Expert Guidance on the Evolving Therapeutic Landscape for








![APHINITY: Safety Pertuzumab (n = 2364) Placebo (n = 2405) Primary cardiac endpoint[1] § APHINITY: Safety Pertuzumab (n = 2364) Placebo (n = 2405) Primary cardiac endpoint[1] §](https://slidetodoc.com/presentation_image_h/09d69ae7a0b1a4f788139ad317707dd9/image-31.jpg)



![Future Directions: Ongoing Clinical Trials in HER 2+ EBC Trial Name IMpassion 050[1] Phase Future Directions: Ongoing Clinical Trials in HER 2+ EBC Trial Name IMpassion 050[1] Phase](https://slidetodoc.com/presentation_image_h/09d69ae7a0b1a4f788139ad317707dd9/image-58.jpg)
- Slides: 60

Expert Guidance on the Evolving Therapeutic Landscape for HER 2 -Positive Early-Stage Breast Cancer Lee Schwartzberg, MD, FACP Medical Director The West Clinical Professor of Medicine University of Tennessee College of Medicine Memphis, Tennessee Supported by an educational grant from Puma Biotechnology.

About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details

Faculty Lee Schwartzberg, MD, FACP Medical Director West Cancer Center and Research Institute Professor of Medicine University of Tennessee Health Science Center Memphis, Tennessee Lee Schwartzberg, MD, FACP, has disclosed that he has received funds for research support from Amgen; consulting fees from Amgen, Astra. Zeneca, Bristol-Myers Squibb, Genentech/Roche, Genomic Health, Helsinn, Myriad, Napo, Spectrum, and Pfizer; and other financial or material support from Bayer.

Agenda § Assessing risk and planning neoadjuvant and adjuvant therapy § Managing lower-risk EBC § Treatment strategies for high-risk EBC § Managing AEs associated with HER 2 -targeted therapy

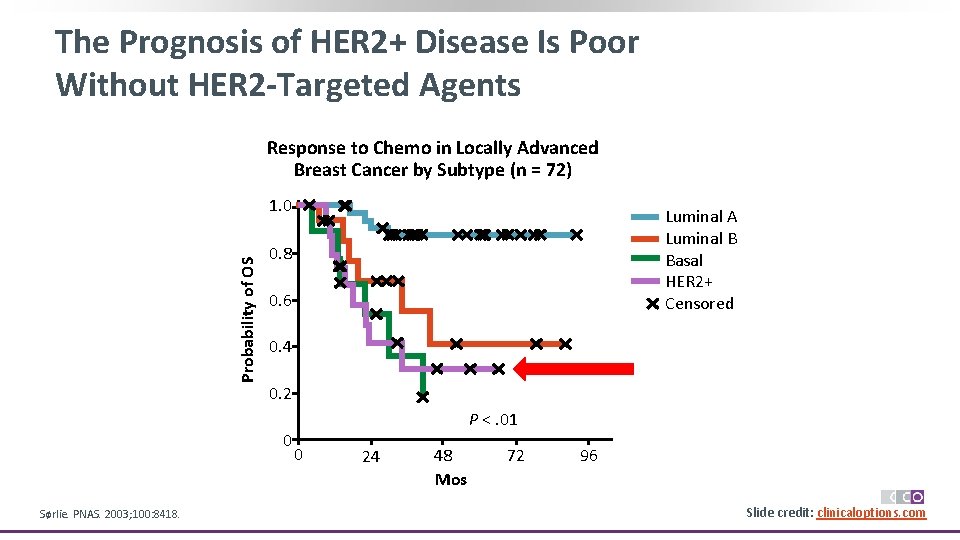
The Prognosis of HER 2+ Disease Is Poor Without HER 2 -Targeted Agents Response to Chemo in Locally Advanced Breast Cancer by Subtype (n = 72) Probability of OS 1. 0 0. 8 0. 6 0. 4 0. 2 0 Sørlie. PNAS. 2003; 100: 8418. Luminal A Luminal B Basal HER 2+ Censored P <. 01 0 24 48 Mos 72 96 Slide credit: clinicaloptions. com

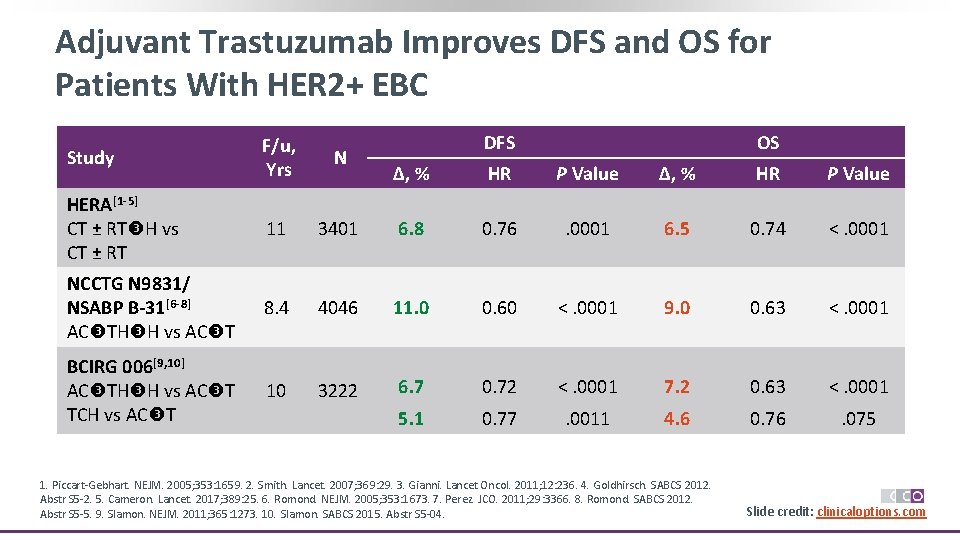
Adjuvant Trastuzumab Improves DFS and OS for Patients With HER 2+ EBC Study F/u, Yrs N HERA[1 -5] CT ± RT H vs CT ± RT 11 NCCTG N 9831/ NSABP B-31[6 -8] AC TH H vs AC T BCIRG 006[9, 10] AC TH H vs AC T TCH vs AC T DFS OS Δ, % HR P Value 3401 6. 8 0. 76 . 0001 6. 5 0. 74 <. 0001 8. 4 4046 11. 0 0. 60 <. 0001 9. 0 0. 63 <. 0001 10 3222 6. 7 0. 72 <. 0001 7. 2 0. 63 <. 0001 5. 1 0. 77 . 0011 4. 6 0. 76 . 075 1. Piccart-Gebhart. NEJM. 2005; 353: 1659. 2. Smith. Lancet. 2007; 369: 29. 3. Gianni. Lancet Oncol. 2011; 12: 236. 4. Goldhirsch. SABCS 2012. Abstr S 5 -2. 5. Cameron. Lancet. 2017; 389: 25. 6. Romond. NEJM. 2005; 353: 1673. 7. Perez. JCO. 2011; 29: 3366. 8. Romond. SABCS 2012. Abstr S 5 -5. 9. Slamon. NEJM. 2011; 365: 1273. 10. Slamon. SABCS 2015. Abstr S 5 -04. Slide credit: clinicaloptions. com

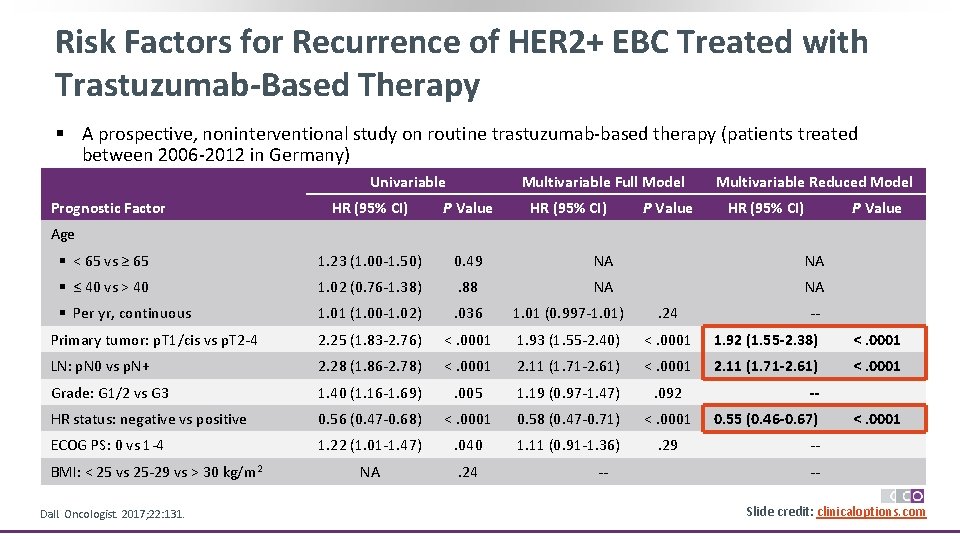
Risk Factors for Recurrence of HER 2+ EBC Treated with Trastuzumab-Based Therapy § A prospective, noninterventional study on routine trastuzumab-based therapy (patients treated between 2006 -2012 in Germany) Univariable Prognostic Factor Multivariable Full Model HR (95% CI) P Value Multivariable Reduced Model HR (95% CI) P Value § < 65 vs ≥ 65 1. 23 (1. 00 -1. 50) 0. 49 NA NA § ≤ 40 vs > 40 1. 02 (0. 76 -1. 38) . 88 NA NA § Per yr, continuous 1. 01 (1. 00 -1. 02) . 036 1. 01 (0. 997 -1. 01) . 24 Primary tumor: p. T 1/cis vs p. T 2 -4 2. 25 (1. 83 -2. 76) <. 0001 1. 93 (1. 55 -2. 40) <. 0001 1. 92 (1. 55 -2. 38) <. 0001 LN: p. N 0 vs p. N+ 2. 28 (1. 86 -2. 78) <. 0001 2. 11 (1. 71 -2. 61) <. 0001 Grade: G 1/2 vs G 3 1. 40 (1. 16 -1. 69) . 005 1. 19 (0. 97 -1. 47) . 092 HR status: negative vs positive 0. 56 (0. 47 -0. 68) <. 0001 0. 58 (0. 47 -0. 71) <. 0001 ECOG PS: 0 vs 1 -4 1. 22 (1. 01 -1. 47) . 040 1. 11 (0. 91 -1. 36) . 29 NA . 24 Age BMI: < 25 vs 25 -29 vs > 30 kg/m 2 Dall. Oncologist. 2017; 22: 131. -- -- -0. 55 (0. 46 -0. 67) <. 0001 --Slide credit: clinicaloptions. com

Adjuvant Therapy: Patients With Lower-Risk HER 2+ EBC

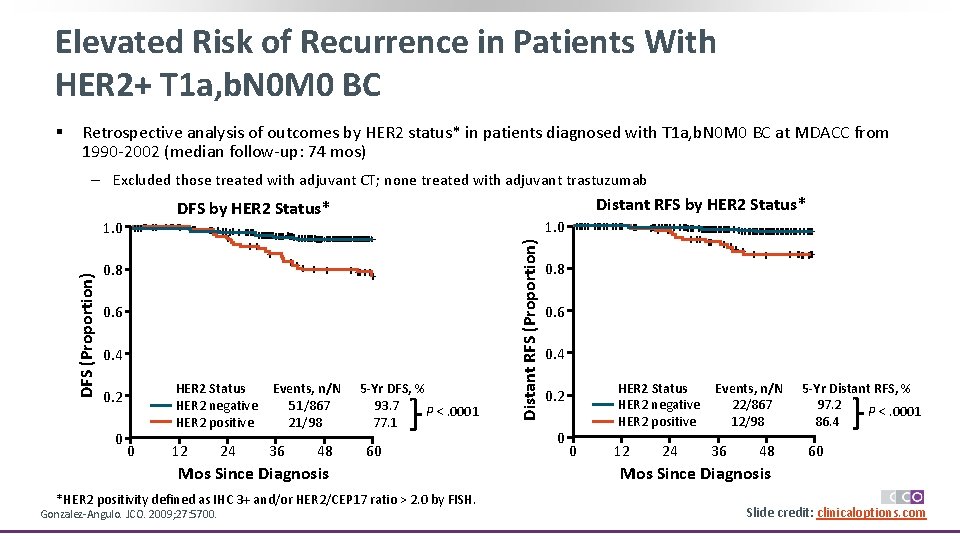
Elevated Risk of Recurrence in Patients With HER 2+ T 1 a, b. N 0 M 0 BC § Retrospective analysis of outcomes by HER 2 status* in patients diagnosed with T 1 a, b. N 0 M 0 BC at MDACC from 1990 -2002 (median follow-up: 74 mos) ‒ Excluded those treated with adjuvant CT; none treated with adjuvant trastuzumab Distant RFS by HER 2 Status* 1. 0 +++++++ ++ +++++++++++ + +++ + + 0. 8 + ++++++ 0. 6 0. 4 HER 2 Status Events, n/N 5 -Yr DFS, % HER 2 negative 51/867 93. 7 P <. 0001 HER 2 positive 21/98 77. 1 0. 2 0 0 12 24 36 48 60 Mos Since Diagnosis *HER 2 positivity defined as IHC 3+ and/or HER 2/CEP 17 ratio > 2. 0 by FISH. Gonzalez-Angulo. JCO. 2009; 27: 5700. Distant RFS (Proportion) DFS by HER 2 Status* 1. 0 +++++++ ++ +++++++++++++++++++++++++++++ ++ + + ++++++ 0. 8 0. 6 0. 4 HER 2 Status Events, n/N 5 -Yr Distant RFS, % HER 2 negative 22/867 97. 2 P <. 0001 HER 2 positive 12/98 86. 4 0. 2 0 0 12 24 36 48 60 Mos Since Diagnosis Slide credit: clinicaloptions. com

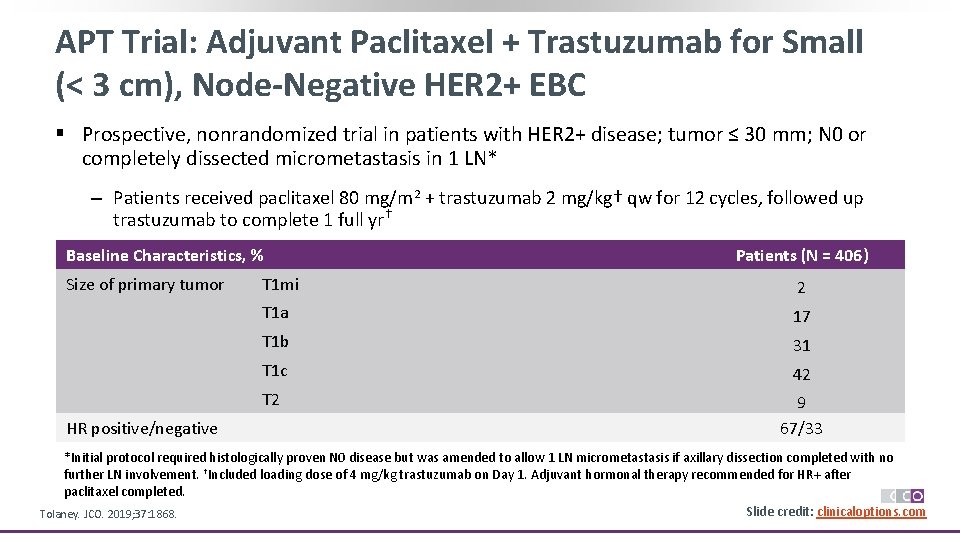
APT Trial: Adjuvant Paclitaxel + Trastuzumab for Small (< 3 cm), Node-Negative HER 2+ EBC § Prospective, nonrandomized trial in patients with HER 2+ disease; tumor ≤ 30 mm; N 0 or completely dissected micrometastasis in 1 LN* ‒ Patients received paclitaxel 80 mg/m 2 + trastuzumab 2 mg/kg† qw for 12 cycles, followed up trastuzumab to complete 1 full yr† Baseline Characteristics, % Size of primary tumor HR positive/negative Patients (N = 406) T 1 mi 2 T 1 a 17 T 1 b 31 T 1 c 42 T 2 9 67/33 *Initial protocol required histologically proven N 0 disease but was amended to allow 1 LN micrometastasis if axillary dissection completed with no further LN involvement. †Included loading dose of 4 mg/kg trastuzumab on Day 1. Adjuvant hormonal therapy recommended for HR+ after paclitaxel completed. Slide credit: clinicaloptions. com Tolaney. JCO. 2019; 37: 1868.

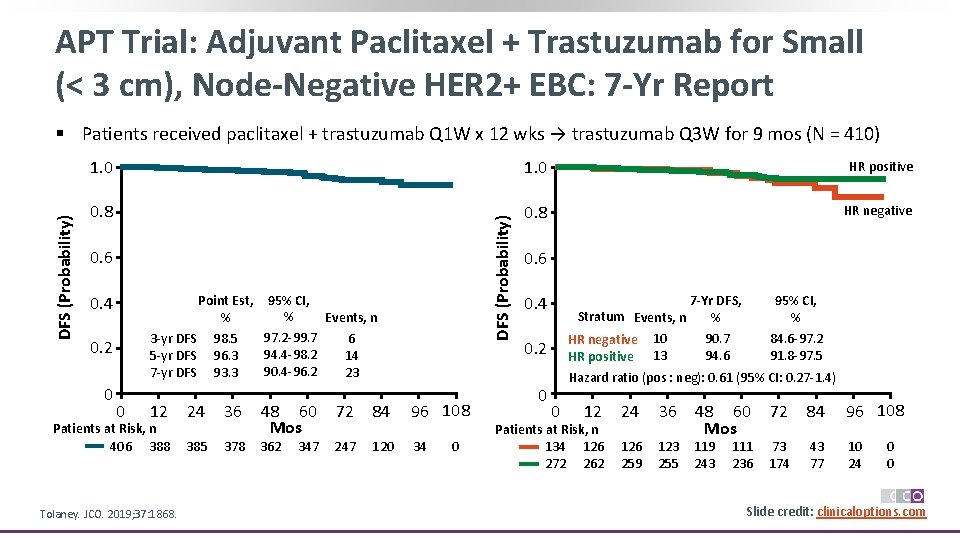
APT Trial: Adjuvant Paclitaxel + Trastuzumab for Small (< 3 cm), Node-Negative HER 2+ EBC: 7 -Yr Report 1. 0 HR positive 0. 8 HR negative DFS (Probability) § Patients received paclitaxel + trastuzumab Q 1 W x 12 wks → trastuzumab Q 3 W for 9 mos (N = 410) 0. 6 0. 4 95% CI, % Events, n 98. 5 96. 3 93. 3 97. 2 -99. 7 94. 4 -98. 2 90. 4 -96. 2 6 14 23 3 -yr DFS 5 -yr DFS 7 -yr DFS 0. 2 0 Point Est, % 0 12 Patients at Risk, n 406 388 Tolaney. JCO. 2019; 37: 1868. 24 36 385 378 48 60 Mos 362 347 72 84 96 108 247 120 34 0 0. 6 7 -Yr DFS, Stratum Events, n % 0. 4 HR negative HR positive 0. 2 0 10 13 95% CI, % 90. 7 94. 6 84. 6 -97. 2 91. 8 -97. 5 Hazard ratio (pos : neg): 0. 61 (95% CI: 0. 27 -1. 4) 0 12 Patients at Risk, n 134 126 272 262 24 36 126 259 123 255 48 60 Mos 119 243 111 236 72 84 96 108 73 174 43 77 10 24 0 0 Slide credit: clinicaloptions. com

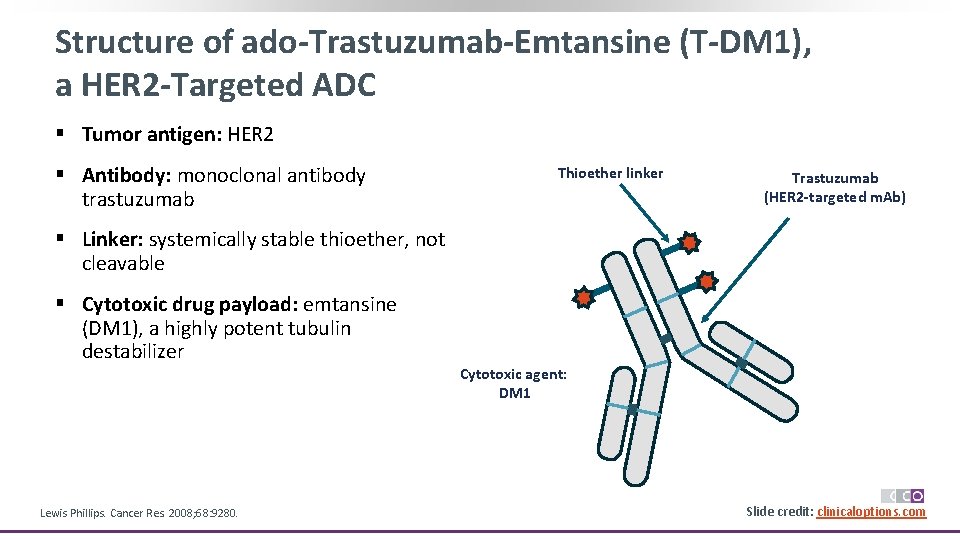
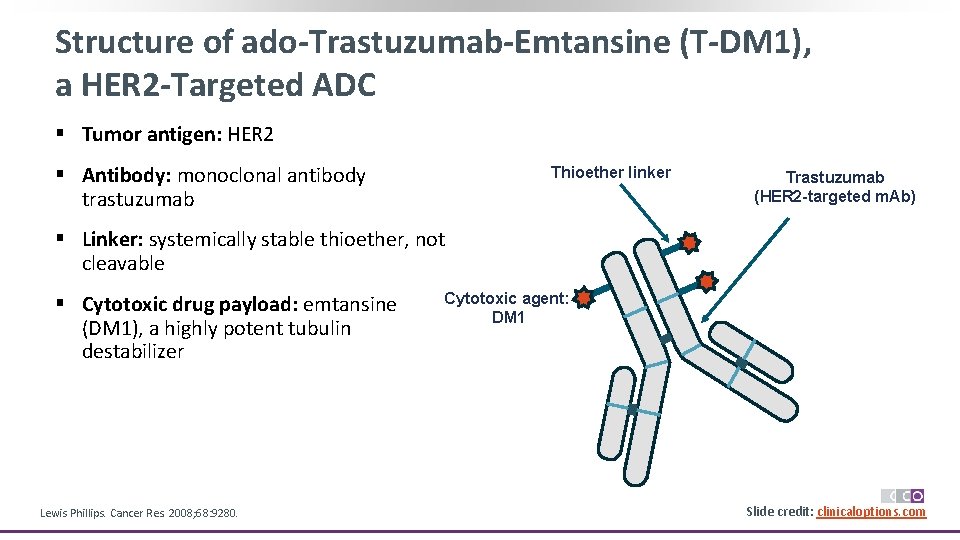
Structure of ado-Trastuzumab-Emtansine (T-DM 1), a HER 2 -Targeted ADC § Tumor antigen: HER 2 § Antibody: monoclonal antibody trastuzumab Thioether linker Trastuzumab (HER 2 -targeted m. Ab) § Linker: systemically stable thioether, not cleavable § Cytotoxic drug payload: emtansine (DM 1), a highly potent tubulin destabilizer Lewis Phillips. Cancer Res. 2008; 68: 9280. Cytotoxic agent: DM 1 Slide credit: clinicaloptions. com

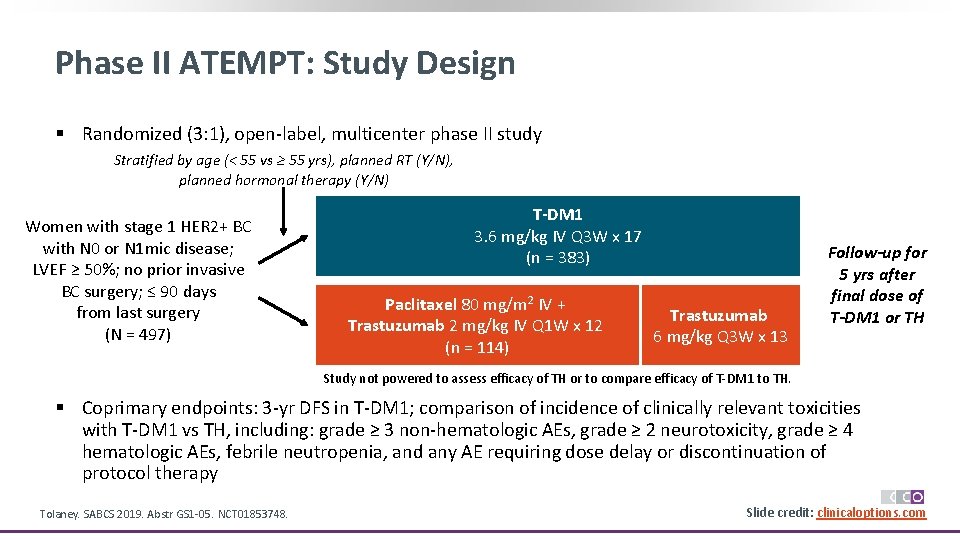
Phase II ATEMPT: Study Design § Randomized (3: 1), open-label, multicenter phase II study Stratified by age (< 55 vs ≥ 55 yrs), planned RT (Y/N), planned hormonal therapy (Y/N) Women with stage 1 HER 2+ BC with N 0 or N 1 mic disease; LVEF ≥ 50%; no prior invasive BC surgery; ≤ 90 days from last surgery (N = 497) T-DM 1 3. 6 mg/kg IV Q 3 W x 17 (n = 383) Paclitaxel 80 mg/m 2 IV + Trastuzumab 2 mg/kg IV Q 1 W x 12 (n = 114) Trastuzumab 6 mg/kg Q 3 W x 13 Follow-up for 5 yrs after final dose of T-DM 1 or TH Study not powered to assess efficacy of TH or to compare efficacy of T-DM 1 to TH. § Coprimary endpoints: 3 -yr DFS in T-DM 1; comparison of incidence of clinically relevant toxicities with T-DM 1 vs TH, including: grade ≥ 3 non-hematologic AEs, grade ≥ 2 neurotoxicity, grade ≥ 4 hematologic AEs, febrile neutropenia, and any AE requiring dose delay or discontinuation of protocol therapy Tolaney. SABCS 2019. Abstr GS 1 -05. NCT 01853748. Slide credit: clinicaloptions. com

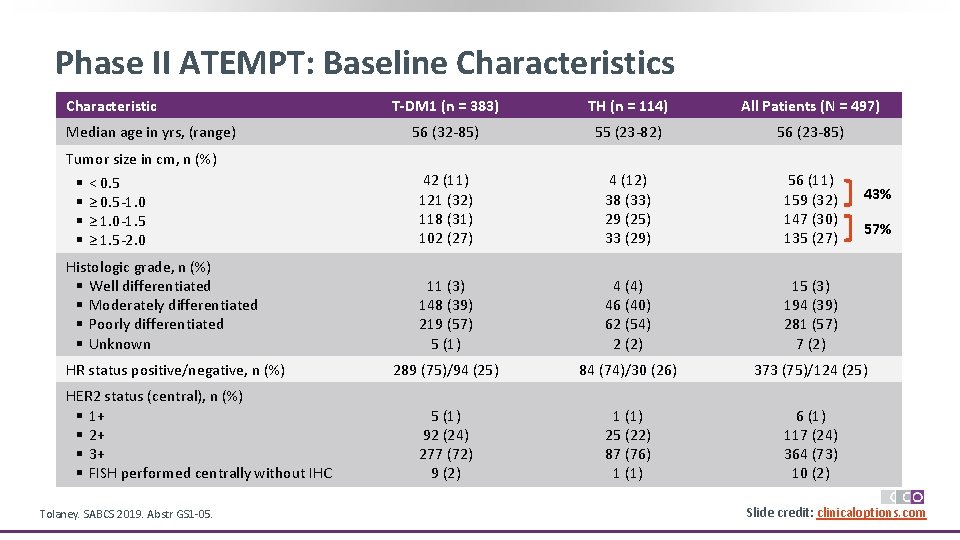
Phase II ATEMPT: Baseline Characteristics Characteristic Median age in yrs, (range) T-DM 1 (n = 383) TH (n = 114) All Patients (N = 497) 56 (32 -85) 55 (23 -82) 56 (23 -85) 42 (11) 121 (32) 118 (31) 102 (27) 4 (12) 38 (33) 29 (25) 33 (29) 56 (11) 159 (32) 147 (30) 135 (27) 11 (3) 148 (39) 219 (57) 5 (1) 4 (4) 46 (40) 62 (54) 2 (2) 15 (3) 194 (39) 281 (57) 7 (2) 289 (75)/94 (25) 84 (74)/30 (26) 373 (75)/124 (25) 5 (1) 92 (24) 277 (72) 9 (2) 1 (1) 25 (22) 87 (76) 1 (1) 6 (1) 117 (24) 364 (73) 10 (2) Tumor size in cm, n (%) § § < 0. 5 ≥ 0. 5 -1. 0 ≥ 1. 0 -1. 5 ≥ 1. 5 -2. 0 Histologic grade, n (%) § Well differentiated § Moderately differentiated § Poorly differentiated § Unknown HR status positive/negative, n (%) HER 2 status (central), n (%) § 1+ § 2+ § 3+ § FISH performed centrally without IHC Tolaney. SABCS 2019. Abstr GS 1 -05. 43% 57% Slide credit: clinicaloptions. com

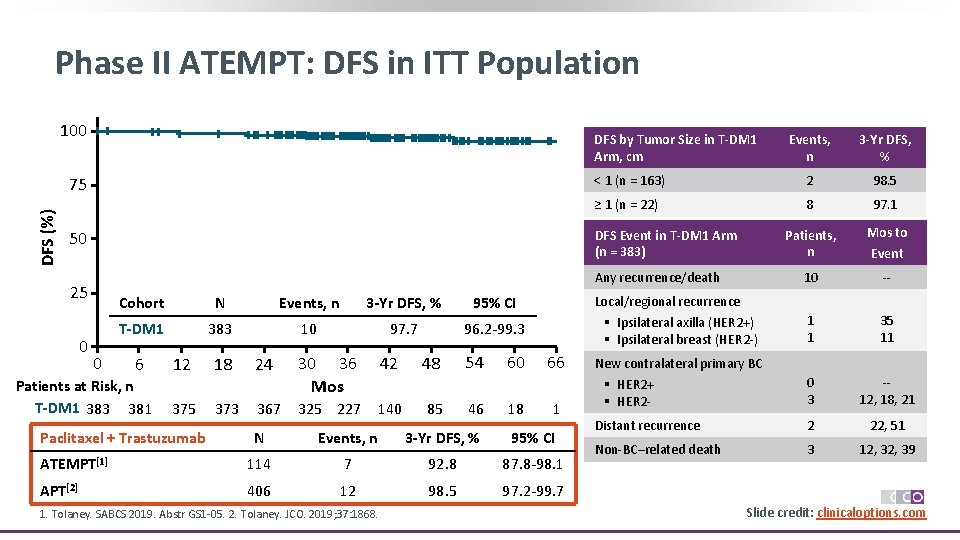
Phase II ATEMPT: DFS in ITT Population 100 DFS by Tumor Size in T-DM 1 Arm, cm DFS (%) 75 3 -Yr DFS, % < 1 (n = 163) 2 98. 5 ≥ 1 (n = 22) 8 97. 1 Patients, n Mos to 10 -- 1 1 35 11 0 3 -12, 18, 21 Distant recurrence 2 22, 51 Non-BC–related death 3 12, 39 DFS Event in T-DM 1 Arm (n = 383) 50 Any recurrence/death 25 0 Events, n 0 Cohort N Events, n 3 -Yr DFS, % 95% CI T-DM 1 383 10 97. 7 96. 2 -99. 3 6 Patients at Risk, n T-DM 1 383 381 12 375 Paclitaxel + Trastuzumab 18 373 24 367 30 36 Mos 42 325 227 140 48 85 54 46 60 18 Local/regional recurrence § Ipsilateral axilla (HER 2+) § Ipsilateral breast (HER 2 -) 66 1 N Events, n 3 -Yr DFS, % 95% CI ATEMPT[1] 114 7 92. 8 87. 8 -98. 1 APT[2] 406 12 98. 5 97. 2 -99. 7 1. Tolaney. SABCS 2019. Abstr GS 1 -05. 2. Tolaney. JCO. 2019; 37: 1868. Event New contralateral primary BC § HER 2+ § HER 2 - Slide credit: clinicaloptions. com

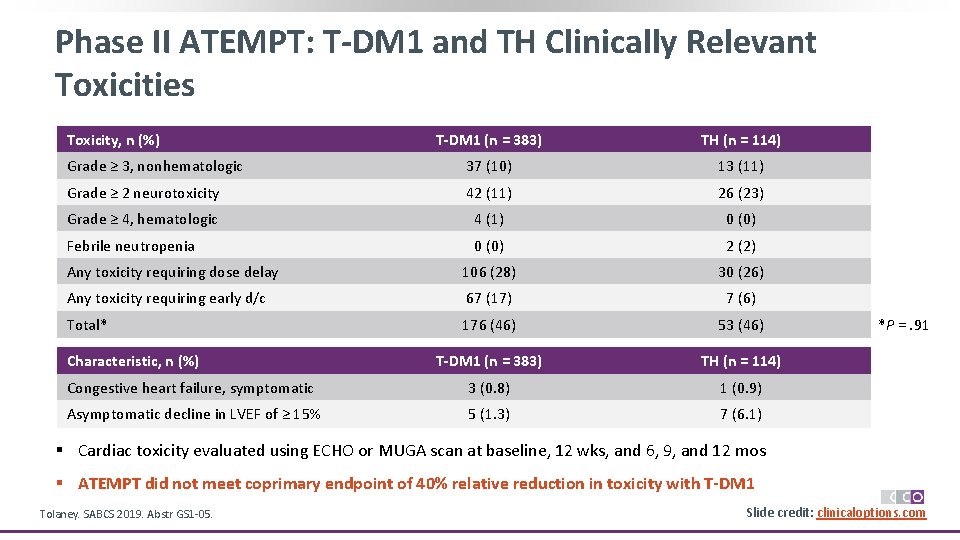
Phase II ATEMPT: T-DM 1 and TH Clinically Relevant Toxicities Toxicity, n (%) T-DM 1 (n = 383) TH (n = 114) Grade ≥ 3, nonhematologic 37 (10) 13 (11) Grade ≥ 2 neurotoxicity 42 (11) 26 (23) Grade ≥ 4, hematologic 4 (1) 0 (0) Febrile neutropenia 0 (0) 2 (2) Any toxicity requiring dose delay 106 (28) 30 (26) Any toxicity requiring early d/c 67 (17) 7 (6) Total* 176 (46) 53 (46) T-DM 1 (n = 383) TH (n = 114) Congestive heart failure, symptomatic 3 (0. 8) 1 (0. 9) Asymptomatic decline in LVEF of ≥ 15% 5 (1. 3) 7 (6. 1) Characteristic, n (%) *P =. 91 § Cardiac toxicity evaluated using ECHO or MUGA scan at baseline, 12 wks, and 6, 9, and 12 mos § ATEMPT did not meet coprimary endpoint of 40% relative reduction in toxicity with T-DM 1 Tolaney. SABCS 2019. Abstr GS 1 -05. Slide credit: clinicaloptions. com

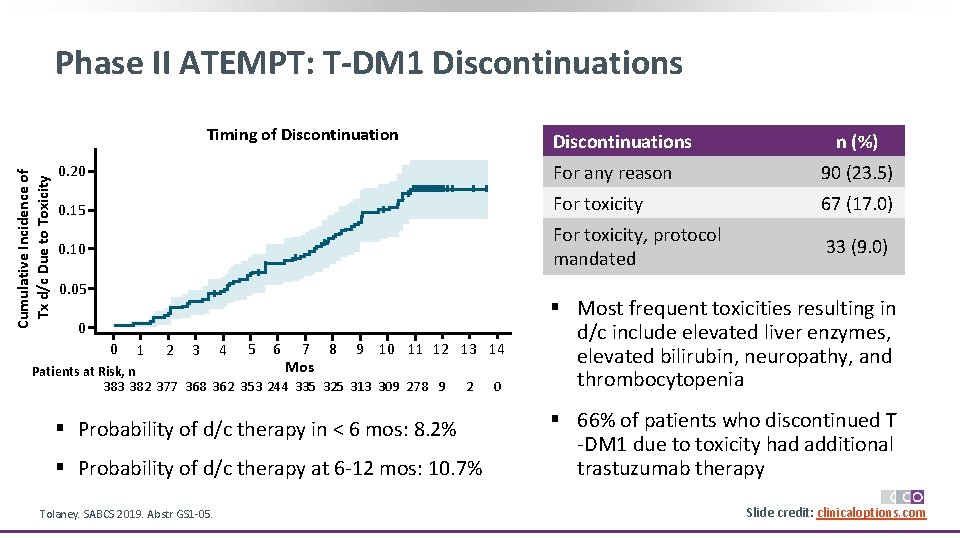
Phase II ATEMPT: T-DM 1 Discontinuations Cumulative Incidence of Tx d/c Due to Toxicity Timing of Discontinuations n (%) 0. 20 For any reason 90 (23. 5) 0. 15 For toxicity 67 (17. 0) 0. 10 For toxicity, protocol mandated 33 (9. 0) 0. 05 0 0 1 2 3 4 5 6 7 8 Mos 9 10 11 12 13 14 Patients at Risk, n 383 382 377 368 362 353 244 335 325 313 309 278 9 2 § Probability of d/c therapy in < 6 mos: 8. 2% § Probability of d/c therapy at 6 -12 mos: 10. 7% Tolaney. SABCS 2019. Abstr GS 1 -05. 0 § Most frequent toxicities resulting in d/c include elevated liver enzymes, elevated bilirubin, neuropathy, and thrombocytopenia § 66% of patients who discontinued T -DM 1 due to toxicity had additional trastuzumab therapy Slide credit: clinicaloptions. com

Neoadjuvant Therapy

Why Use Neoadjuvant Systemic Therapy? § Reduce tumor size, increasing chances that patient can have breast-conserving surgery (lumpectomy) rather than mastectomy; potential for less axillary surgery § Treat patients at high risk for metastatic disease with systemic therapy without delay § Allows patient and doctor to see if the tumor responds to treatment (and allows to change therapy if not working); “response-guided approach” § Assessing pathologic response at surgery (residual cancer vs p. CR) is prognostic and can help guide treatment recommendations § No difference in survival comparing patients who have had chemo before or after surgery Gralow. JCO. 2008; 26: 814. Kaufmann. JCO. 2006; 24: 1940. Slide credit: clinicaloptions. com

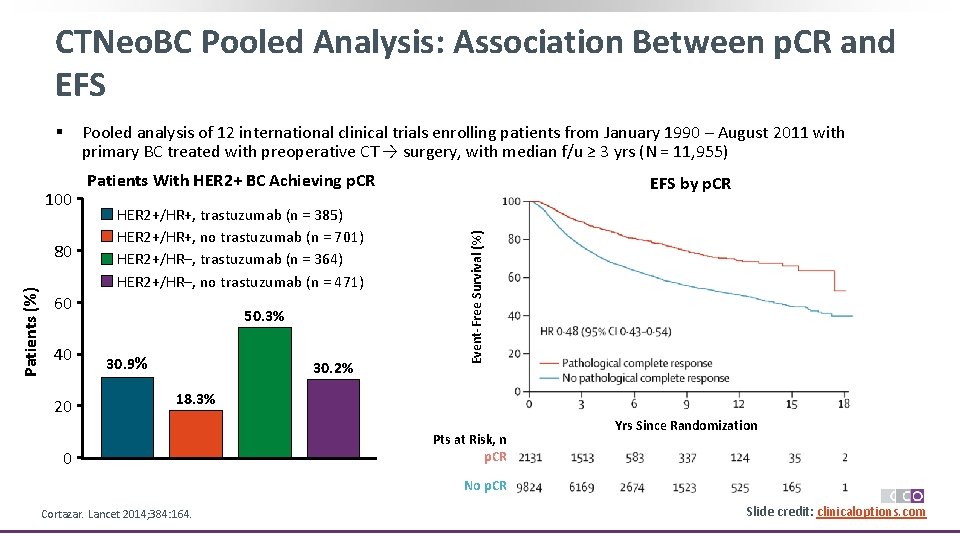
CTNeo. BC Pooled Analysis: Association Between p. CR and EFS 100 Patients (%) 80 Pooled analysis of 12 international clinical trials enrolling patients from January 1990 – August 2011 with primary BC treated with preoperative CT → surgery, with median f/u ≥ 3 yrs (N = 11, 955) Patients With HER 2+ BC Achieving p. CR HER 2+/HR+, trastuzumab (n = 385) HER 2+/HR+, no trastuzumab (n = 701) HER 2+/HR–, trastuzumab (n = 364) HER 2+/HR–, no trastuzumab (n = 471) 60 40 20 50. 3% 30. 9% 30. 2% EFS by p. CR 100 Event-Free Survival (%) § 60 40 20 0 18. 3% 0 80 Pts at Risk, n p. CR Yrs Since Randomization No p. CR Cortazar. Lancet 2014; 384: 164. Slide credit: clinicaloptions. com

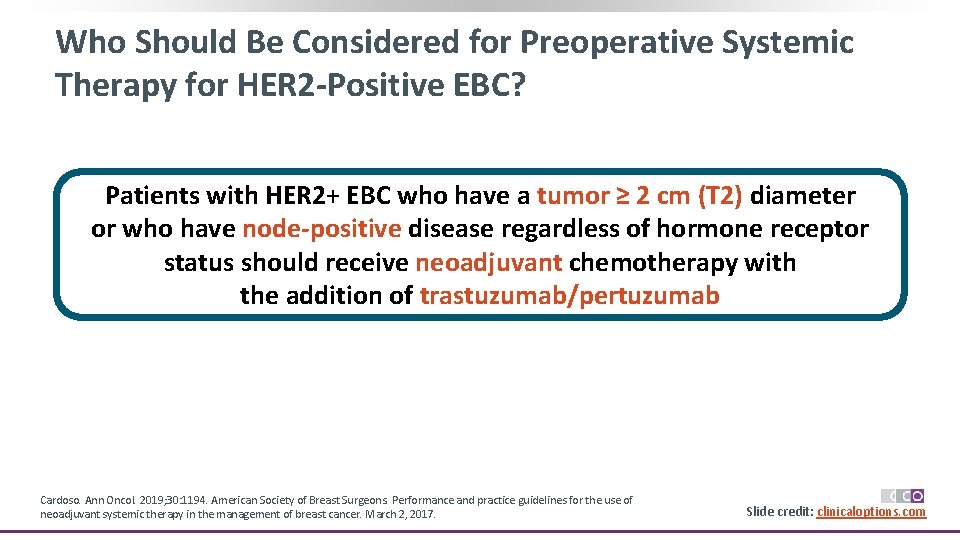
Who Should Be Considered for Preoperative Systemic Therapy for HER 2 -Positive EBC? Patients with HER 2+ EBC who have a tumor ≥ 2 cm (T 2) diameter or who have node-positive disease regardless of hormone receptor status should receive neoadjuvant chemotherapy with the addition of trastuzumab/pertuzumab Cardoso. Ann Oncol. 2019; 30: 1194. American Society of Breast Surgeons. Performance and practice guidelines for the use of neoadjuvant systemic therapy in the management of breast cancer. March 2, 2017. Slide credit: clinicaloptions. com

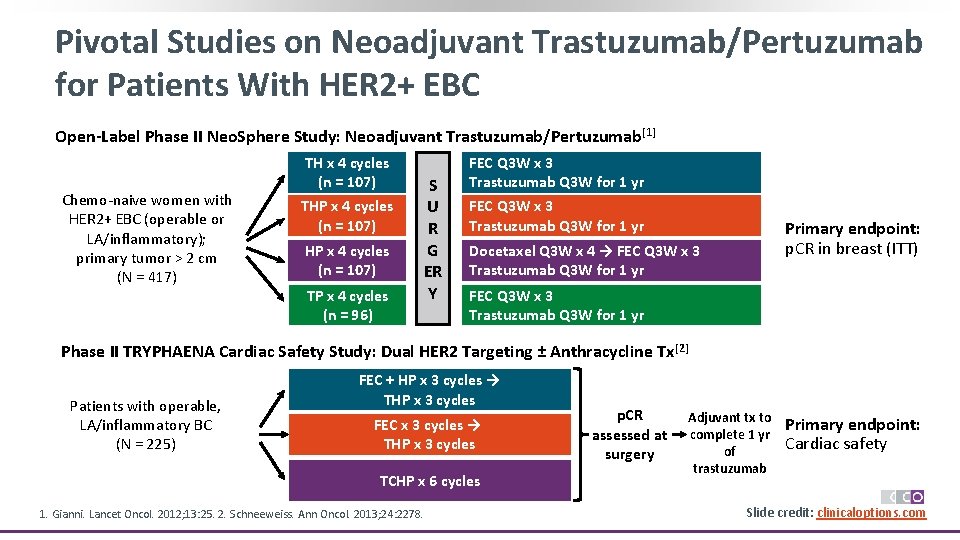
Pivotal Studies on Neoadjuvant Trastuzumab/Pertuzumab for Patients With HER 2+ EBC Open-Label Phase II Neo. Sphere Study: Neoadjuvant Trastuzumab/Pertuzumab [1] Chemo-naive women with HER 2+ EBC (operable or LA/inflammatory); primary tumor > 2 cm (N = 417) TH x 4 cycles (n = 107) THP x 4 cycles (n = 107) TP x 4 cycles (n = 96) S U R G ER Y FEC Q 3 W x 3 Trastuzumab Q 3 W for 1 yr Primary endpoint: p. CR in breast (ITT) Docetaxel Q 3 W x 4 → FEC Q 3 W x 3 Trastuzumab Q 3 W for 1 yr Phase II TRYPHAENA Cardiac Safety Study: Dual HER 2 Targeting ± Anthracycline Tx [2] Patients with operable, LA/inflammatory BC (N = 225) FEC + HP x 3 cycles → THP x 3 cycles FEC x 3 cycles → THP x 3 cycles TCHP x 6 cycles 1. Gianni. Lancet Oncol. 2012; 13: 25. 2. Schneeweiss. Ann Oncol. 2013; 24: 2278. p. CR assessed at surgery Adjuvant tx to complete 1 yr of trastuzumab Primary endpoint: Cardiac safety Slide credit: clinicaloptions. com

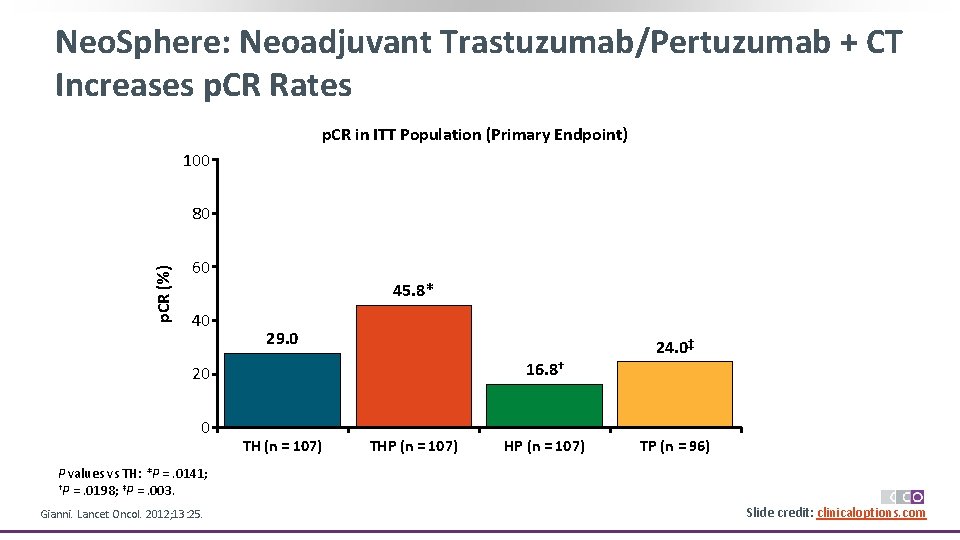
Neo. Sphere: Neoadjuvant Trastuzumab/Pertuzumab + CT Increases p. CR Rates p. CR in ITT Population (Primary Endpoint) 100 p. CR (%) 80 60 45. 8* 40 29. 0 16. 8† 20 0 TH (n = 107) THP (n = 107) 24. 0‡ TP (n = 96) P values vs TH: *P =. 0141; †P =. 0198; ‡P =. 003. Gianni. Lancet Oncol. 2012; 13: 25. Slide credit: clinicaloptions. com

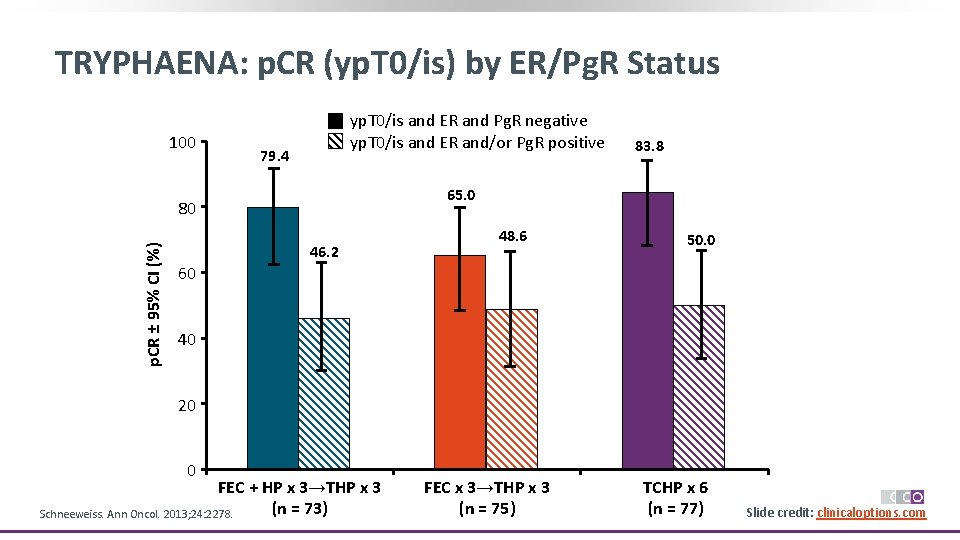
TRYPHAENA: p. CR (yp. T 0/is) by ER/Pg. R Status 100 yp. T 0/is and ER and Pg. R negative yp. T 0/is and ER and/or Pg. R positive 79. 4 65. 0 80 p. CR ± 95% CI (%) 83. 8 46. 2 48. 6 50. 0 60 40 20 0 FEC + HP x 3→THP x 3 (n = 73) Schneeweiss. Ann Oncol. 2013; 24: 2278. FEC x 3→THP x 3 (n = 75) TCHP x 6 (n = 77) Slide credit: clinicaloptions. com

Adjuvant Therapy: Patients with Higher-Risk HER 2+ EBC

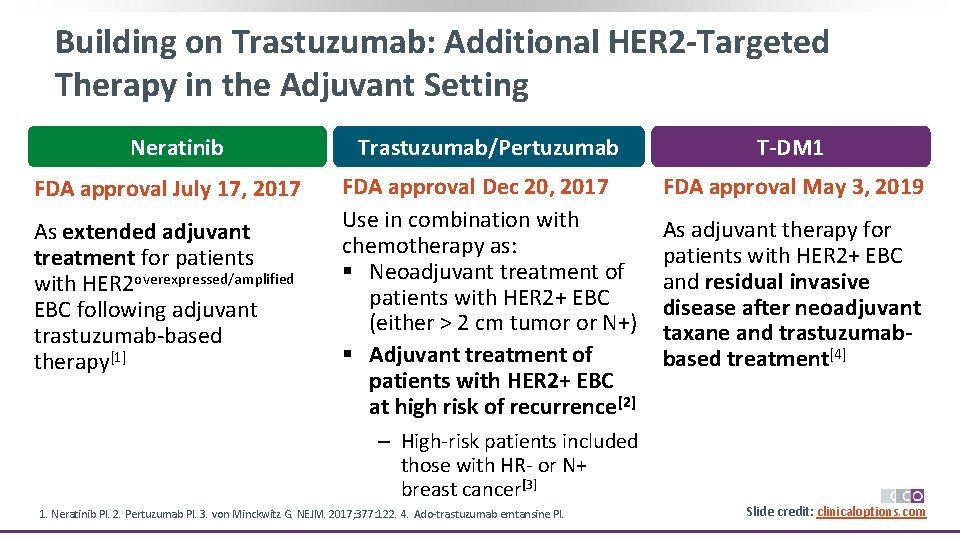
Building on Trastuzumab: Additional HER 2 -Targeted Therapy in the Adjuvant Setting Neratinib FDA approval July 17, 2017 As extended adjuvant treatment for patients with HER 2 overexpressed/amplified EBC following adjuvant trastuzumab-based therapy[1] Trastuzumab/Pertuzumab T-DM 1 FDA approval Dec 20, 2017 Use in combination with chemotherapy as: § Neoadjuvant treatment of patients with HER 2+ EBC (either > 2 cm tumor or N+) § Adjuvant treatment of patients with HER 2+ EBC at high risk of recurrence[2] FDA approval May 3, 2019 As adjuvant therapy for patients with HER 2+ EBC and residual invasive disease after neoadjuvant taxane and trastuzumabbased treatment[4] ‒ High-risk patients included those with HR- or N+ breast cancer[3] 1. Neratinib PI. 2. Pertuzumab PI. 3. von Minckwitz G. NEJM. 2017; 377: 122. 4. Ado-trastuzumab emtansine PI. Slide credit: clinicaloptions. com

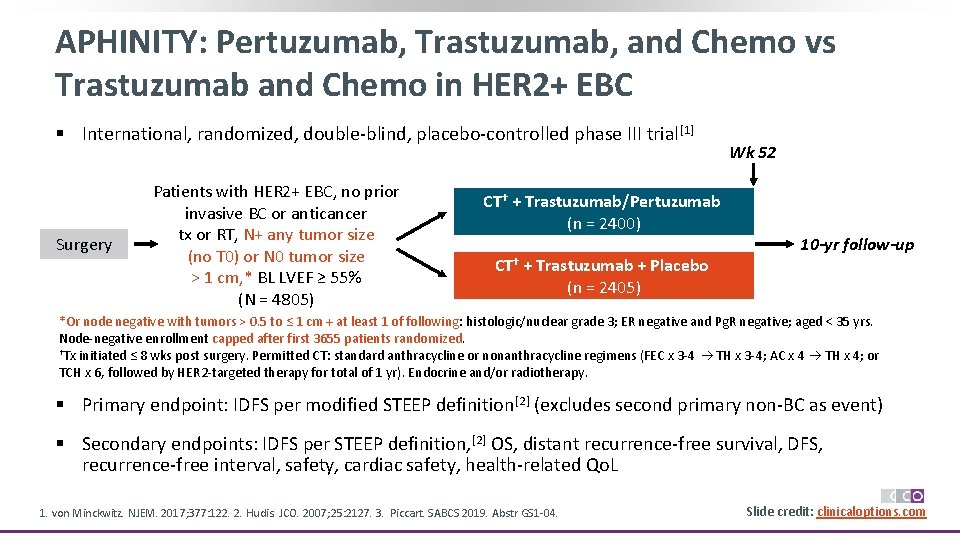
APHINITY: Pertuzumab, Trastuzumab, and Chemo vs Trastuzumab and Chemo in HER 2+ EBC § International, randomized, double-blind, placebo-controlled phase III trial [1] Surgery Patients with HER 2+ EBC, no prior invasive BC or anticancer tx or RT, N+ any tumor size (no T 0) or N 0 tumor size > 1 cm, * BL LVEF ≥ 55% (N = 4805) CT† + Trastuzumab/Pertuzumab (n = 2400) CT† + Trastuzumab + Placebo (n = 2405) Wk 52 10 -yr follow-up *Or node negative with tumors > 0. 5 to ≤ 1 cm + at least 1 of following: histologic/nuclear grade 3; ER negative and Pg. R negative; aged < 35 yrs. Node-negative enrollment capped after first 3655 patients randomized. †Tx initiated ≤ 8 wks post surgery. Permitted CT: standard anthracycline or nonanthracycline regimens (FEC x 3 -4 TH x 3 -4; AC x 4 TH x 4; or TCH x 6, followed by HER 2 -targeted therapy for total of 1 yr). Endocrine and/or radiotherapy. could be started at end of adjuvant CT. § Primary endpoint: IDFS per modified STEEP definition[2] (excludes second primary non-BC as event) § Secondary endpoints: IDFS per STEEP definition, [2] OS, distant recurrence-free survival, DFS, recurrence-free interval, safety, cardiac safety, health-related Qo. L 1. von Minckwitz. NJEM. 2017; 377: 122. 2. Hudis. JCO. 2007; 25: 2127. 3. Piccart. SABCS 2019. Abstr GS 1 -04. Slide credit: clinicaloptions. com

APHINITY: Updated Analysis of IDFS in ITT Population Yr 3 100 Yr 6 94. 1% 90. 6% 93. 2% 87. 8% IDFS (%) 80 60 Events, n (%) 40 Pertuzumab (n = 2400) Placebo (n = 2404) 221 (9. 2) 287 (11. 9) Stratified HR 0. 76 (95% CI: 0. 64 -0. 91) Median follow-up, mos 20 74. 1 6 -yr duration Difference in event-free rate, % 0 0 Patients at Risk, n Pertuzumab 2400 Placebo 2404 2. 8 1 2 3 4 5 6 2277 2312 2198 2215 2122 2134 2055 2039 1978 1967 1482 1421 Yrs From Randomization § OS data immature at time of analysis (maturity: 42. 5%); Stratified HR: 0. 85 (95% CI: 0. 67 -1. 07); P =. 170 (P value of. 0012 required for statistically significant difference ) Piccart. SABCS 2019. Abstr GS 1 -04. Slide credit: clinicaloptions. com

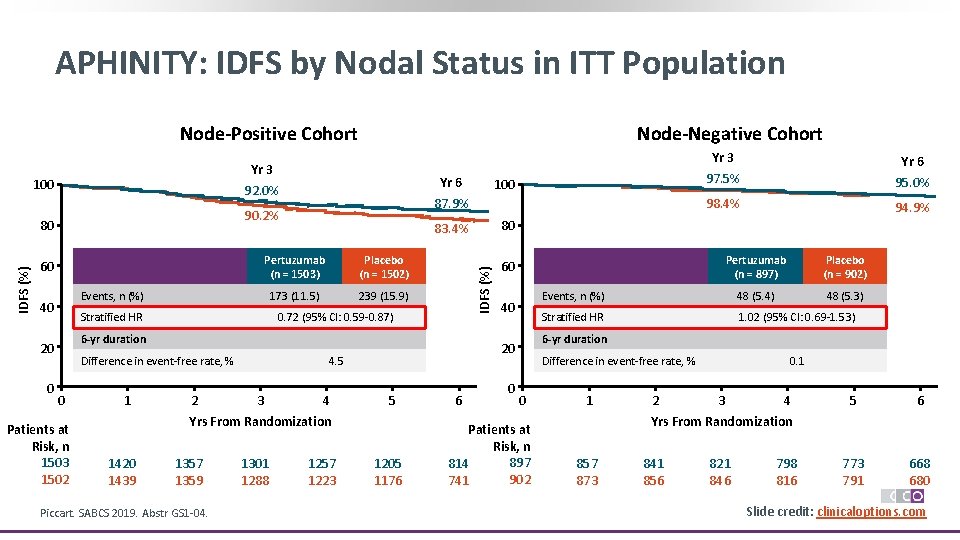
APHINITY: IDFS by Nodal Status in ITT Population Node-Positive Cohort 90. 2% IDFS (%) 80 60 Pertuzumab (n = 1503) Placebo (n = 1502) 173 (11. 5) 239 (15. 9) Events, n (%) 40 Stratified HR Yr 6 87. 9% 100 83. 4% 80 IDFS (%) Yr 3 92. 0% 100 0. 72 (95% CI: 0. 59 -0. 87) 6 -yr duration 20 0 Node-Negative Cohort Difference in event-free rate, % 0 Patients at Risk, n 1503 1502 1 2 3 4 5 Yrs From Randomization 1420 1439 1357 1359 Piccart. SABCS 2019. Abstr GS 1 -04. 1301 1288 Mos 1257 1223 1205 1176 6 Yr 6 95. 0% 98. 4% 94. 9% Pertuzumab (n = 897) Placebo (n = 902) Events, n (%) 48 (5. 4) 48 (5. 3) Stratified HR 1. 02 (95% CI: 0. 69 -1. 53) 60 40 6 -yr duration 20 4. 5 Yr 3 97. 5% 0 Difference in event-free rate, % 0 Patients at Risk, n 897 814 902 741 1 2 0. 1 3 4 5 6 773 791 668 680 Yrs From Randomization 857 873 841 856 821 846 798 816 Slide credit: clinicaloptions. com

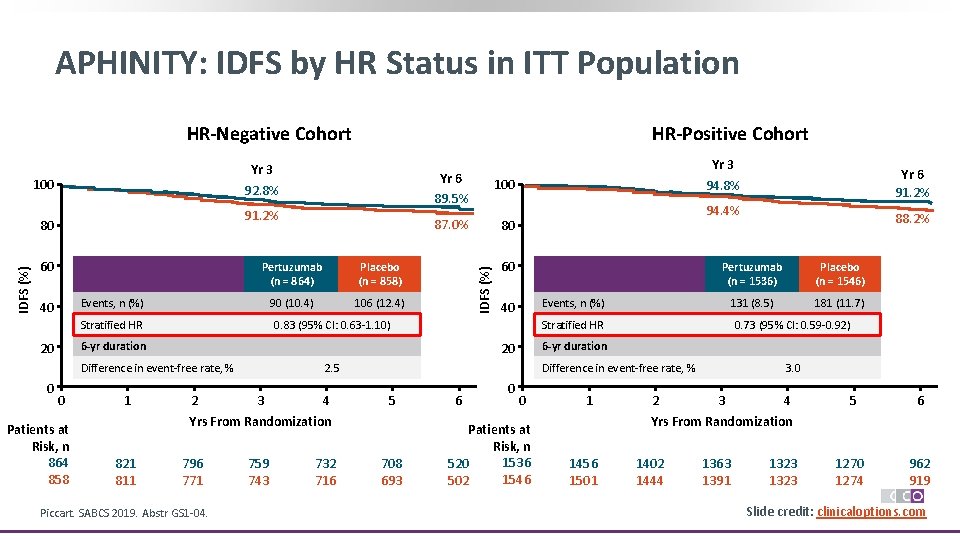
APHINITY: IDFS by HR Status in ITT Population HR-Negative Cohort 91. 2% IDFS (%) 80 60 Pertuzumab (n = 864) Placebo (n = 858) 90 (10. 4) 106 (12. 4) Events, n (%) 40 Stratified HR Yr 6 89. 5% 100 87. 0% 80 IDFS (%) Yr 3 92. 8% 100 Patients at Risk, n 864 858 1 2 4 796 771 Piccart. SABCS 2019. Abstr GS 1 -04. 759 743 732 716 Pertuzumab (n = 1536) Placebo (n = 1546) 131 (8. 5) 181 (11. 7) Events, n (%) 40 88. 2% Stratified HR 0. 73 (95% CI: 0. 59 -0. 92) 6 -yr duration Difference in event-free rate, % 5 Yrs From Randomization 821 811 60 2. 5 3 Yr 6 91. 2% 94. 4% 20 Difference in event-free rate, % 0 Yr 3 94. 8% 0. 83 (95% CI: 0. 63 -1. 10) 6 -yr duration 20 0 HR-Positive Cohort 708 693 6 0 0 Patients at Risk, n 1536 520 1546 502 1 2 3. 0 3 4 5 6 Yrs From Randomization 1456 1501 1402 1444 1363 1391 Mos 1323 1270 1274 962 919 Slide credit: clinicaloptions. com
![APHINITY Safety Pertuzumab n 2364 Placebo n 2405 Primary cardiac endpoint1 APHINITY: Safety Pertuzumab (n = 2364) Placebo (n = 2405) Primary cardiac endpoint[1] §](https://slidetodoc.com/presentation_image_h/09d69ae7a0b1a4f788139ad317707dd9/image-31.jpg)
APHINITY: Safety Pertuzumab (n = 2364) Placebo (n = 2405) Primary cardiac endpoint[1] § Heart failure NYHA III/IV + LVEF drop* § Cardiac death† 18 (0. 8) 16 (0. 7) 2 (0. 1) 8 (0. 3) 6 (0. 2) 2 (0. 1) Secondary cardiac endpoint: asymptomatic or mildly symptomatic LVEF drop ‡[1] 65 (2. 7) 68 (2. 8) 385 (16. 3) 287 (12. 1) 228 (9. 6) 232 (9. 8) 137 (7. 5) 95 (18. 0) 163 (6. 9) 377 (15. 7) 266 (11. 1) 230 (9. 6) 90 (3. 7) 59 (3. 1) 31 (6. 1) 113 (4. 7) 18 (0. 8) 20 (0. 8) Safety Outcome, n (%) Grade ≥ 3 AEs[2] § Neutropenia § Febrile neutropenia § Decreased neutrophil count § Diarrhea • Anthracycline CT • Nonanthracycline CT § Anemia Fatal AE[2] *LVEF drop defined as ejection fraction decrease ≥ 10% from BL to below 50%. †Determined by Cardiac Advisory Board per prospective definition. 1. Piccart. SABCS 2019. Abstr GS 1 -04. 2. von Minckwitz. NJEM. 2017; 377: 122 Slide credit: clinicaloptions. com

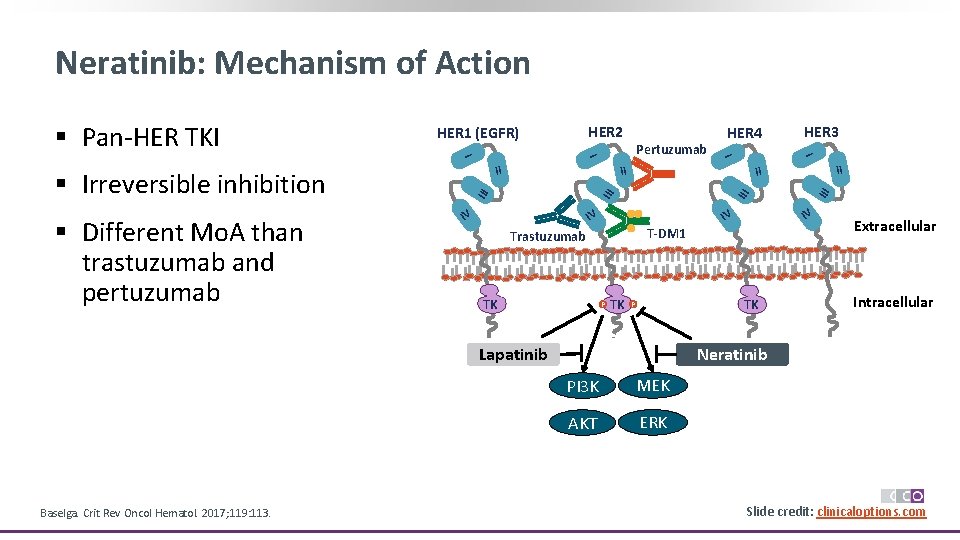
Neratinib: Mechanism of Action IV T-DM 1 P TK III IV IV Trastuzumab TK II II III IV HER 3 I II TK P Lapatinib Baselga. Crit Rev Oncol Hematol. 2017; 119: 113. HER 4 I Pertuzumab I I II § Irreversible inhibition § Different Mo. A than trastuzumab and pertuzumab HER 2 HER 1 (EGFR) III § Pan-HER TKI Extracellular Intracellular Neratinib PI 3 K MEK AKT ERK Slide credit: clinicaloptions. com

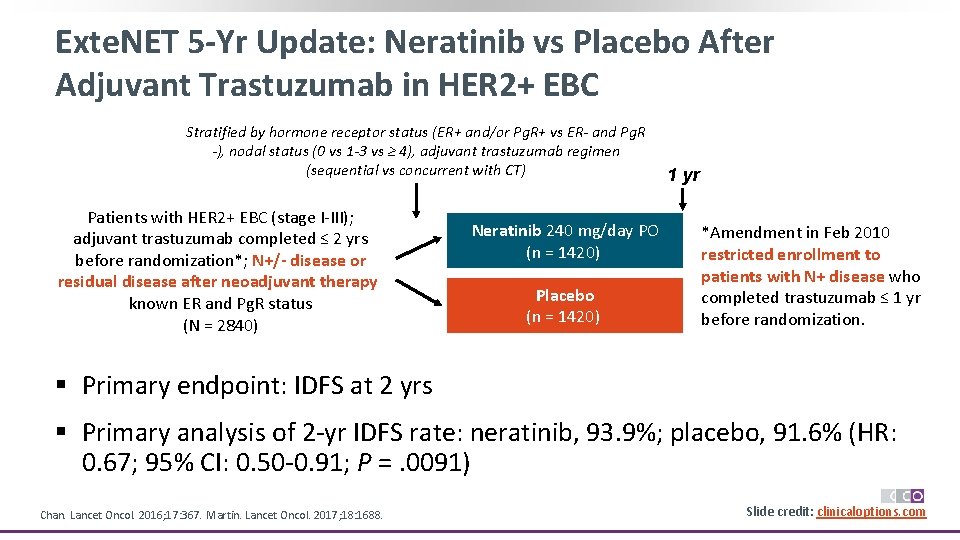
Exte. NET 5 -Yr Update: Neratinib vs Placebo After Adjuvant Trastuzumab in HER 2+ EBC Stratified by hormone receptor status (ER+ and/or Pg. R+ vs ER- and Pg. R -), nodal status (0 vs 1 -3 vs ≥ 4), adjuvant trastuzumab regimen (sequential vs concurrent with CT) Patients with HER 2+ EBC (stage I-III); adjuvant trastuzumab completed ≤ 2 yrs before randomization*; N+/- disease or residual disease after neoadjuvant therapy; known ER and Pg. R status (N = 2840) § Primary endpoint: IDFS at 2 yrs 1 yr Neratinib 240 mg/day PO (n = 1420) Placebo (n = 1420) *Amendment in Feb 2010 restricted enrollment to patients with N+ disease who completed trastuzumab ≤ 1 yr before randomization. Endocrine therapy given according to local practice § Primary analysis of 2 -yr IDFS rate: neratinib, 93. 9%; placebo, 91. 6% (HR: 0. 67; 95% CI: 0. 50 -0. 91; P =. 0091) Chan. Lancet Oncol. 2016; 17: 367. Martin. Lancet Oncol. 2017; 18: 1688. Slide credit: clinicaloptions. com

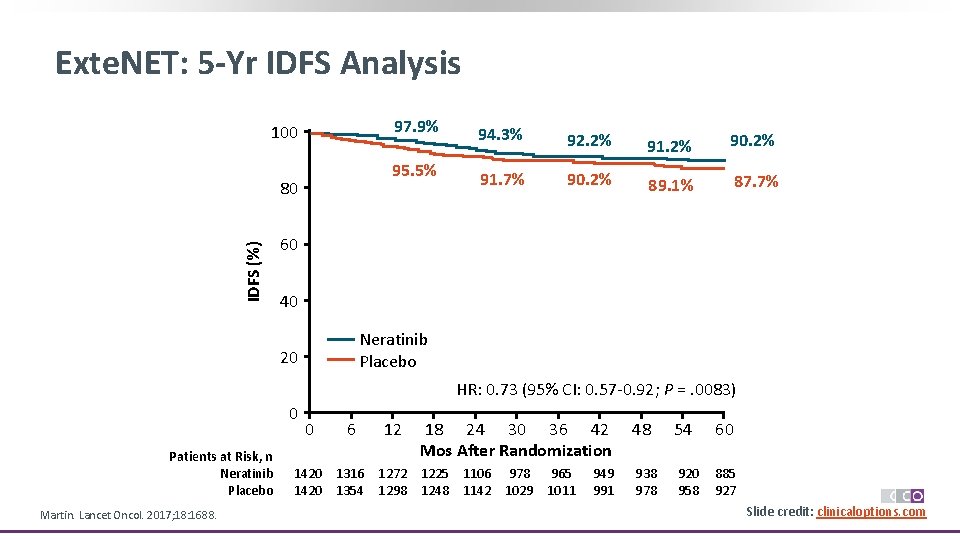
Exte. NET: 5 -Yr IDFS Analysis 97. 9% 100 95. 5% IDFS (%) 80 94. 3% 92. 2% 91. 2% 90. 2% 91. 7% 90. 2% 89. 1% 87. 7% 60 40 Neratinib Placebo 20 HR: 0. 73 (95% CI: 0. 57 -0. 92; P =. 0083) 0 Patients at Risk, n Neratinib Placebo Martin. Lancet Oncol. 2017; 18: 1688. 0 6 12 18 24 30 36 42 Mos After Randomization 1420 1316 1272 1225 1106 978 965 1420 1354 1298 1248 1142 1029 1011 949 991 48 54 60 938 978 920 958 885 927 Slide credit: clinicaloptions. com

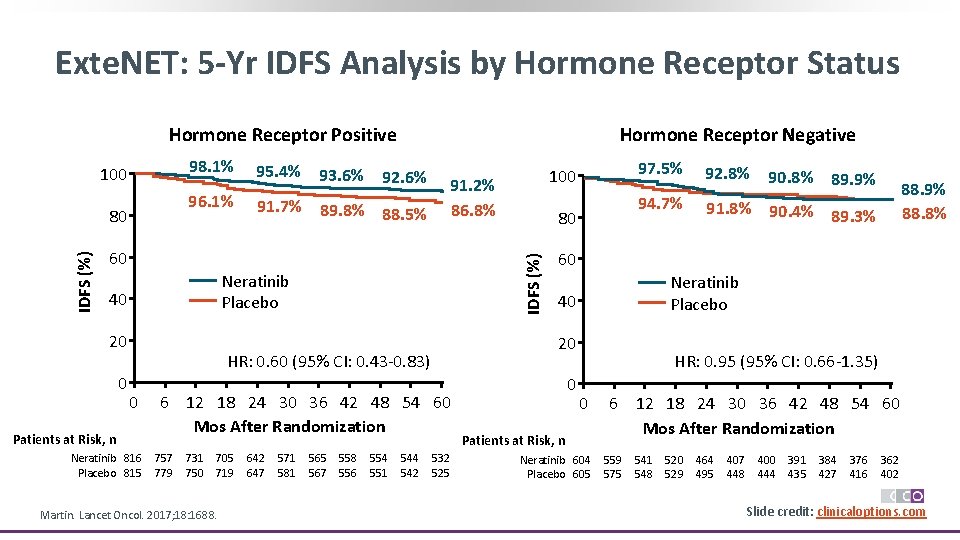
Exte. NET: 5 -Yr IDFS Analysis by Hormone Receptor Status Hormone Receptor Positive 98. 1% 100 96. 1% 93. 6% 92. 6% 91. 7% 89. 8% 88. 5% 91. 2% 86. 8% 60 Neratinib Placebo 40 20 0 Patients at Risk, n Neratinib 816 Placebo 815 6 12 18 24 30 36 42 48 54 60 Mos After Randomization 757 779 731 750 705 719 Martin. Lancet Oncol. 2017; 18: 1688. 642 647 571 581 80 565 567 558 556 554 551 544 542 97. 5% 92. 8% 94. 7% 91. 8% 90. 4% 89. 3% 532 525 90. 8% 89. 9% 88. 8% 60 Neratinib Placebo 40 20 HR: 0. 60 (95% CI: 0. 43 -0. 83) 0 100 IDFS (%) 80 95. 4% Hormone Receptor Negative 0 HR: 0. 95 (95% CI: 0. 66 -1. 35) 0 Patients at Risk, n Neratinib 604 Placebo 605 6 12 18 24 30 36 42 48 54 60 Mos After Randomization 559 575 541 548 520 529 464 495 407 448 400 444 391 435 384 427 376 416 362 402 Slide credit: clinicaloptions. com

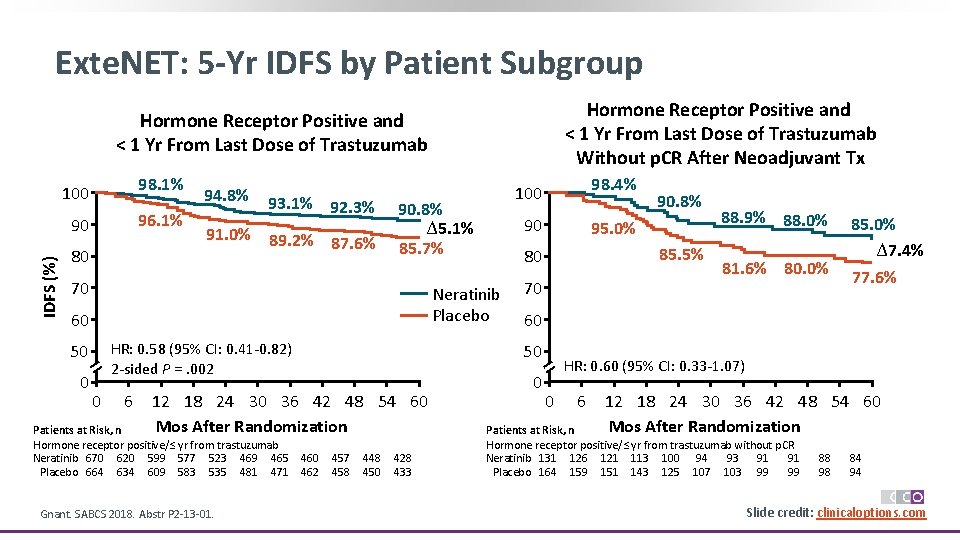
Exte. NET: 5 -Yr IDFS by Patient Subgroup Hormone Receptor Positive and < 1 Yr From Last Dose of Trastuzumab Without p. CR After Neoadjuvant Tx IDFS (%) Hormone Receptor Positive and < 1 Yr From Last Dose of Trastuzumab 100 98. 1% 90 96. 1% 80 94. 8% 93. 1% 92. 3% 91. 0% 89. 2% 87. 6% 90. 8% ∆5. 1% 85. 7% 70 HR: 0. 58 (95% CI: 0. 41 -0. 82) 2 -sided P =. 002 50 0 0 6 Gnant. SABCS 2018. Abstr P 2 -13 -01. 90 95. 0% 460 462 457 458 448 450 428 433 90. 8% 85. 5% 70 88. 9% 88. 0% 81. 6% 80. 0% 85. 0% ∆7. 4% 77. 6% 60 50 12 18 24 30 36 42 48 54 60 Mos After Randomization Patients at Risk, n Hormone receptor positive/≤ yr from trastuzumab Neratinib 670 620 599 577 523 469 465 Placebo 664 634 609 583 535 481 471 98. 4% 80 Neratinib Placebo 60 100 0 HR: 0. 60 (95% CI: 0. 33 -1. 07) 0 6 12 18 24 30 36 42 48 54 60 Mos After Randomization Patients at Risk, n Hormone receptor positive/≤ yr from trastuzumab without p. CR Neratinib 131 126 121 113 100 94 93 91 91 Placebo 164 159 151 143 125 107 103 99 99 88 98 84 94 Slide credit: clinicaloptions. com

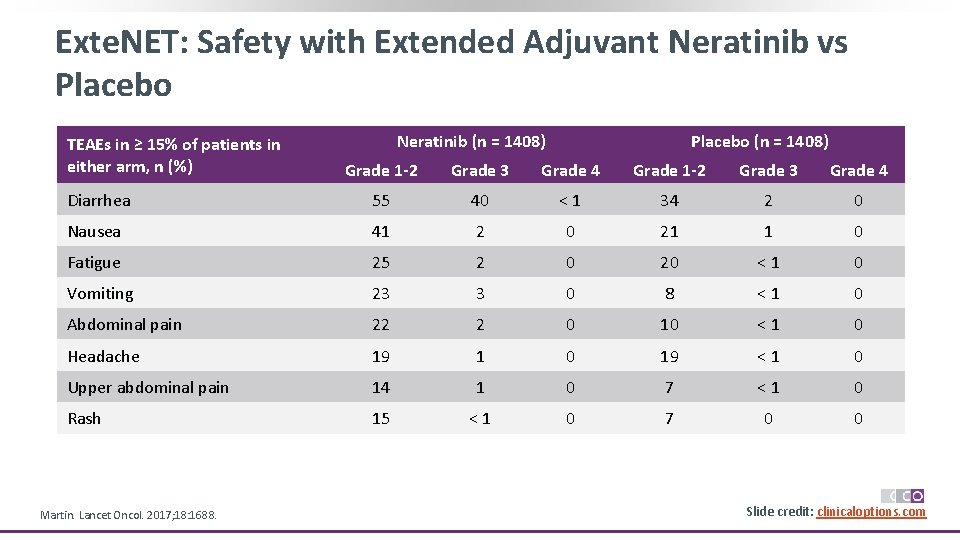
Exte. NET: Safety with Extended Adjuvant Neratinib vs Placebo TEAEs in ≥ 15% of patients in either arm, n (%) Neratinib (n = 1408) Placebo (n = 1408) Grade 1 -2 Grade 3 Grade 4 Diarrhea 55 40 < 1 34 2 0 Nausea 41 2 0 21 1 0 Fatigue 25 2 0 20 < 1 0 Vomiting 23 3 0 8 < 1 0 Abdominal pain 22 2 0 10 < 1 0 Headache 19 1 0 19 < 1 0 Upper abdominal pain 14 1 0 7 < 1 0 Rash 15 < 1 0 7 0 0 Martin. Lancet Oncol. 2017; 18: 1688. Slide credit: clinicaloptions. com

Structure of ado-Trastuzumab-Emtansine (T-DM 1), a HER 2 -Targeted ADC § Tumor antigen: HER 2 § Antibody: monoclonal antibody trastuzumab Thioether linker Trastuzumab (HER 2 -targeted m. Ab) § Linker: systemically stable thioether, not cleavable § Cytotoxic drug payload: emtansine (DM 1), a highly potent tubulin destabilizer Lewis Phillips. Cancer Res. 2008; 68: 9280. Cytotoxic agent: DM 1 Slide credit: clinicaloptions. com

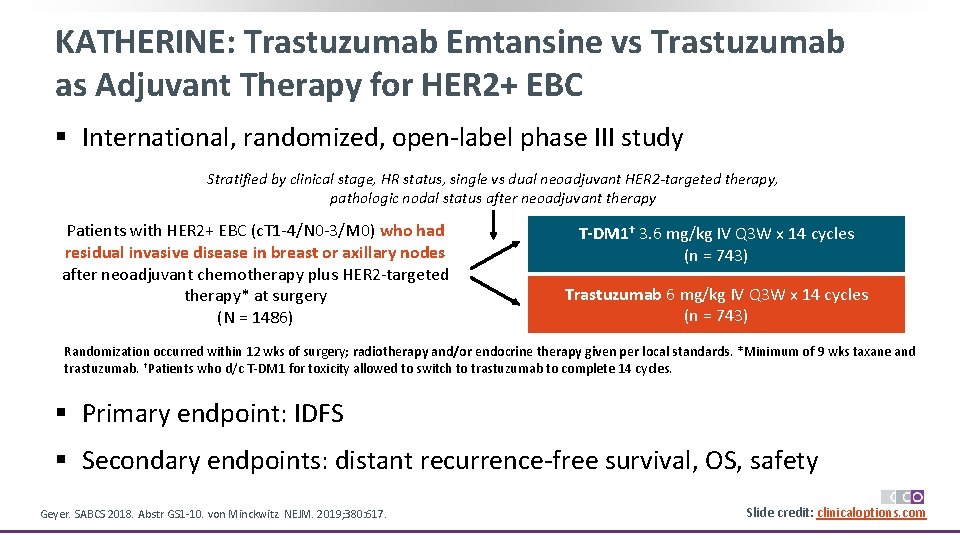
KATHERINE: Trastuzumab Emtansine vs Trastuzumab as Adjuvant Therapy for HER 2+ EBC § International, randomized, open-label phase III study Stratified by clinical stage, HR status, single vs dual neoadjuvant HER 2 -targeted therapy, pathologic nodal status after neoadjuvant therapy Patients with HER 2+ EBC (c. T 1 -4/N 0 -3/M 0) who had residual invasive disease in breast or axillary nodes after neoadjuvant chemotherapy plus HER 2 -targeted therapy* at surgery (N = 1486) T-DM 1† 3. 6 mg/kg IV Q 3 W x 14 cycles (n = 743) Trastuzumab 6 mg/kg IV Q 3 W x 14 cycles (n = 743) Randomization occurred within 12 wks of surgery; radiotherapy and/or endocrine therapy given per local standards. *Minimum of 9 wks taxane and trastuzumab. †Patients who d/c T-DM 1 for toxicity allowed to switch to trastuzumab to complete 14 cycles. § Primary endpoint: IDFS § Secondary endpoints: distant recurrence-free survival, OS, safety Geyer. SABCS 2018. Abstr GS 1 -10. von Minckwitz. NEJM. 2019; 380: 617. Slide credit: clinicaloptions. com

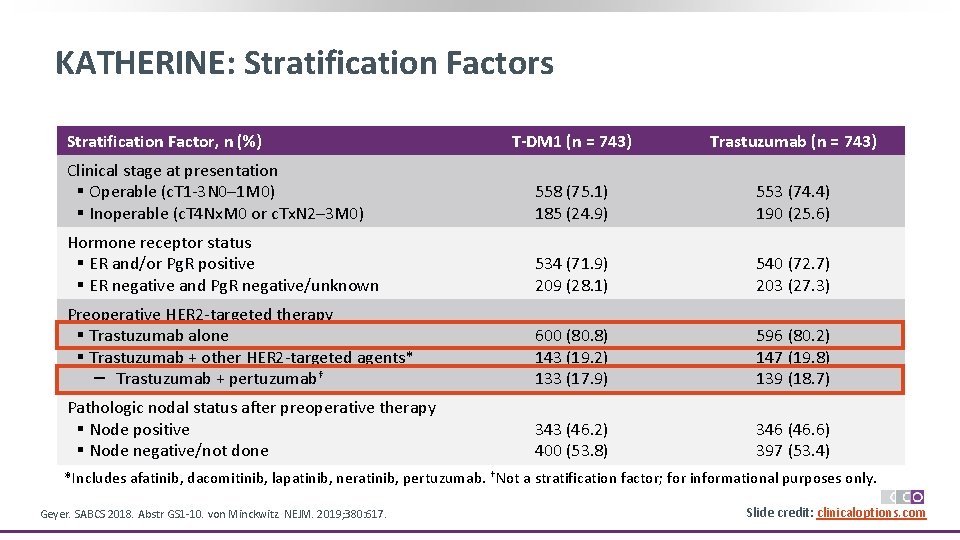
KATHERINE: Stratification Factors Stratification Factor, n (%) T-DM 1 (n = 743) Trastuzumab (n = 743) Clinical stage at presentation § Operable (c. T 1 -3 N 0– 1 M 0) § Inoperable (c. T 4 Nx. M 0 or c. Tx. N 2– 3 M 0) 558 (75. 1) 185 (24. 9) 553 (74. 4) 190 (25. 6) Hormone receptor status § ER and/or Pg. R positive § ER negative and Pg. R negative/unknown 534 (71. 9) 209 (28. 1) 540 (72. 7) 203 (27. 3) Preoperative HER 2 -targeted therapy § Trastuzumab alone § Trastuzumab + other HER 2 -targeted agents* – Trastuzumab + pertuzumab† 600 (80. 8) 143 (19. 2) 133 (17. 9) 596 (80. 2) 147 (19. 8) 139 (18. 7) Pathologic nodal status after preoperative therapy § Node positive § Node negative/not done 343 (46. 2) 400 (53. 8) 346 (46. 6) 397 (53. 4) *Includes afatinib, dacomitinib, lapatinib, neratinib, pertuzumab. †Not a stratification factor; for informational purposes only. Geyer. SABCS 2018. Abstr GS 1 -10. von Minckwitz. NEJM. 2019; 380: 617. Slide credit: clinicaloptions. com

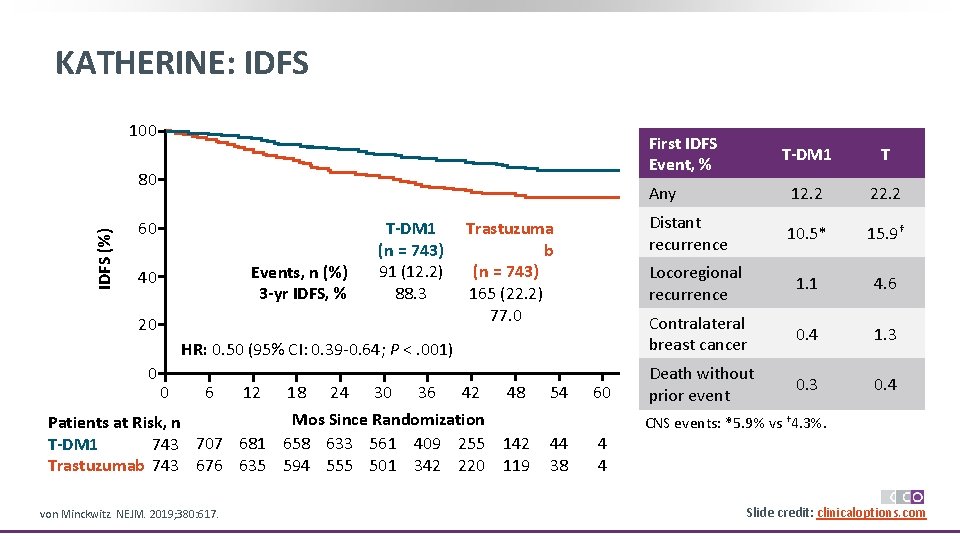
KATHERINE: IDFS 100 First IDFS Event, % IDFS (%) 80 60 Events, n (%) 3 -yr IDFS, % 40 T-DM 1 (n = 743) 91 (12. 2) 88. 3 20 Trastuzuma b (n = 743) 165 (22. 2) 77. 0 HR: 0. 50 (95% CI: 0. 39 -0. 64; P <. 001) 0 0 6 12 18 24 30 36 42 48 Mos Since Randomization Patients at Risk, n 743 707 681 658 633 561 409 255 142 T-DM 1 Trastuzumab 743 676 635 594 555 501 342 220 119 von Minckwitz. NEJM. 2019; 380: 617. 54 60 T-DM 1 T Any 12. 2 22. 2 Distant recurrence 10. 5* 15. 9† Locoregional recurrence 1. 1 4. 6 Contralateral breast cancer 0. 4 1. 3 Death without prior event 0. 3 0. 4 CNS events: *5. 9% vs † 4. 3%. 44 38 4 4 Slide credit: clinicaloptions. com

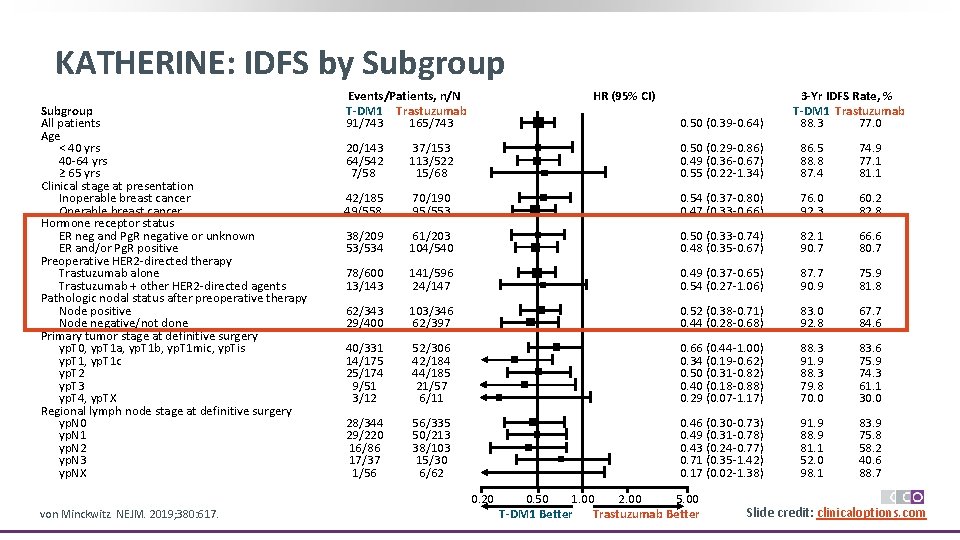
KATHERINE: IDFS by Subgroup All patients Age < 40 yrs 40 -64 yrs ≥ 65 yrs Clinical stage at presentation Inoperable breast cancer Operable breast cancer Hormone receptor status ER neg and Pg. R negative or unknown ER and/or Pg. R positive Preoperative HER 2 -directed therapy Trastuzumab alone Trastuzumab + other HER 2 -directed agents Pathologic nodal status after preoperative therapy Node positive Node negative/not done Primary tumor stage at definitive surgery yp. T 0, yp. T 1 a, yp. T 1 b, yp. T 1 mic, yp. Tis yp. T 1, yp. T 1 c yp. T 2 yp. T 3 yp. T 4, yp. TX Regional lymph node stage at definitive surgery yp. N 0 yp. N 1 yp. N 2 yp. N 3 yp. NX Events/Patients, n/N T-DM 1 Trastuzumab 91/743 165/743 HR (95% CI) 0. 50 (0. 39 -0. 64) 20/143 64/542 7/58 37/153 113/522 15/68 0. 50 (0. 29 -0. 86) 0. 49 (0. 36 -0. 67) 0. 55 (0. 22 -1. 34) 86. 5 88. 8 87. 4 74. 9 77. 1 81. 1 42/185 49/558 70/190 95/553 0. 54 (0. 37 -0. 80) 0. 47 (0. 33 -0. 66) 76. 0 92. 3 60. 2 82. 8 38/209 53/534 61/203 104/540 0. 50 (0. 33 -0. 74) 0. 48 (0. 35 -0. 67) 82. 1 90. 7 66. 6 80. 7 78/600 13/143 141/596 24/147 0. 49 (0. 37 -0. 65) 0. 54 (0. 27 -1. 06) 87. 7 90. 9 75. 9 81. 8 62/343 29/400 103/346 62/397 0. 52 (0. 38 -0. 71) 0. 44 (0. 28 -0. 68) 83. 0 92. 8 67. 7 84. 6 40/331 14/175 25/174 9/51 3/12 52/306 42/184 44/185 21/57 6/11 0. 66 (0. 44 -1. 00) 0. 34 (0. 19 -0. 62) 0. 50 (0. 31 -0. 82) 0. 40 (0. 18 -0. 88) 0. 29 (0. 07 -1. 17) 88. 3 91. 9 88. 3 79. 8 70. 0 83. 6 75. 9 74. 3 61. 1 30. 0 28/344 29/220 16/86 17/37 1/56 56/335 50/213 38/103 15/30 6/62 0. 46 (0. 30 -0. 73) 0. 49 (0. 31 -0. 78) 0. 43 (0. 24 -0. 77) 0. 71 (0. 35 -1. 42) 0. 17 (0. 02 -1. 38) 91. 9 88. 9 81. 1 52. 0 98. 1 83. 9 75. 8 58. 2 40. 6 88. 7 0. 20 von Minckwitz. NEJM. 2019; 380: 617. 3 -Yr IDFS Rate, % T-DM 1 Trastuzumab 88. 3 77. 0 0. 50 2. 00 1. 00 5. 00 Trastuzumab Better T-DM 1 Better Slide credit: clinicaloptions. com

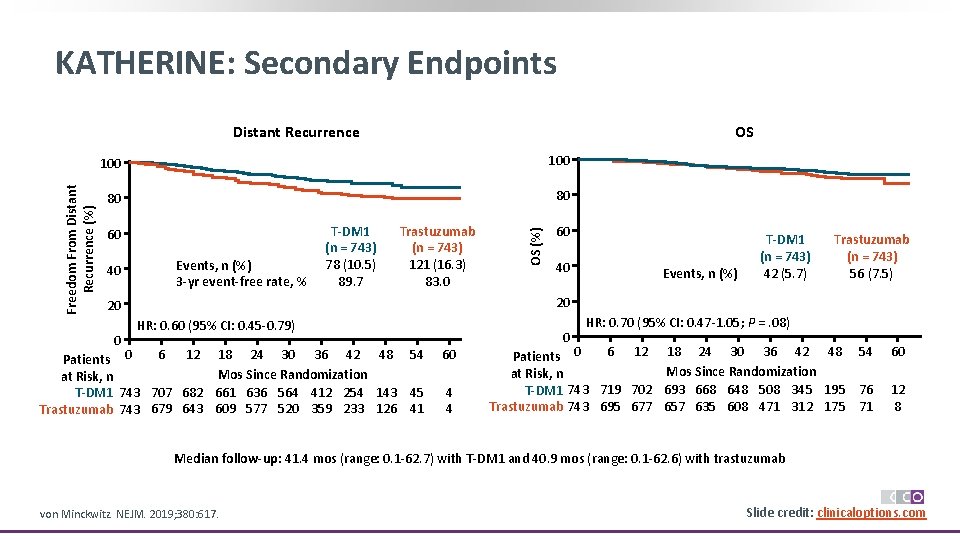
KATHERINE: Secondary Endpoints OS 100 80 80 60 40 Events, n (%) 3 -yr event-free rate, % T-DM 1 (n = 743) 78 (10. 5) 89. 7 Trastuzumab (n = 743) 121 (16. 3) 83. 0 60 40 Events, n (%) T-DM 1 (n = 743) 42 (5. 7) Trastuzumab (n = 743) 56 (7. 5) 20 20 0 OS (%) Freedom From Distant Recurrence (%) Distant Recurrence HR: 0. 60 (95% CI: 0. 45 -0. 79) 6 12 18 24 30 36 42 48 54 Patients 0 Mos Since Randomization at Risk, n T-DM 1 743 707 682 661 636 564 412 254 143 45 Trastuzumab 743 679 643 609 577 520 359 233 126 41 60 4 4 0 HR: 0. 70 (95% CI: 0. 47 -1. 05; P =. 08) 6 12 18 24 30 36 42 48 54 Patients 0 Mos Since Randomization at Risk, n T-DM 1 743 719 702 693 668 648 508 345 195 76 Trastuzumab 743 695 677 657 635 608 471 312 175 71 60 12 8 Median follow-up: 41. 4 mos (range: 0. 1 -62. 7) with T-DM 1 and 40. 9 mos (range: 0. 1 -62. 6) with trastuzumab von Minckwitz. NEJM. 2019; 380: 617. Slide credit: clinicaloptions. com

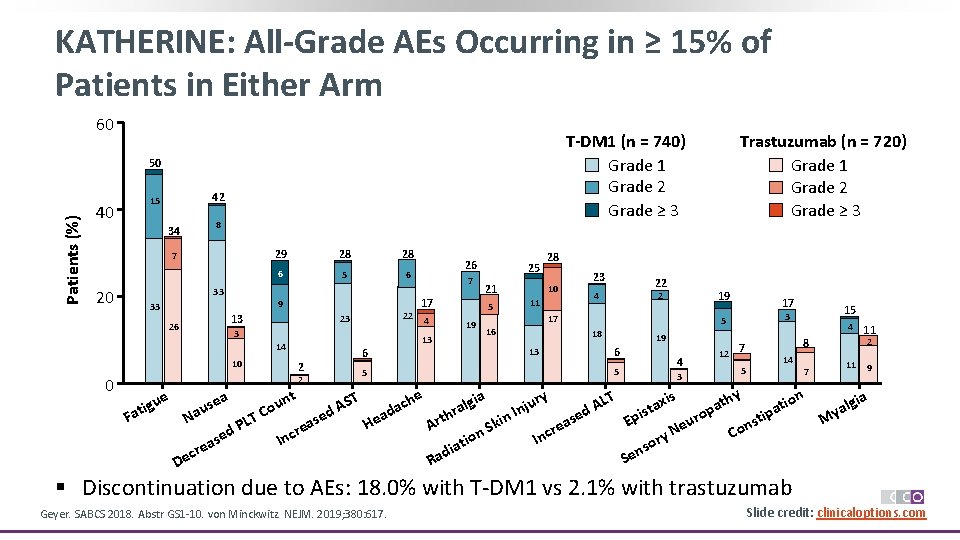
KATHERINE: All-Grade AEs Occurring in ≥ 15% of Patients in Either Arm 60 T-DM 1 (n = 740) Grade 1 Grade 2 Grade ≥ 3 Patients (%) 50 40 42 15 8 34 7 20 29 28 28 6 5 6 33 9 33 13 26 22 23 3 14 10 0 ue se au ig Fat N LT P d se ea ecr D a 6 2 t un o C 5 2 T se a e r Inc S d. A 26 7 17 4 13 25 21 5 19 28 10 11 23 22 4 19 2 17 16 17 19 6 5 4 3 12 15 3 5 18 13 Trastuzumab (n = 720) Grade 1 Grade 2 Grade ≥ 3 7 5 4 11 8 14 2 11 7 ry LT xis thy gia ion u che l t j A a a t a n p hr ed ad ro n. I pis tip s i s u E a k He n Art e e S N Co y ncr on r I i t so n dia e a S R 9 ia g yal M § Discontinuation due to AEs: 18. 0% with T-DM 1 vs 2. 1% with trastuzumab Geyer. SABCS 2018. Abstr GS 1 -10. von Minckwitz. NEJM. 2019; 380: 617. Slide credit: clinicaloptions. com

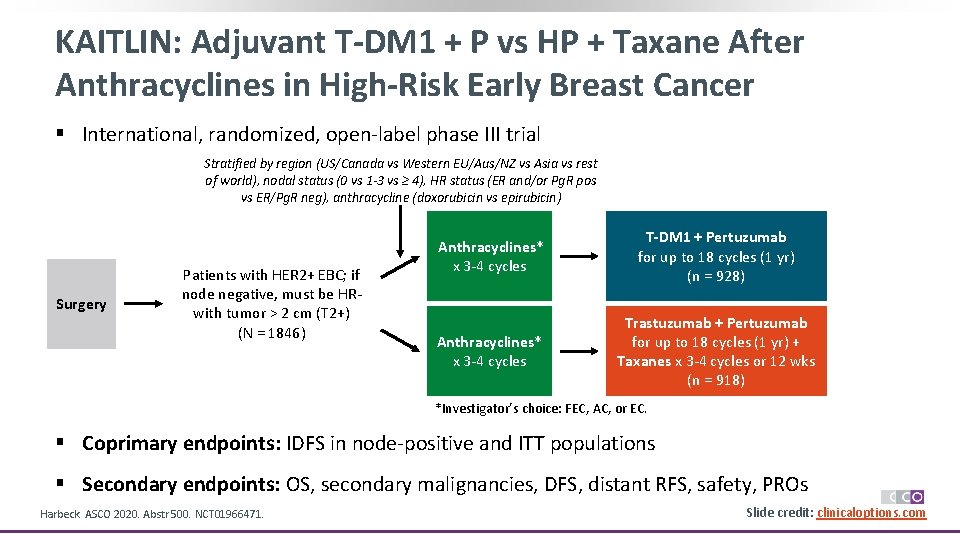
KAITLIN: Adjuvant T-DM 1 + P vs HP + Taxane After Anthracyclines in High-Risk Early Breast Cancer § International, randomized, open-label phase III trial Stratified by region (US/Canada vs Western EU/Aus/NZ vs Asia vs rest of world), nodal status (0 vs 1 -3 vs ≥ 4), HR status (ER and/or Pg. R pos vs ER/Pg. R neg), anthracycline (doxorubicin vs epirubicin) Surgery Patients with HER 2+ EBC; if node negative, must be HR- with tumor > 2 cm (T 2+) (N = 1846) Anthracyclines* x 3 -4 cycles T-DM 1 + Pertuzumab for up to 18 cycles (1 yr) (n = 928) Anthracyclines* x 3 -4 cycles Trastuzumab + Pertuzumab for up to 18 cycles (1 yr) + Taxanes x 3 -4 cycles or 12 wks (n = 918) *Investigator’s choice: FEC, AC, or EC. § Coprimary endpoints: IDFS in node-positive and ITT populations § Secondary endpoints: OS, secondary malignancies, DFS, distant RFS, safety, PROs Harbeck. ASCO 2020. Abstr 500. NCT 01966471. Slide credit: clinicaloptions. com

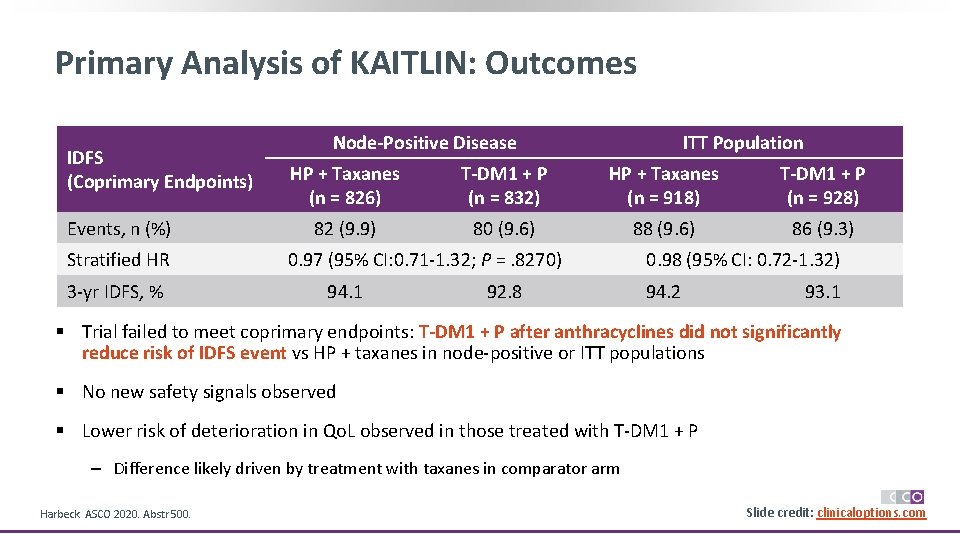
Primary Analysis of KAITLIN: Outcomes IDFS (Coprimary Endpoints) Events, n (%) Stratified HR 3 -yr IDFS, % Node-Positive Disease ITT Population HP + Taxanes (n = 826) T-DM 1 + P (n = 832) HP + Taxanes (n = 918) T-DM 1 + P (n = 928) 82 (9. 9) 80 (9. 6) 88 (9. 6) 86 (9. 3) 0. 97 (95% CI: 0. 71 -1. 32; P =. 8270) 94. 1 92. 8 0. 98 (95% CI: 0. 72 -1. 32) 94. 2 93. 1 § Trial failed to meet coprimary endpoints: T-DM 1 + P after anthracyclines did not significantly reduce risk of IDFS event vs HP + taxanes in node-positive or ITT populations § No new safety signals observed § Lower risk of deterioration in Qo. L observed in those treated with T-DM 1 + P ‒ Difference likely driven by treatment with taxanes in comparator arm Harbeck. ASCO 2020. Abstr 500. Slide credit: clinicaloptions. com

So, What Do We Do?

Proposed Strategy for Managing Patients With Stage I-III HER 2+ EBC c. T 1 a/b, c. N 0* c. T 1 c c. N 0 ≥ c. T 2 or ≥ c. N 1 Surgery → trastuzumabbased therapy (observation only; TH followed by H* 1 yr) Assess Risk* and Patient Preference Neoadjuvant TCHP or AC-THP *High-risk patients (age < 35 yrs, grade 3, hormone receptor negative, multifocal disease) could be considered for neoadjuvant therapy. ‡In the phase II CONTROL trial assessing the effectiveness of antidiarrheal prophylaxis for neratinib-induced diarrhea, 54% of patients received pertuzumab as part of (neo)adjuvant therapy prior to extended adjuvant therapy with neratinib. [1] 1. Barcenas. Ann Oncol. 2020; [Epub]. Residual invasive disease T-DM 1 x 14 p. CR (yp. T 0/is yp. N 0) HR+ H ± P‡ x 1 yr ? ? Neratinib if HR+ HR− H±P x 1 yr Neratinib Slide credit: clinicaloptions. com

Managing AEs Associated With HER 2 -Targeted Therapy in EBC

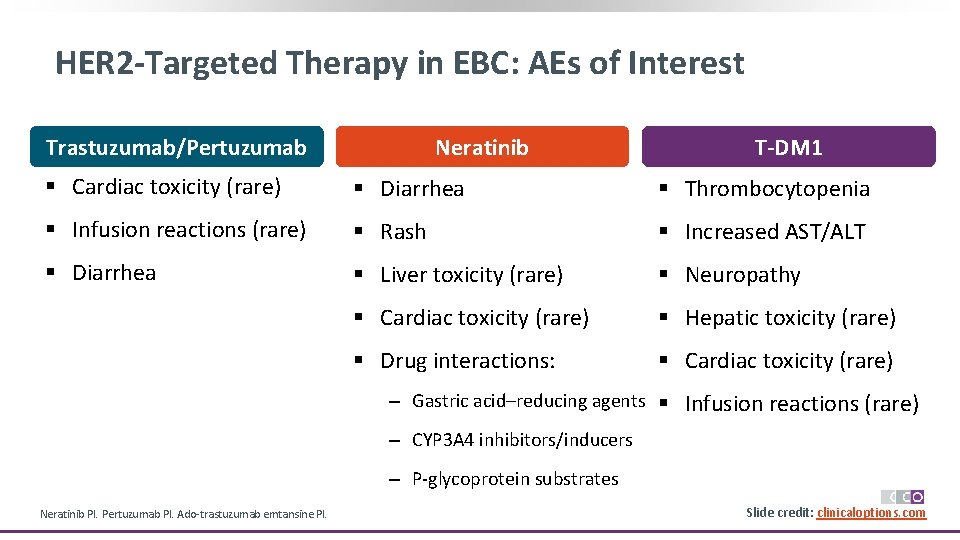
HER 2 -Targeted Therapy in EBC: AEs of Interest Trastuzumab/Pertuzumab Neratinib T-DM 1 § Cardiac toxicity (rare) § Diarrhea § Thrombocytopenia § Infusion reactions (rare) § Rash § Increased AST/ALT § Diarrhea § Liver toxicity (rare) § Neuropathy § Cardiac toxicity (rare) § Hepatic toxicity (rare) § Drug interactions: § Cardiac toxicity (rare) ‒ Gastric acid–reducing agents § Infusion reactions (rare) ‒ CYP 3 A 4 inhibitors/inducers ‒ P-glycoprotein substrates Neratinib PI. Pertuzumab PI. Ado-trastuzumab emtansine PI. Slide credit: clinicaloptions. com

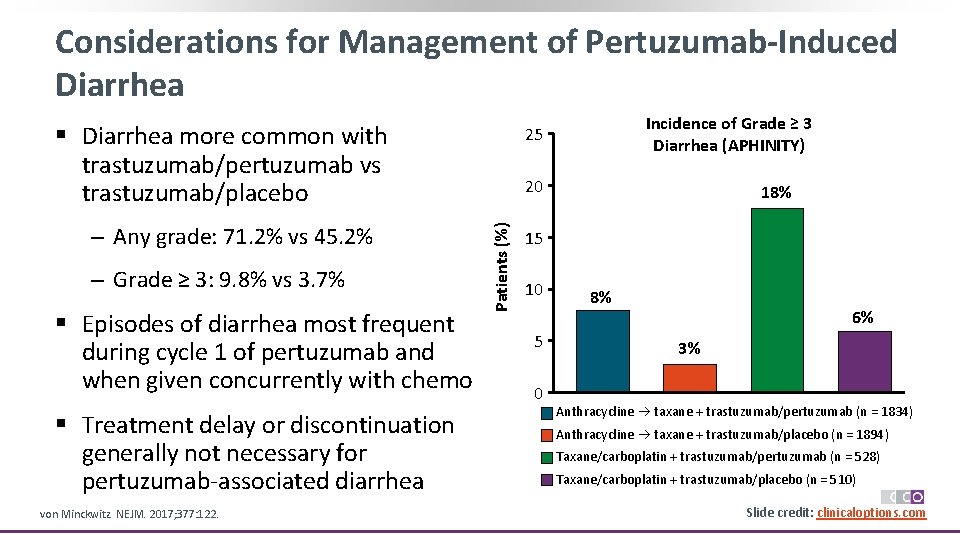
Considerations for Management of Pertuzumab-Induced Diarrhea § Diarrhea more common with trastuzumab/pertuzumab vs trastuzumab/placebo ‒ Grade ≥ 3: 9. 8% vs 3. 7% § Episodes of diarrhea most frequent during cycle 1 of pertuzumab and when given concurrently with chemo § Treatment delay or discontinuation generally not necessary for pertuzumab-associated diarrhea von Minckwitz. NEJM. 2017; 377: 122. 20 Patients (%) ‒ Any grade: 71. 2% vs 45. 2% Incidence of Grade ≥ 3 Diarrhea (APHINITY) 25 18% 15 10 5 0 8% 6% 3% Anthracycline taxane + trastuzumab/pertuzumab (n = 1834) Anthracycline taxane + trastuzumab/placebo (n = 1894) Taxane/carboplatin + trastuzumab/pertuzumab (n = 528) Taxane/carboplatin + trastuzumab/placebo (n = 510) Slide credit: clinicaloptions. com

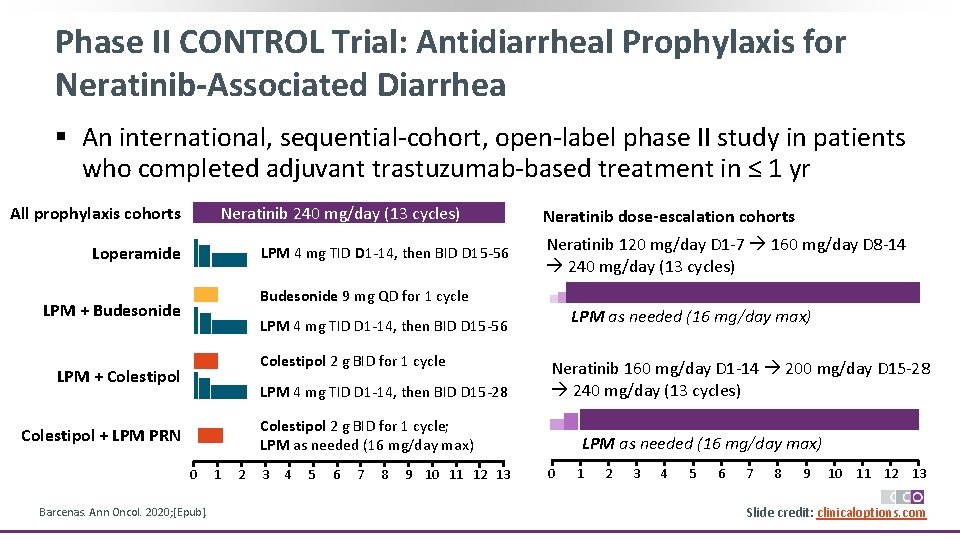
Phase II CONTROL Trial: Antidiarrheal Prophylaxis for Neratinib-Associated Diarrhea § An international, sequential-cohort, open-label phase II study in patients who completed adjuvant trastuzumab-based treatment in ≤ 1 yr All prophylaxis cohorts Neratinib 240 mg/day (13 cycles) Loperamide LPM 4 mg TID D 1 -14, then BID D 15 -56 Neratinib dose-escalation cohorts Neratinib 120 mg/day D 1 -7 160 mg/day D 8 -14 240 mg/day (13 cycles) Budesonide 9 mg QD for 1 cycle LPM + Budesonide LPM as needed (16 mg/day max) LPM 4 mg TID D 1 -14, then BID D 15 -56 Colestipol 2 g BID for 1 cycle LPM + Colestipol LPM 4 mg TID D 1 -14, then BID D 15 -28 Neratinib 160 mg/day D 1 -14 200 mg/day D 15 -28 240 mg/day (13 cycles) Colestipol 2 g BID for 1 cycle; LPM as needed (16 mg/day max) Colestipol + LPM PRN 0 Barcenas. Ann Oncol. 2020; [Epub]. 1 2 3 4 5 6 7 8 9 10 11 12 13 LPM as needed (16 mg/day max) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Slide credit: clinicaloptions. com

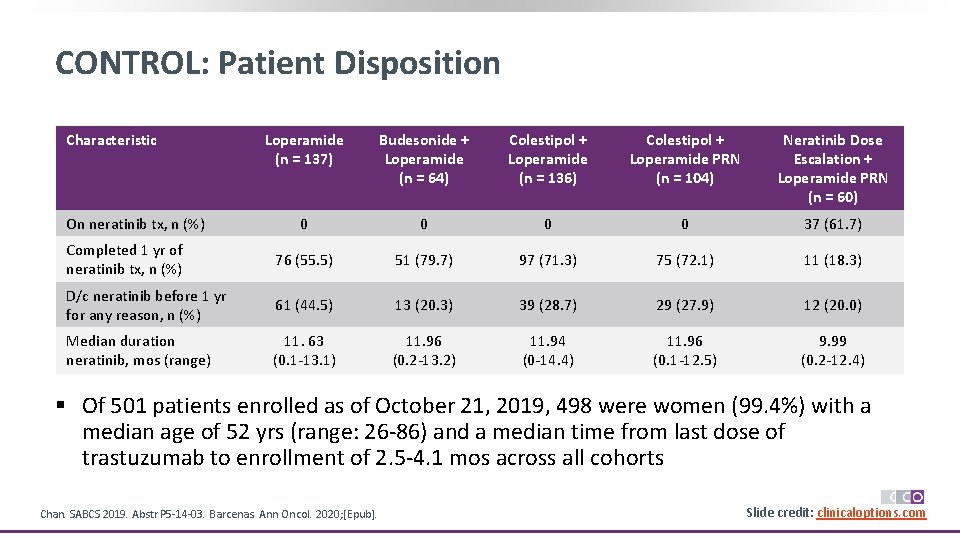
CONTROL: Patient Disposition Characteristic Loperamide (n = 137) Budesonide + Loperamide (n = 64) Colestipol + Loperamide (n = 136) Colestipol + Loperamide PRN (n = 104) Neratinib Dose Escalation + Loperamide PRN (n = 60) 0 0 37 (61. 7) Completed 1 yr of neratinib tx, n (%) 76 (55. 5) 51 (79. 7) 97 (71. 3) 75 (72. 1) 11 (18. 3) D/c neratinib before 1 yr for any reason, n (%) 61 (44. 5) 13 (20. 3) 39 (28. 7) 29 (27. 9) 12 (20. 0) Median duration neratinib, mos (range) 11. 63 (0. 1 -13. 1) 11. 96 (0. 2 -13. 2) 11. 94 (0 -14. 4) 11. 96 (0. 1 -12. 5) 9. 99 (0. 2 -12. 4) On neratinib tx, n (%) § Of 501 patients enrolled as of October 21, 2019, 498 were women (99. 4%) with a median age of 52 yrs (range: 26 -86) and a median time from last dose of trastuzumab to enrollment of 2. 5 -4. 1 mos across all cohorts Chan. SABCS 2019. Abstr P 5 -14 -03. Barcenas. Ann Oncol. 2020; [Epub]. Slide credit: clinicaloptions. com

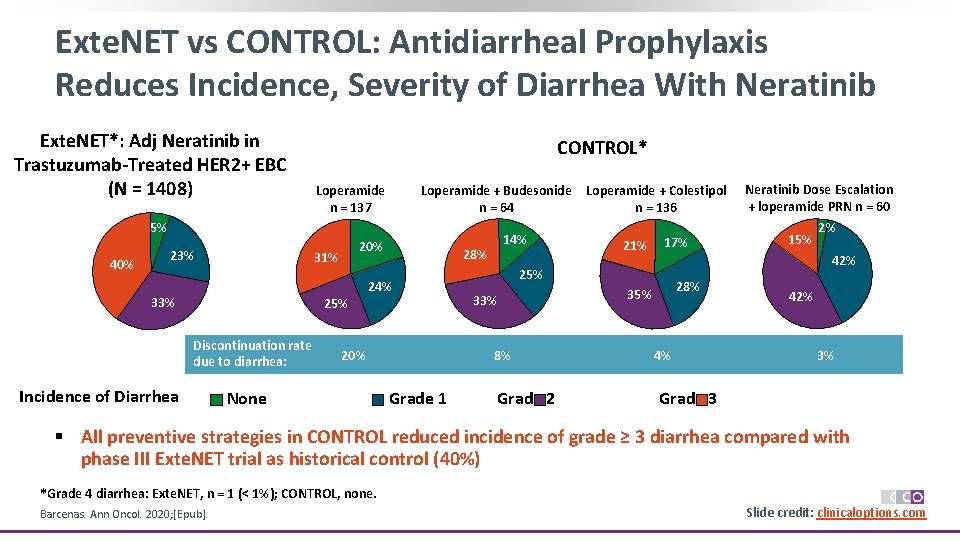
Exte. NET vs CONTROL: Antidiarrheal Prophylaxis Reduces Incidence, Severity of Diarrhea With Neratinib Exte. NET*: Adj Neratinib in Trastuzumab-Treated HER 2+ EBC (N = 1408) CONTROL* Loperamide n = 137 Loperamide + Budesonide n = 64 5% 40% 23% 20% 31% 33% 25% Discontinuation rate due to diarrhea: Incidence of Diarrhea 24% 20% None 14% 28% 21% 25% 33% 8% Grade 1 Loperamide + Colestipol Sales n = 136 Grade 2 None Grade 1 17% Neratinib Dose Escalation + loperamide PRN n = 60 2% 15% 42% 28% Grade 2 4% 42% Grade 3 3% Grade 3 § All preventive strategies in CONTROL reduced incidence of grade ≥ 3 diarrhea compared with phase III Exte. NET trial as historical control (40%) *Grade 4 diarrhea: Exte. NET, n = 1 (< 1%); CONTROL, none. Barcenas. Ann Oncol. 2020; [Epub]. Slide credit: clinicaloptions. com

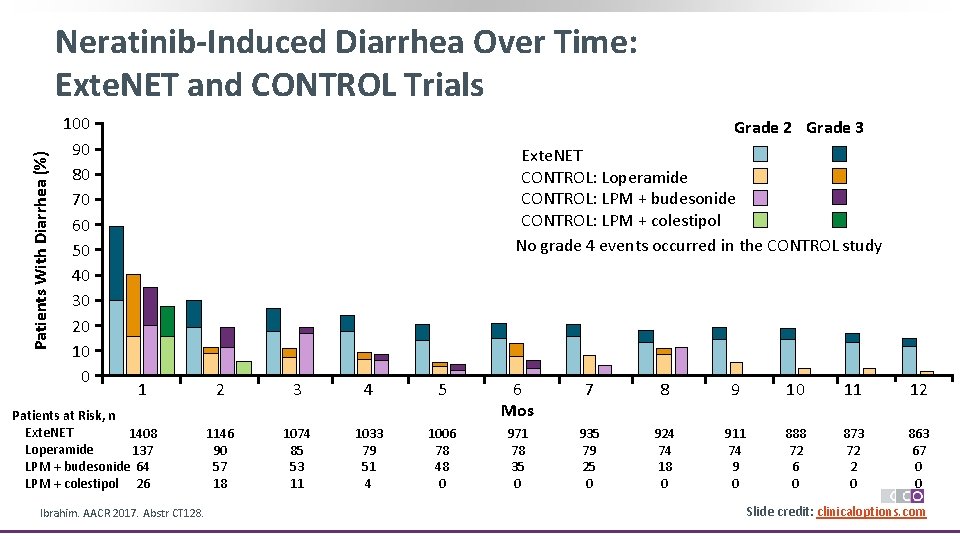
Patients With Diarrhea (%) Neratinib-Induced Diarrhea Over Time: Exte. NET and CONTROL Trials 100 90 80 70 60 50 40 30 20 10 0 Grade 2 Grade 3 Exte. NET CONTROL: Loperamide CONTROL: LPM + budesonide CONTROL: LPM + colestipol No grade 4 events occurred in the CONTROL study 1 Patients at Risk, n Exte. NET 1408 Loperamide 137 LPM + budesonide 64 LPM + colestipol 26 Ibrahim. AACR 2017. Abstr CT 128. 2 3 4 5 6 Mos 7 8 9 10 11 12 1146 90 57 18 1074 85 53 11 1033 79 51 4 1006 78 48 0 971 78 35 0 935 79 25 0 924 74 18 0 911 74 9 0 888 72 6 0 873 72 2 0 863 67 0 0 Slide credit: clinicaloptions. com

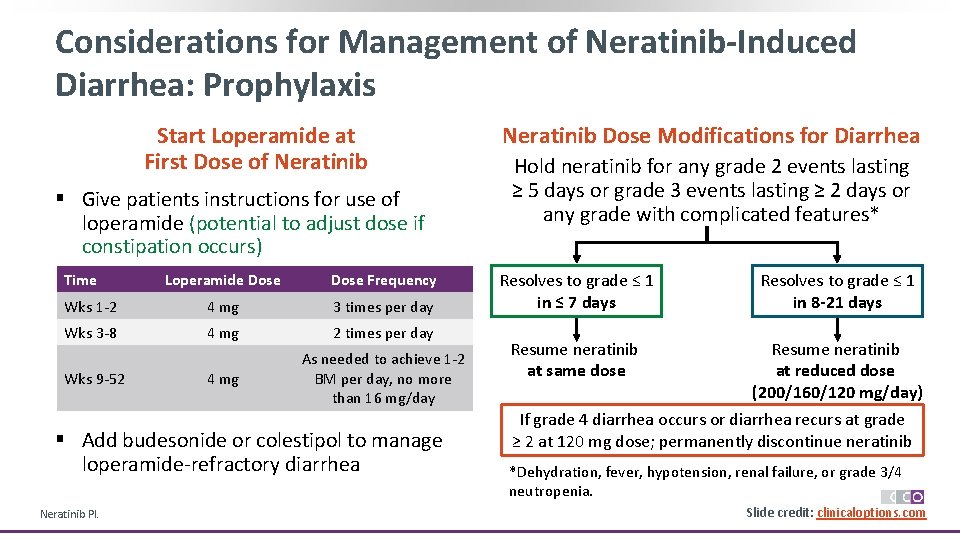
Considerations for Management of Neratinib-Induced Diarrhea: Prophylaxis Start Loperamide at First Dose of Neratinib § Give patients instructions for use of loperamide (potential to adjust dose if constipation occurs) Time Loperamide Dose Frequency Wks 1 -2 4 mg 3 times per day Wks 3 -8 4 mg 2 times per day 4 mg As needed to achieve 1 -2 BM per day, no more than 16 mg/day Wks 9 -52 § Add budesonide or colestipol to manage loperamide-refractory diarrhea Neratinib PI. Neratinib Dose Modifications for Diarrhea Hold neratinib for any grade 2 events lasting ≥ 5 days or grade 3 events lasting ≥ 2 days or any grade with complicated features* Resolves to grade ≤ 1 in ≤ 7 days Resolves to grade ≤ 1 in 8 -21 days Resume neratinib at same dose Resume neratinib at reduced dose (200/160/120 mg/day) If grade 4 diarrhea occurs or diarrhea recurs at grade ≥ 2 at 120 mg dose; permanently discontinue neratinib *Dehydration, fever, hypotension, renal failure, or grade 3/4 neutropenia. Slide credit: clinicaloptions. com

Considerations for Cardiac Dysfunction During Adjuvant Trastuzumab/Pertuzumab or T-DM 1 § Both HER 2 -targeted therapy and anthracyclines can result in decreased LVEF and CHF (subclinical or clinical cardiac failure) Trastuzumab/Pertuzumab T-DM 1 Baseline Assessment of LVEF Pretreatment: LVEF ≥ 55% or ≥ 50% after anthracyclines Monitor LVEF every 12 wks during therapy For LVEF decrease to Resume tx if < 50% with ≥ 10% LVEF improves to decrease from baseline: ≥ 50% or < 10% hold HER 2 -targeted tx for below baseline at least 3 wks Baseline Assessment of LVEF Pertuzumab PI. Ado-trastuzumab emtansine PI. Pretreatment: LVEF ≥ 50% Monitor LVEF at regular intervals during For LVEF decreasetherapy of Resume tx if < 40% or 45% with LVEF improves to ≥ 10% decrease from ≥ 40% or within baseline: hold T-DM 1 for 10% of baseline at least 3 wks Slide credit: clinicaloptions. com
![Future Directions Ongoing Clinical Trials in HER 2 EBC Trial Name IMpassion 0501 Phase Future Directions: Ongoing Clinical Trials in HER 2+ EBC Trial Name IMpassion 050[1] Phase](https://slidetodoc.com/presentation_image_h/09d69ae7a0b1a4f788139ad317707dd9/image-58.jpg)
Future Directions: Ongoing Clinical Trials in HER 2+ EBC Trial Name IMpassion 050[1] Phase Setting Treatment Arms Primary Endpoint III Neoadjuvant; T 2 -4, N 1 -3, M 0 with known HER 2, HR, PD-L 1 status AC + atezolizumab THP + atezolizumab vs AC + Pbo THP + Pbo p. CR Neoadjuvant; early high-risk (T 1 c-2 N 1 TCHP vs TCHP + atezolizumab or T 3 N 0) or LA disease suitable for vs AC + atezolizumab neoadjuvant tx TCHP + atezolizumab EFS APTneo[2] III PALTAN[3] II Neoadjuvant; stage II-III ER+ HER 2+ (tumor ≥ 2 cm) Palbociclib + letrozole + H ± goserelin p. CR NA-PHER 2[4, 5] II Neoadjuvant; early ER+ HER 2+ (tumor > 1. 5 cm) HP + palbociclib ± fulvestrant Ki 67 1. NCT 03726879. 2. NCT 03595592. 3. NCT 02907918. 4. NCT 02530424. 5. Gianni. ASCO 2019. Abstr 527. Slide credit: clinicaloptions. com

Summary § For patients with HER 2 -positive BC, the availability of HER 2 -targeted agents have markedly and rapidly improved overall outcomes § For patients with small (< 3 cm), node negative HER 2 positive EBC, consider combination of paclitaxel and trastuzumab based on APT trial § Neoadj chemo plus trastuzumab/pertuzumab for patients with HER 2+ EBC and a tumor ≥ 2 cm (T 2) diameter or with node-positive disease § For adjuvant therapy: ‒ APHINITY: dual HER 2 -therapy with trastuzumab and pertuzumab (either continued for a total of 1 year after neoadj chemo or with adj chemo for a total of 1 year) ‒ KATHERINE: T-DM 1 as adjuvant therapy for patients with residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment ‒ Exte. NET: extended adjuvant therapy with neratinib after anti-HER 2 antibody therapy, particularly hormone receptor positive disease and without p. CR after neoadj therapy

Go Online for More CCO Coverage of Breast Cancer! Downloadable slides and on-demand Webcast from recent Webinars Capsule Summaries of the most clinically relevant studies from ASCO 2020 selected by breast cancer experts clinicaloptions. com/oncology