtagene CART infusion Dose escalation: 50, 150, 450, or 800 x 106 cells Dose expansion: 150 -450 x 106 cells Lymphodepletion Flu 30 mg/m 2; Cy 300 mg/m 2 Days -5, -4, -3 Day 0 Wk 2: BM biopsy Wk 4: BM biopsy, response § Primary endpoints: Safety and tolerability § Secondary endpoints: Response per IMWG Uniform Response Criteria for Multiple Myeloma Raje. ASCO 2018. Abstr 8007. Raje. NEJM. 2019; 380: 1726. Slide credit: clinicaloptions. com

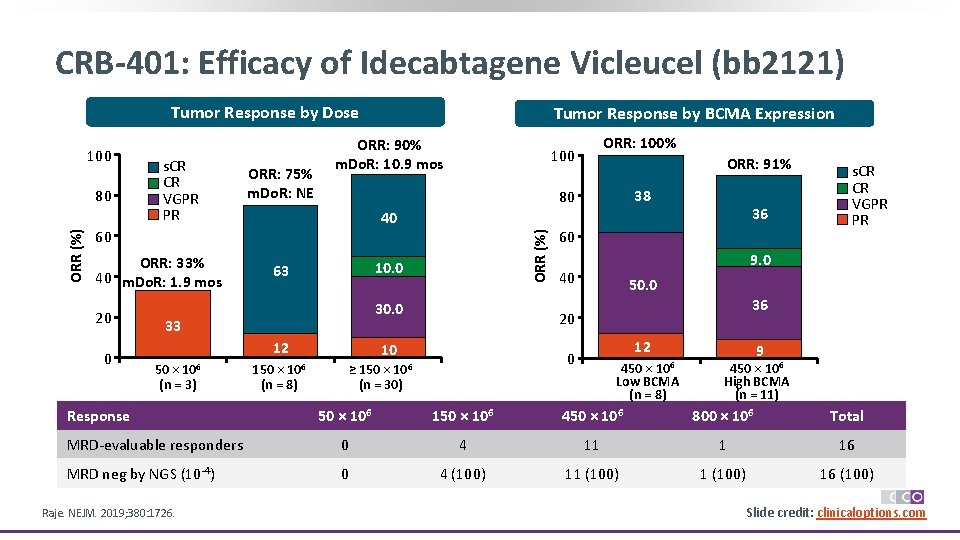
CRB-401: Efficacy of Idecabtagene Vicleucel (bb 2121) Tumor Response by Dose ORR (%) 80 s. CR CR VGPR PR ORR: 75% m. Do. R: NE ORR: 90% m. Do. R: 10. 9 mos 20 0 10. 0 63 30. 0 12 Response 38 36 40 33 50 × 106 (n = 3) ORR: 91% 80 60 ORR: 33% 40 m. Do. R: 1. 9 mos ORR: 100% 100 ORR (%) 100 Tumor Response by BCMA Expression 150 × 106 (n = 8) 60 9. 0 40 50. 0 36 20 10 12 0 ≥ 150 × 106 (n = 30) s. CR CR VGPR PR 450 × 106 Low BCMA (n = 8) 9 450 × 106 High BCMA (n = 11) 50 × 106 150 × 106 450 × 106 800 × 106 Total MRD-evaluable responders 0 4 11 1 16 MRD neg by NGS (10 -4) 0 4 (100) 11 (100) 16 (100) Raje. NEJM. 2019; 380: 1726. Slide credit: clinicaloptions. com

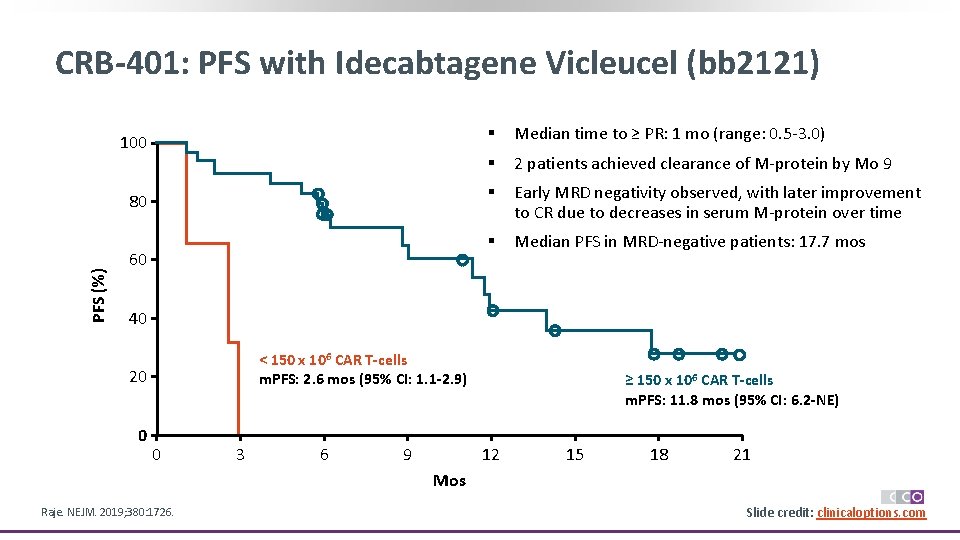
PFS (%) CRB-401: PFS with Idecabtagene Vicleucel (bb 2121) 100 § Median time to ≥ PR: 1 mo (range: 0. 5 -3. 0) § 2 patients achieved clearance of M-protein by Mo 9 80 § Early MRD negativity observed, with later improvement to CR due to decreases in serum M-protein over time § Median PFS in MRD-negative patients: 17. 7 mos 60 40 < 150 x 106 CAR T-cells m. PFS: 2. 6 mos (95% CI: 1. 1 -2. 9) 20 0 0 3 6 9 ≥ 150 x 106 CAR T-cells m. PFS: 11. 8 mos (95% CI: 6. 2 -NE) 12 15 18 21 Mos Raje. NEJM. 2019; 380: 1726. Slide credit: clinicaloptions. com

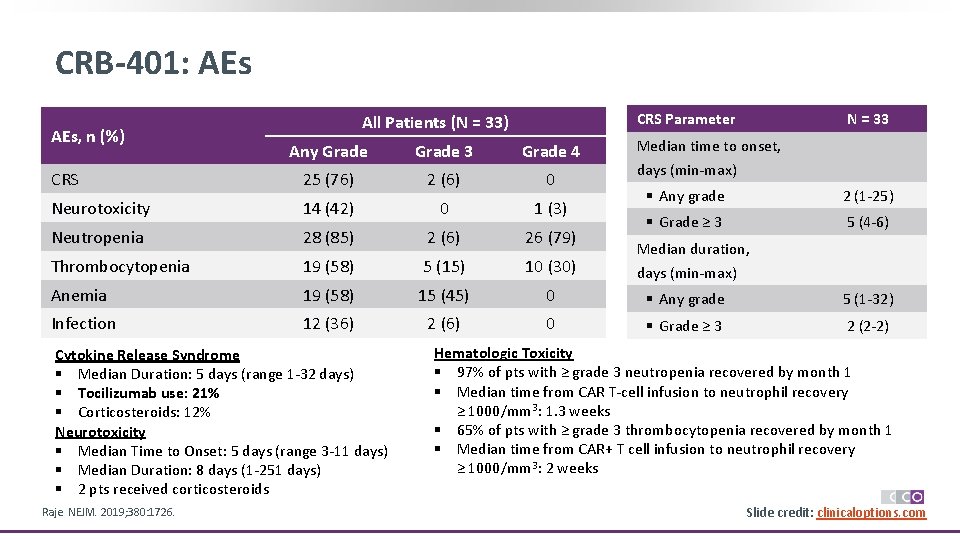
CRB-401: AEs, n (%) CRS Parameter All Patients (N = 33) N = 33 Median time to onset, Any Grade 3 Grade 4 CRS 25 (76) 2 (6) 0 Neurotoxicity 14 (42) 0 1 (3) Neutropenia 28 (85) 2 (6) 26 (79) Thrombocytopenia 19 (58) 5 (15) 10 (30) Anemia 19 (58) 15 (45) 0 § Any grade 5 (1 -32) Infection 12 (36) 2 (6) 0 § Grade ≥ 3 2 (2 -2) Cytokine Release Syndrome § Median Duration: 5 days (range 1 -32 days) § Tocilizumab use: 21% § Corticosteroids: 12% Neurotoxicity § Median Time to Onset: 5 days (range 3 -11 days) § Median Duration: 8 days (1 -251 days) § 2 pts received corticosteroids Raje. NEJM. 2019; 380: 1726. days (min-max) § Any grade 2 (1 -25) § Grade ≥ 3 5 (4 -6) Median duration, days (min-max) Hematologic Toxicity § 97% of pts with ≥ grade 3 neutropenia recovered by month 1 § Median time from CAR T-cell infusion to neutrophil recovery ≥ 1000/mm 3: 1. 3 weeks § 65% of pts with ≥ grade 3 thrombocytopenia recovered by month 1 § Median time from CAR+ T cell infusion to neutrophil recovery ≥ 1000/mm 3: 2 weeks Slide credit: clinicaloptions. com

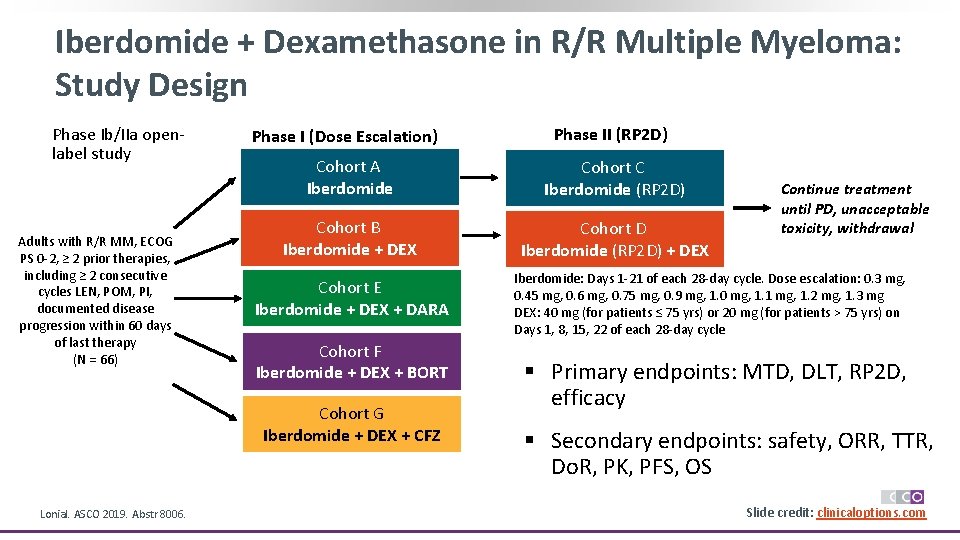
Iberdomide + Dexamethasone in R/R Multiple Myeloma: Study Design Phase Ib/IIa openlabel study Adults with R/R MM, ECOG PS 0 -2, ≥ 2 prior therapies, including ≥ 2 consecutive cycles LEN, POM, PI, documented disease progression within 60 days of last therapy (N = 66) Phase I (Dose Escalation) Cohort A Iberdomide Cohort C Iberdomide (RP 2 D) Cohort B Iberdomide + DEX Cohort D Iberdomide (RP 2 D) + DEX Cohort E Iberdomide + DEX + DARA Cohort F Iberdomide + DEX + BORT Cohort G Iberdomide + DEX + CFZ Lonial. ASCO 2019. Abstr 8006. Phase II (RP 2 D) Continue treatment until PD, unacceptable toxicity, withdrawal Iberdomide: Days 1 -21 of each 28 -day cycle. Dose escalation: 0. 3 mg, 0. 45 mg, 0. 6 mg, 0. 75 mg, 0. 9 mg, 1. 0 mg, 1. 1 mg, 1. 2 mg, 1. 3 mg DEX: 40 mg (for patients ≤ 75 yrs) or 20 mg (for patients > 75 yrs) on Days 1, 8, 15, 22 of each 28 -day cycle § Primary endpoints: MTD, DLT, RP 2 D, efficacy § Secondary endpoints: safety, ORR, TTR, Do. R, PK, PFS, OS Slide credit: clinicaloptions. com

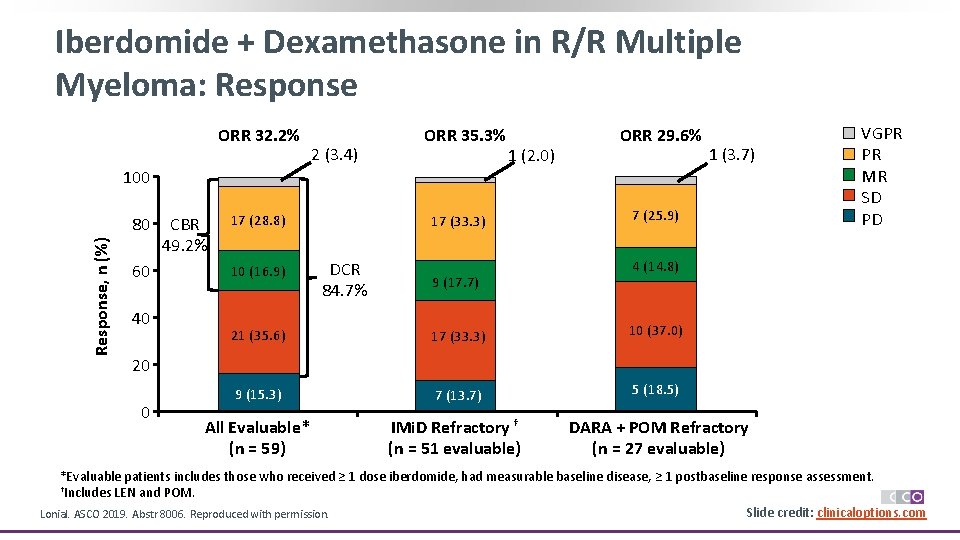
Iberdomide + Dexamethasone in R/R Multiple Myeloma: Response ORR 32. 2% 2 (3. 4) ORR 35. 3% 1 (2. 0) ORR 29. 6% 1 (3. 7) 100 Response, n (%) 80 60 40 CBR 49. 2% 17 (28. 8) 10 (16. 9) 17 (33. 3) DCR 84. 7% 9 (17. 7) 7 (25. 9) VGPR PR MR SD PD 4 (14. 8) 21 (35. 6) 17 (33. 3) 10 (37. 0) 9 (15. 3) 7 (13. 7) 5 (18. 5) All Evaluable* (n = 59) IMi. D Refractory † (n = 51 evaluable) DARA + POM Refractory (n = 27 evaluable) 20 0 *Evaluable patients includes those who received ≥ 1 dose iberdomide, had measurable baseline disease, ≥ 1 postbaseline response assessment. †Includes LEN and POM. Slide credit: clinicaloptions. com Lonial. ASCO 2019. Abstr 8006. Reproduced with permission.

Alkylating Agents in Relapsed/Refractory Myeloma § Alkylating agents have long been a standard of care for myeloma, and are used alone and in combination regimens § While these are no longer category 1 recommendations, they are still used for certain patients with relapsed/refractory myeloma ‒ Bendamustine, in combination with bortezomib/dex or len/dex ‒ Cyclophosphamide, in combination with IMi. Ds and Pis ‒ Intense alkylator-based chemotherapy regimens: DCEP, VTD-PACE, and high-dose cyclophosphamide § Melflufen: An investigational peptide-conjugated alkylating agent being developed for the treatment of relapsed/refractory myeloma Slide credit: clinicaloptions. com

Summary: R/R MM § For relapse after frontline therapy, treatment options should include regimens with different agents than those used for initial treatment ‒ For example, if relapsing on VRd or lenalidomide maintenance, consider pomalidomide or carfilzomib-based regimens with anti-CD 38 m. Ab § At each relapse, treatment regimens should include at least 1 new therapy ‒ Consider venetoclax-based therapy for patients with t(11; 14) ‒ Consider selinexor-based therapy for patients after ≥ 4 previous lines of therapy ‒ Consider enrollment on clinical trials for all patients with MM § At first relapse and in subsequent relapses, clinicians should consider restaging and reassessing cytogenetics § Heavily pretreated patients need individualized supportive care due to disease burden and multiple comorbidities and/or AEs from previous therapy

Go Online for More CCO Coverage of Myeloma! Expert Insights on Treatment Options for Myeloma, an Interactive Decision Support Tool Clinical. Thought Commentaries with perspectives on managing patients with myeloma from different experts Downloadable Resources with proposed MM treatment algorithms clinicaloptions. com/oncology clinicaloptions. com/myelomatool
 Future perfect continuous worksheet
Future perfect continuous worksheet Future perfect e future continuous
Future perfect e future continuous Balanced vs lingualized occlusion
Balanced vs lingualized occlusion Indirect guidance
Indirect guidance What are the current trends in media and information
What are the current trends in media and information Line current and phase current
Line current and phase current Line current and phase current
Line current and phase current Drift current and diffusion current in semiconductor
Drift current and diffusion current in semiconductor Ac systems lesson 4
Ac systems lesson 4 Drift current and diffusion current in semiconductor
Drift current and diffusion current in semiconductor What is diffusion current and drift current
What is diffusion current and drift current In a delta connected source feeding a y connected load
In a delta connected source feeding a y connected load Holding current and latching current
Holding current and latching current Diffusion current density
Diffusion current density Current and future issues in corrections
Current and future issues in corrections Pinch off voltage
Pinch off voltage Welding chapter 3
Welding chapter 3 Touch current vs leakage current
Touch current vs leakage current Non planar circuit
Non planar circuit Future perfect and future continuous examples
Future perfect and future continuous examples Tenses in english
Tenses in english Future plans and finished future actions
Future plans and finished future actions Budoucí čas will
Budoucí čas will Kondicional engleski
Kondicional engleski Current state vs future state diagram
Current state vs future state diagram Introduction of milieu therapy
Introduction of milieu therapy 7 rights of medication administration in order
7 rights of medication administration in order Association for applied and therapeutic humor
Association for applied and therapeutic humor Future vs future perfect
Future vs future perfect Swing past participle
Swing past participle Future nurse future midwife
Future nurse future midwife Future past continuous tense examples
Future past continuous tense examples Present continuous for future plan
Present continuous for future plan Therapeutic orientations
Therapeutic orientations Penyakit masyarakat sasaran bintibluh
Penyakit masyarakat sasaran bintibluh Therapeutic exercise chapter 1 mcqs
Therapeutic exercise chapter 1 mcqs Microwave diathermy block diagram
Microwave diathermy block diagram Therapeutic drift
Therapeutic drift Introduction of therapeutic communication
Introduction of therapeutic communication Therapeutic triangle
Therapeutic triangle Therapeutic story writing
Therapeutic story writing Therapeutic communication elements
Therapeutic communication elements Principles of therapeutic exercise
Principles of therapeutic exercise Potency vs efficacy
Potency vs efficacy High therapeutic index
High therapeutic index High therapeutic index
High therapeutic index Classification of therapeutic exercise
Classification of therapeutic exercise Intentional relationship model
Intentional relationship model 5 health care career pathways
5 health care career pathways Reproductive vs therapeutic cloning
Reproductive vs therapeutic cloning Multicultural therapeutic communication skills
Multicultural therapeutic communication skills Jobs in health science career cluster
Jobs in health science career cluster Therapeutic index
Therapeutic index Therapeutic substitution vs interchange
Therapeutic substitution vs interchange Therapeutic environment in hospital
Therapeutic environment in hospital Therapeutic value of play
Therapeutic value of play Non therapeutic
Non therapeutic Competitive and non competitive antagonist
Competitive and non competitive antagonist What is habilitative exercise?
What is habilitative exercise? Kelly chaplin
Kelly chaplin Therapeutic drift
Therapeutic drift Therapeutic communication goals
Therapeutic communication goals Therapeutic window
Therapeutic window Ichaemic
Ichaemic Chapter 4 therapeutic communication skills
Chapter 4 therapeutic communication skills Therapeutic recreation program sydney
Therapeutic recreation program sydney Caries stabilization
Caries stabilization Btpb repertory rubrics
Btpb repertory rubrics Roger verbeeck
Roger verbeeck Therapeutic window
Therapeutic window Therapeutic touch
Therapeutic touch Therapeutic photography exercises
Therapeutic photography exercises Therapeutic diet for fever
Therapeutic diet for fever Prehospital emergency care 11th edition
Prehospital emergency care 11th edition Physical incompatibilities
Physical incompatibilities Therapeutic services examples
Therapeutic services examples Essex steps
Essex steps Chapter 8 health team communication
Chapter 8 health team communication Therapeutic recreation models
Therapeutic recreation models Therapeutic interview
Therapeutic interview Faradic current
Faradic current Therapeutic dialogue
Therapeutic dialogue Therapeutic window
Therapeutic window Therapeutic exercise definition
Therapeutic exercise definition Therapeutic privilege example
Therapeutic privilege example Physical incompatibility examples
Physical incompatibility examples What is therapeutic equivalence?
What is therapeutic equivalence?