FDA Regulations Investigational Drugs and Devices Presented by

FDA Regulations: Investigational Drugs and Devices Presented by: Karen Jeans Program Analyst, COACH Marian Serge Program Analyst, COACH

What This Presentation Will Cover • Conducting research with investigational drugs • Conducting research with investigational devices

Prologue FDA regulations are unlike the human research regulations known as the Common Rule regulations. This can create a lot of issues that are unique and yet similar to conduct of non-FDA regulated human research studies. Knowing what is and is not regulated by FDA can make your life a lot easier, regardless of your role.

What is FDA?

FDA Regulates Products • Drugs, biologics, medical devices (diagnostic and therapeutic), foods: nearly 25% of the U. S. economy • FDA has responsibility for clinical investigations of FDAregulated products • Irrespective of study funding (unlike OHRP) • Irrespective of study location within the U. S. • Irrespective of whether for commercialization/marketing or for scientific knowledge

It is Sometimes Hard to Figure out What FDA Regulates

How Did it All Begin? • 1937: Sulfanilamide – the first “wonder drug” for strep throat and gonorrhea • Made into Elixir of Sulfanilamide

Elixir of Sulfanilamide Incident • 240 gallons shipped • Resulted in 107 deaths • No laws regarding safety • Congress got involved

Levels of Authority at FDA • Law: Passed by Congress; Governs the U. S. Public AND FDA • Federal Food, Drug, and Cosmetic Act • Regulation: Promulgated by FDA to implement the law; Carries the force of law • Code of Federal Regulations • Guidance: FDA’s best advice; Alternate methods may be used to meet regulation • Information Sheet Guidance for IRBs, Clinical Investigators, and Sponsors: Significant Risk and Non. Significant Risk Medical Device Studies

Basic FDA Human Research Protections Governing the Conduct of Clinical Investigations in Human Subjects • FDA regulations directed towards protection of human research subjects • 21 CFR Part 50: Informed Consent • 21 CFR Part 56: IRB Regulations • These regulations are near-identical to the “Common Rule” which governs protection of subjects in federally funded research
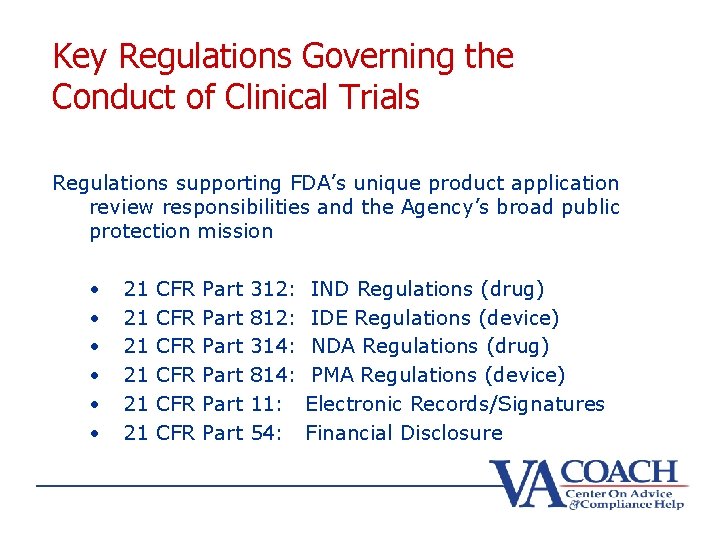
Key Regulations Governing the Conduct of Clinical Trials Regulations supporting FDA’s unique product application review responsibilities and the Agency’s broad public protection mission • • • 21 21 21 CFR CFR CFR Part Part 312: 812: 314: 814: 11: 54: IND Regulations (drug) IDE Regulations (device) NDA Regulations (drug) PMA Regulations (device) Electronic Records/Signatures Financial Disclosure

What is FDA regulated research involving human subjects? Definition of Clinical Investigation: Clinical investigation means any experiment that involves a test article and one or more human participants and that is one of the following: [21 CFR § 50. 3(c)] [21 CFR § 56. 102(c)] • Subject to requirements for prior submission to the Food and Drug Administration under § 505(i) or § 520(g) of the act. • Not subject to requirements for prior submission to the Food and Drug Administration under these sections of the act, but the results of which are intended to be submitted later to, or held for inspection by, the Food and Drug Administration as part of an application for a research or marketing permit. Definition of Human Subject: Individual who is or becomes a participant in research, either as a recipient of the test article or as a control (21 CFR § 50. 3(g)) or on whom or on whose specimen an investigational device is used or as a control. A subject may be in normal health or may have a medical condition or disease (21 CFR § 812. 3(p))

What is an investigational drug? • A new drug or biological drug that is used in a clinical investigation. The term also includes a biological term that is used in vitro for diagnostic purposes. The terms “investigational drug” and “investigational new drug” are deemed to be synonymous for purposes of this part. • • 21 CFR 312. 3(b) Overseen by • Center for Drug Evaluation & Research (CDER) • Center for Biologics Evaluation & Research (CBER)
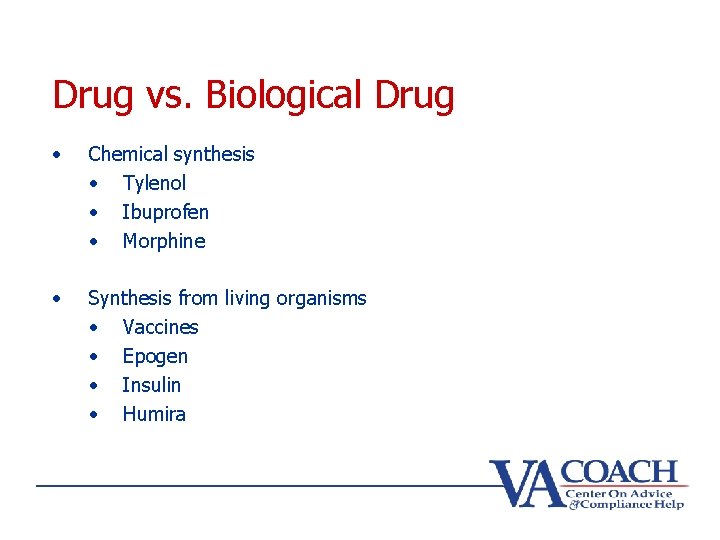
Drug vs. Biological Drug • Chemical synthesis • Tylenol • Ibuprofen • Morphine • Synthesis from living organisms • Vaccines • Epogen • Insulin • Humira

What Causes Trouble? Drug vs. Cosmetic Drug • “Articles intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease” and • “Articles (other than Food) intended to affect the structure or function of the body of man or other animals” • FD&C Act, Section 201(g)(1) Cosmetic • “Articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body. . . for cleaning, beautifying, promoting attractiveness, or altering the appearance” • FD&C Act, Section 201(i)

Drug or Cosmetic? 1. 2. 3. 4. 5. 6. Shampoo Deodorants Antiperspirants Toothpastes with fluoride Aromatherapy fragrances Collagen

Why is this Important to Differentiate Drugs from Cosmetics? • • Differences in intended uses Approval requirements are different • FDA • IRB

Now That You’ve Figure Out it is a Drug Is it unapproved or approved?

“New” Unapproved Drugs New Unapproved Drug Requires an IND from FDA for clinical investigations to be conducted in human subjects IRB reviews clinical investigation to determine if it meets approval criteria

What is an Investigational New Drug (IND) Application? • Affirms a body of knowledge about the manufacturing, pharmacology, and toxicology of the drug to support its use in human testing • Requires that the clinical investigation(s) be performed in accordance with Good Clinical Practice (GCP) • Provides an additional level of protection through FDA oversight • An IND is required when an unapproved drug or biologic is used in a clinical investigation
Common Types of INDs • Commercial • Noncommercial research • Emergency • Treatment

Phases of an Investigation Involving an IND • INDs can be submitted for one or more phases • Phase 1: Initial Introduction • Phase 2: Dose escalation and effectiveness • Phase 3: Safety and effectiveness to evaluate overall risk-benefit relationship of the drug • Phase 4: Do not involve INDs
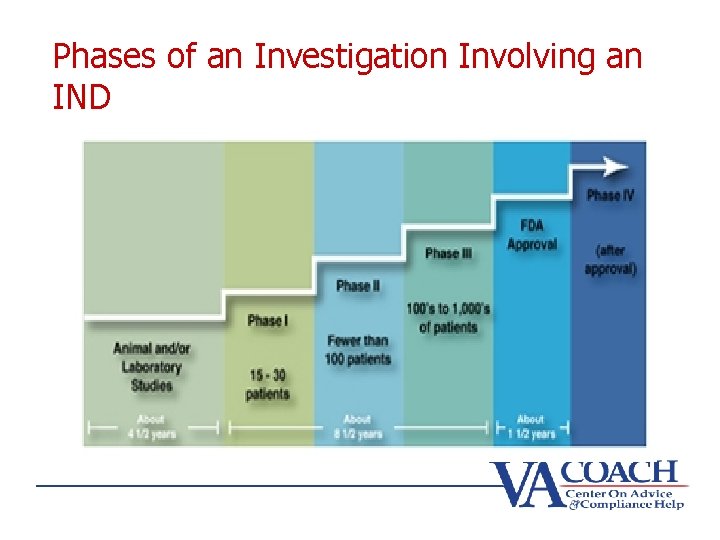
Phases of an Investigation Involving an IND

Phases of an Investigation: Examples of Studies Phase I Study of MT 110 in Colorectal Cancer (CRC), Gastrointestinal (GI) and Lung Cancer (MT 110 -101) Phase II: Phase II Trial of a 96 -Hour Continuous Infusion of Paclitaxel Followed by Cisplatin for Patients With Stage III/IV and Relapsed NSCLC Phase III Controlled Clinical Study of NPO-11 in Patients Undergoing Gastric Endoscopy Evaluation of Satisfaction Regarding Patient's Management of Ocular Surface Diseases

“New” Unapproved Drugs Investigational Drug Requires an IND from FDA for clinical investigations to be conducted in human subjects IRB reviews clinical investigation to determine if it meets approval criteria

Approved Drugs: The Issue Investigational Clinical Investigation Approved Drug Not Investigational Off-Label Clinical Care As described Not in Product Investigational Insert

Marketed Drugs in Off-Label Use vs. Clinical Investigation • Dr. Sue Yason calls you wanting to use Botox for a patient in her clinic. She wants to inject Botox into the muscles around the right eye of a 81 -year old male patient who has severe migraines. • Botox is only approved for use of treatment of wrinkles between the eyebrows in people ages 18 -65 years old. It has not been tested on people under 18 or over 65. • She wants to know whether she needs to submit this to the IRB Committee or call FDA for permission.

But What If. . .

Is an IND always required? • No IND is needed when an approved product is used in the course of medical practice (even for an indication different from the approved indication) • But an IND may be required when an approved product is used in a clinical investigation

IND Needed? Five conditions for no IND needed in clinical investigations involving human subjects with an approved drug • • • Not intended to support labeling change Not intended to support advertising change No significant increase in risk (administration, dosage, subject population) In compliance with FDA regulations regarding informed consent and Institutional Review Boards In compliance with FDA regulations regarding advertising

How Does One Get an IND? Submit • • • an Application to FDA with 9 components: Cover Sheet and Form FDA 1571 Table of Contents Introductory Statement and General Investigational Plan Investigator’s Brochure Clinical Protocol Chemistry, Manufacturing and Control Information Pharmacology and Toxicology Information Previous Human Experience Additional Information • 21 CFR 312. 23

When Does the IND Go Into Effect? • FDA notifies sponsors in writing the date it receives the IND application • Receives an IND number • 30 -day rule • Earlier notification • Clinical hold 21 CFR 312. 40(b)

What We Have Covered • • Drugs vs. Biologics Drugs vs. Cosmetics Off-Label vs. Clinical Investigations of Marketed Drugs Investigational New Drug Applications

Review of INDs • • Commercial IND Noncommercial Research IND

IRB Issues • Does the IRB need to review the IND application? • What is “validating” an IND?

IRB Issues • Does the IRB need to review the IND application? NO

Organizational Issues What is “validating” an IND? • Accreditation requirement: Verifying that the IND number supplied is on a document originating from either FDA, a sponsor, or a Contract Research Organization (CRO) • Source document: • Industry-sponsored clinical trials: • Clinical Protocol • Sponsor or CRO correspondence or e-mail • FDA correspondence • Investigator-initiated • FDA correspondence

Summary of Drug Issues • • • Drugs vs. Biologics Drugs vs. Cosmetics Off-Label vs. Clinical Investigations of Marketed Drugs IND Determinations Types of INDs • Commercial • Noncommercial
- Slides: 38